Don’t Go Breaking My Heart: MCMV as a Model for HCMV-Associated Cardiovascular Diseases
Abstract
1. Introduction
2. HCMV and Cardiovascular Diseases
3. MCMV and Cardiovascular Research
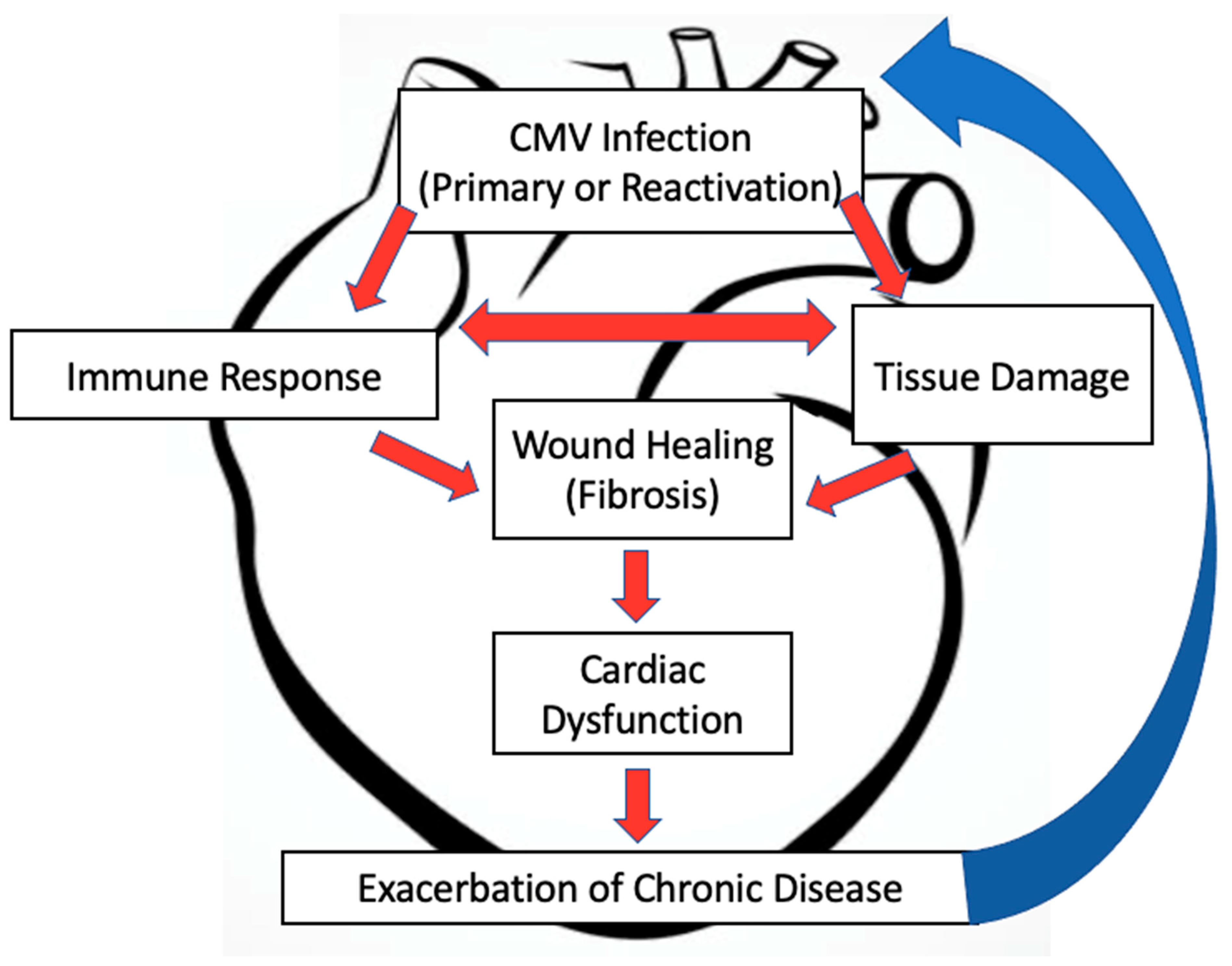
4. MCMV and Cardiac Dysfunction
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Staras, S.A.S.; Flanders, W.D.; Dollard, S.C.; Pass, R.F.; McGowan, J.E.; Cannon, M.J. Cytomegalovirus Seroprevalence and Childhood Sources of Infection: A Population-Based Study among Pre-Adolescents in the United States. J. Clin. Virol. 2008, 43, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the Worldwide Seroprevalence of Cytomegalovirus: A Systematic Review and Meta-Analysis. Rev. Med. Virol. 2019, 29, e2034. [Google Scholar] [CrossRef]
- Bate, S.L.; Dollard, S.C.; Cannon, M.J. Cytomegalovirus Seroprevalence in the United States: The National Health and Nutrition Examination Surveys, 1988–2004. Clin. Infect. Dis. 2010, 50, 1439–1447. [Google Scholar] [CrossRef]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of Cytomegalovirus Seroprevalence and Demographic Characteristics Associated with Infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Soderberg-Naucler, C. Does Cytomegalovirus Play a Causative Role in the Development of Various Inflammatory Diseases and Cancer? J. Intern. Med. 2006, 259, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Vescovini, R.; Telera, A.R.; Pedrazzoni, M.; Abbate, B.; Rossetti, P.; Verzicco, I.; Arcangeletti, M.C.; Medici, M.C.; Calderaro, A.; Volpi, R.; et al. Impact of Persistent Cytomegalovirus Infection on Dynamic Changes in Human Immune System Profile. PLoS ONE 2016, 11, e0151965. [Google Scholar] [CrossRef] [PubMed]
- Savva, G.M.; Pachnio, A.; Kaul, B.; Morgan, K.; Huppert, F.A.; Brayne, C.; Moss, P.A.H. The Medical Research Council Cognitive Function and Ageing Study Cytomegalovirus Infection Is Associated with Increased Mortality in the Older Population. Aging Cell 2013, 12, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Okedele, O.O.; Nelson, H.H.; Oyenuga, M.L.; Thyagarajan, B.; Prizment, A. Cytomegalovirus and Cancer-Related Mortality in the National Health and Nutritional Examination Survey. Cancer Causes Control 2020, 31, 541–547. [Google Scholar] [CrossRef]
- Simanek, A.M.; Dowd, J.B.; Pawelec, G.; Melzer, D.; Dutta, A.; Aiello, A.E. Seropositivity to Cytomegalovirus, Inflammation, All-Cause and Cardiovascular Disease-Related Mortality in the United States. PLoS ONE 2011, 6, e16103. [Google Scholar] [CrossRef]
- Wilson, R.S.E.; Morris, T.H.; Rees, J.R. Cytomegalovirus Myocarditis. Br. Heart J. 1972, 4, 865–868. [Google Scholar] [CrossRef][Green Version]
- Petrie, B.L.; Melnick, J.L.; Adam, E.; Burek, J.; McCollum, C.H.; DeBakey, M.E. Nucleic Acid Sequences of Cytomegalovirus in Cells Cultured from Human Arterial Tissue. J. Infect. Dis. 1987, 155, 158–159. [Google Scholar] [CrossRef]
- Sinzger, C.; Grefte, A.; Plachter, B.; Gouw, A.S.H.; The, T.H.; Jahn, G. Fibroblasts, Epithelial Cells, Endothelial Cells and Smooth Muscle Cells Are Major Targets of Human Cytomegalovirus Infection in Lung and Gastrointestinal Tissues. J. Gen. Virol. 1995, 76, 741–750. [Google Scholar] [CrossRef]
- Soderberg-Naucler, C.; Larsson, S.; Bergstedt-Lindqvist, S.; Moller, E. Identification of Blood Mononuclear Cells Permissive of Cytomegalovirus Infection in Vitro. Transplant. Proc. 1993, 25, 1416–1418. [Google Scholar]
- Schönian, U.; Crombach, M.; Maisch, B. Assessment of Cytomegalovirus DNA and Protein Expression in Patients with Myocarditis. Clin. Immunol. Immunopathol. 1993, 68, 229–233. [Google Scholar] [CrossRef]
- Melnick, J. Cytomegalovirus Antigen within Human Arterial Smooth Muscle Cells. Lancet 1983, 322, 644–647. [Google Scholar] [CrossRef]
- Kyto, V.; Vuorinen, T.; Saukko, P.; Lautenschlager, I.; Lignitz, E.; Saraste, A.; Voipio-Pulkki, L.-M. Cytomegalovirus Infection of the Heart Is Common in Patients with Fatal Myocarditis. Clin. Infect. Dis. 2005, 40, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Magno Palmeira, M.; Umemura Ribeiro, H.Y.; Garcia Lira, Y.; Machado Jucá Neto, F.O.; da Silva Rodrigues, I.A.; Fernandes da Paz, L.N.; da Nascimento Pinheiro, M.C. Heart Failure Due to Cytomegalovirus Myocarditis in Immunocompetent Young Adults: A Case Report. BMC Res. Notes 2016, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.K.; Kumar, A.; Padala, S. Fulminant Cytomegalovirus Myocarditis in an Immunocompetent Host: Resolution with Oral Valganciclovir. Texas Heart Inst. J. 2014, 41, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Kotton, C.N.; Kumar, D.; Caliendo, A.M.; Huprikar, S.; Chou, S.; Danziger-Isakov, L.; Humar, A. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-Organ Transplantation. Transplantation 2018, 102, 900–931. [Google Scholar] [CrossRef]
- Campos, C.F.; Leite, L.; Pereira, P.; Vaz, C.P.; Branca, R.; Campilho, F.; Freitas, F.; Ligeiro, D.; Marques, A.; Torrado, E.; et al. PTX3 Polymorphisms Influence Cytomegalovirus Reactivation After Stem-Cell Transplantation. Front. Immunol. 2019, 10, 88. [Google Scholar] [CrossRef]
- Grattan, M.; Moreno-Cabral, C.; Starnes, V.; Oyer, P.; Stinson, E.B.; Shumway, N. Cytomegalovirus Infection Is Associated with Cardiac Allograft Rejection and Atherosclerosis. JAMA 1989, 261, 3561–3566. [Google Scholar] [CrossRef]
- Valantine, H.A.; Gao, S.-Z.; Menon, S.G.; Renlund, D.G.; Hunt, S.A.; Oyer, P.; Stinson, E.B.; Brown, B.W.; Merigan, T.C.; Schroeder, J.S. Impact of Prophylactic Immediate Posttransplant Ganciclovir on Development of Transplant Atherosclerosis: A Post Hoc Analysis of a Randomized, Placebo-Controlled Study. Circulation 1999, 100, 61–66. [Google Scholar] [CrossRef]
- Weill, D. Role of Cytomegalovirus in Cardiac Allograft Vasculopathy. Transpl. Infect. Dis. 2001, 3, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Andersson, R.; Friman, V.; Selimovic, N.; Hanzen, L.; Nasic, S.; Nyström, U.; Sigurdardottir, V. Cytomegalovirus Infection and Disease Reduce 10-Year Cardiac Allograft Vasculopathy-Free Survival in Heart Transplant Recipients. BMC Infect. Dis. 2015, 15, 582. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, P.K.; Kallio, E.A.; Tikkanen, J.M.; Sihvola, R.K.; Hayry, P.J.; Lemstrom, K.B. Cytomegalovirus Infection and Cardiac Allograft Vasculopathy. Transpl. Infect. Dis. 1999, 1, 115–126. [Google Scholar] [CrossRef]
- Merigan, T.C.; Renlund, D.G.; Keay, S.; Bristow, M.; Starnes, V.; O’Connell, J.; Resta, S.; Dunn, D.; Gamberg, P.; Ratkovec, R.; et al. A Controlled Trial of Ganciclovir to Prevent Cytomegalovirus Disease after Heart Transplantation. N. Engl. J. Med. 1992, 326, 1182–1186. [Google Scholar] [CrossRef]
- Streblow, D.; Orloff, S.; Nelson, J. Acceleration of Allograft Failure by Cytomegalovirus. Curr. Opin. Immunol. 2007, 19, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Atabani, S.F.; Smith, C.; Atkinson, C.; Aldridge, R.W.; Rodriguez-Perálvarez, M.; Rolando, N.; Harber, M.; Jones, G.; O’Riordan, A.; Burroughs, A.K.; et al. Cytomegalovirus Replication Kinetics in Solid Organ Transplant Recipients Managed by Preemptive Therapy: CMV and Preemptive Antiviral Therapy. Am. J. Transplant. 2012, 12, 2457–2464. [Google Scholar] [CrossRef]
- Lee, S.; Brook, E.; Affandi, J.; Howson, P.; Tanudjaja, S.A.; Dhaliwal, S.; Irish, A.; Price, P. A High Burden of Cytomegalovirus Marks Poor Vascular Health in Transplant Recipients More Clearly than in the General Population. Clin. Transl. Immunol. 2019, 8, e1043. [Google Scholar] [CrossRef] [PubMed]
- Streblow, D.N.; Kreklywich, C.N.; Andoh, T.; Moses, A.V.; Dumortier, J.; Smith, P.P.; Defilippis, V.; Fruh, K.; Nelson, J.A.; Orloff, S.L. The Role of Angiogenic and Wound Repair Factors During CMV-Accelerated Transplant Vascular Sclerosis in Rat Cardiac Transplants: CMV Accelerated TVS Involves Angiogenesis and Wound Healing. Am. J. Transplant. 2008, 8, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, J.; Zhang, W.; Chen, Y.; Zhang, K.; Popescu, L.M.; Ma, X.; Bond Lau, W.; Rong, R.; Yu, X.; et al. Signature MicroRNA Expression Profile of Essential Hypertension and Its Novel Link to Human Cytomegalovirus Infection. Circulation 2011, 124, 175–184. [Google Scholar] [CrossRef]
- Li, C.; Samaranayake, N.R.; Ong, K.L.; Wong, H.K.; Cheung, B.M.Y. Is Human Cytomegalovirus Infection Associated with Hypertension? The United States National Health and Nutrition Examination Survey 1999–2002. PLoS ONE 2012, 7, e39760. [Google Scholar] [CrossRef] [PubMed]
- Firth, C.; Harrison, R.; Ritchie, S.; Wardlaw, J.; Ferro, C.J.; Starr, J.M.; Deary, I.J.; Moss, P. Cytomegalovirus Infection Is Associated with an Increase in Systolic Blood Pressure in Older Individuals. QJM 2016, 109, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Speir, E.; Modali, R.; Huang, E.-S.; Leon, M.B.; Shawl, F.; Finkel, T.; Epstein, S.E. Potential Role of Human Cytomegalovirus and P53 Interaction in Coronary Restenosis. Science 1994, 265, 5. [Google Scholar] [CrossRef] [PubMed]
- Dummer, S.; Lee, A.; Breinig, M.; Kormos, R.; Ho, M.; Griffiths, B. Investigation of Cytomegalovirus-Infection as a Risk Factor for Coronary Atherosclerosis in the Explanted Hearts of Patients Undergoing Heart-Transplantation. J. Med. Virol. 1994, 44, 305–309. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Finkel, T. Association between Prior Cytomegalovirus Infection and the Risk of Restenosis after Coronary Atherectomy. N. Engl. J. Med. 1996, 335, 624–630. [Google Scholar] [CrossRef]
- Blum, A.; Peleg, A.; Weinberg, M. Anti-Cytomegalovirus (CMV) IgG Antibody Titer in Patients with Risk Factors to Atherosclerosis. Clin. Exp. Med. 2003, 3, 157–160. [Google Scholar] [CrossRef]
- Olson, N.C.; Doyle, M.F.; Jenny, N.S.; Huber, S.A.; Psaty, B.M.; Kronmal, R.A.; Tracy, R.P. Decreased Naive and Increased Memory CD4+ T Cells Are Associated with Subclinical Atherosclerosis: The Multi-Ethnic Study of Atherosclerosis. PLoS ONE 2013, 8, e71498. [Google Scholar] [CrossRef]
- Streblow, D.N.; Soderberg-Naucler, C.; Vieira, J.; Smith, P.; Wakabayashi, E.; Ruchti, F.; Mattison, K.; Altschuler, Y.; Nelson, J.A. The Human Cytomegalovirus Chemokine Receptor US28 Mediates Vascular Smooth Muscle Cell Migration. Cell 1999, 99, 511–520. [Google Scholar] [CrossRef]
- Reinhardt, B.; Mertens, T.; Mayrbeyrle, U.; Frank, H.; Luske, A.; Schierling, K.; Waltenberger, J. HCMV Infection of Human Vascular Smooth Muscle Cells Leads to Enhanced Expression of Functionally Intact PDGF β-Receptor. Cardiovasc. Res. 2005, 67, 151–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reinhardt, B. Human Cytomegalovirus-Induced Reduction of Extracellular Matrix Proteins in Vascular Smooth Muscle Cell Cultures: A Pathomechanism in Vasculopathies? J. Gen. Virol. 2006, 87, 2849–2858. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, J.; Streblow, D.N.; Moses, A.V.; Jacobs, J.M.; Kreklywich, C.N.; Camp, D.; Smith, R.D.; Orloff, S.L.; Nelson, J.A. Human Cytomegalovirus Secretome Contains Factors That Induce Angiogenesis and Wound Healing. JVI 2008, 82, 6524–6535. [Google Scholar] [CrossRef]
- Beyaz, M.O.; Ugurlucan, M.; Oztas, D.M.; Meric, M.; Conkbayir, C.; Agacfidan, A.; Onel, M.; Alpagut, U. Evaluation of the Relationship between Plaque Formation Leading to Symptomatic Carotid Artery Stenosis and Cytomegalovirus by Investigating the Virus DNA. AMSAD 2019, 4, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xiong, S.; Yang, Y.; Fu, W.; Wang, Y.; Ge, J. The relationship between human cytomegalovirus infection and atherosclerosis development. In Biochemistry of Diabetes and Atherosclerosis; Gilchrist, J.S.C., Tappia, P.S., Netticadan, T., Eds.; Springer: Boston, MA, USA, 2003; pp. 91–96. ISBN 978-1-4613-4852-8. [Google Scholar]
- Dogra, P.; Sparer, T.E. What We Have Learned from Animal Models of HCMV. In Human Cytomegaloviruses; Yurochko, A.D., Miller, W.E., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2014; Volume 1119, pp. 267–288. ISBN 978-1-62703-787-7. [Google Scholar]
- Vink, C.; Beuken, E.; Bruggeman, C.A. Complete DNA Sequence of the Rat Cytomegalovirus Genome. J. Virol. 2000, 74, 7656–7665. [Google Scholar] [CrossRef] [PubMed]
- Price, P.; Olver, S.D. Syndromes Induced by Cytomegalovirus Infection. Clin. Immunol. Immunopathol. 1996, 80, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Lenzo, J.C.; Fairweather, D.; Cull, V.; Shellam, G.R.; James(Lawson), C.M. Characterisation of Murine Cytomegalovirus Myocarditis: Cellular Infiltration of the Heart and Virus Persistence. J. Mol. Cell. Cardiol. 2002, 34, 629–640. [Google Scholar] [CrossRef]
- Vliegen, I.; Duijvestijn, A.; Grauls, G.; Herngreen, S.; Bruggeman, C.; Stassen, F. Cytomegalovirus Infection Aggravates Atherogenesis in ApoE Knockout Mice by Both Local and Systemic Immune Activation. Microbes Infect. 2004, 6, 17–24. [Google Scholar] [CrossRef]
- Ritter, J.T.; Tang-Feldman, Y.J.; Lochhead, G.R.; Estrada, M.; Lochhead, S.; Yu, C.; Ashton-Sager, A.; Tuteja, D.; Leutenegger, C.; Pomeroy, C. In Vivo Characterization of Cytokine Profiles and Viral Load during Murine Cytomegalovirus-Induced Acute Myocarditis. Cardiovasc. Pathol. 2010, 19, 83–93. [Google Scholar] [CrossRef]
- Rawlinson, W.D.; Farrell, H.E.; Barrell, B.G. Analysis of the Complete DNA Sequence of Murine Cytomegalovirus. J. Virol. 1996, 70, 8833–8849. [Google Scholar] [CrossRef]
- Reddehase, M.J.; Podlech, J.; Grzimek, N.K.A. Mouse Models of Cytomegalovirus Latency: Overview. J. Clin. Virol. 2002, 25, 23–36. [Google Scholar] [CrossRef]
- Weisblum, Y.; Panet, A.; Haimov-Kochman, R.; Wolf, D.G. Models of Vertical Cytomegalovirus (CMV) Transmission and Pathogenesis. Semin. Immunopathol. 2014, 36, 615–625. [Google Scholar] [CrossRef]
- Cook, C.H.; Bickerstaff, A.A.; Wang, J.-J.; Zimmerman, P.D.; Forster, M.R.; Nadasdy, T.; Colvin, R.B.; Hadley, G.A.; Orosz, C.G. Disruption of Murine Cardiac Allograft Acceptance by Latent Cytomegalovirus: Disruption of Murine Cardiac Allograft. Am. J. Transplant. 2008, 9, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ke, Q.; Jin, Z.; Wang, H.; Kocher, O.; Morgan, J.P.; Zhang, J.; Crumpacker, C.S. Cytomegalovirus Infection Causes an Increase of Arterial Blood Pressure. PLoS Pathog. 2009, 5, e1000427. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.M.; O’Donoghue, H.; Bartholomaeus, W.N.; Reed, W.D. Genetic Control of Mouse Cytomegalovirus-Induced Myocarditis. Immunology 1990, 69, 20–26. [Google Scholar]
- Bonavita, C.M.; White, T.M.; Francis, J.; Cardin, R.D. Heart Dysfunction Following Long-Term Murine Cytomegalovirus Infection: Fibrosis, Hypertrophy, and Tachycardia. Viral Immunol. 2020, 33, 237–245. [Google Scholar] [CrossRef]
- Lenzo, J.C.; Shellam, G.R.; Lawson, C.M. Ganciclovir and Cidofovir Treatment of Cytomegalovirus-Induced Myocarditis in Mice. Antimicrob. Agents Chemother. 2001, 45, 1444–1449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iglezias, S.D.; Benvenuti, L.A.; Calabrese, F.; Salemi, V.M.C.; Silva, A.M.G.; Carturan, E.; de Oliveira, S.A.; Thiene, G.; De Brito, T. Endomyocardial Fibrosis: Pathological and Molecular Findings of Surgically Resected Ventricular Endomyocardium. Virchows Arch. 2008, 453, 233–241. [Google Scholar] [CrossRef]
- Lenzo, J.C.; Mansfield, J.P.; Sivamoorthy, S.; Cull, V.S.; James, C.M. Cytokine Expression in Murine Cytomegalovirus-Induced Myocarditis: Modulation with Interferon-α Therapy. Cell. Immunol. 2003, 223, 77–86. [Google Scholar] [CrossRef]
- Cull, V.S.; Broomfield, S.; Bartlett, E.J.; Brekalo, N.L.; James, C.M. Coimmunisation with Type I IFN Genes Enhances Protective Immunity against Cytomegalovirus and Myocarditis in GB DNA-Vaccinated Mice. Gene Ther. 2002, 9, 1369–1378. [Google Scholar] [CrossRef][Green Version]
- Heim, C.; Abele-Ohl, S.; Eckl, S.; Ramsperger-Gleixner, M.; Mahmoudian, S.; Weyand, M.; Stamminger, T.; Ensminger, S.M. Murine Cytomegalovirus Infection Leads to Increased Levels of Transplant Arteriosclerosis in a Murine Aortic Allograft Model. Transplantation 2010, 90, 373–379. [Google Scholar] [CrossRef]
- Khoretonenko, M.V.; Leskov, I.L.; Jennings, S.R.; Yurochko, A.D.; Stokes, K.Y. Cytomegalovirus Infection Leads to Microvascular Dysfunction and Exacerbates Hypercholesterolemia-Induced Responses. Am. J. Pathol. 2010, 177, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Senchenkov, E.; Khoretonenko, M.V.; Leskov, I.L.; Ostanin, D.V.; Stokes, K.Y. P-Selectin Mediates the Microvascular Dysfunction Associated with Persistent Cytomegalovirus Infection in Normocholesterolemic and Hypercholesterolemic Mice: P-Selectin in Persistent CMV Infection. Microcirculation 2011, 18, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Presti, R.M.; Pollock, J.L.; Canto, A.J.D.; O’Guin, A.K. Interferon Regulates Acute and Latent Murine Cytomegalovirus Infection and Chronic Disease of the Great Vessels. J. Exp. Med. 1998, 188, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Vliegen, I.; Stassen, F.; Grauls, G.; Blok, R.; Bruggeman, C. MCMV Infection Increases Early T-Lymphocyte Influx in Atherosclerotic Lesions in ApoE Knockout Mice. J. Clin. Virol. 2002, 25, 159–171. [Google Scholar] [CrossRef]
- Vliegen, I.; Duijvestijn, A.; Stassen, F.; Bruggeman, C. Murine Cytomegalovirus Infection Directs Macrophage Differentiation into a Pro-Inflammatory Immune Phenotype: Implications for Atherogenesis. Microbes Infect. 2004, 6, 1056–1062. [Google Scholar] [CrossRef]
- Froberg, M.K.; Adams, A.; Seacotte, N.; Parker-Thornburg, J.; Kolattukudy, P. Cytomegalovirus Infection Accelerates Inflammation in Vascular Tissue Overexpressing Monocyte Chemoattractant Protein-1. Circ. Res. 2001, 89, 1224–1230. [Google Scholar] [CrossRef]
- Burnett, M.S.; Durrani, S.; Stabile, E.; Saji, M.; Lee, C.W.; Kinnaird, T.D.; Hoffman, E.P.; Epstein, S.E. Murine Cytomegalovirus Infection Increases Aortic Expression of Proatherosclerotic Genes. Circulation 2004, 109, 893–897. [Google Scholar] [CrossRef]
- Tang-Feldman, Y.J.; Lochhead, S.R.; Lochhead, G.R.; Yu, C.; George, M.; Villablanca, A.C.; Pomeroy, C. Murine Cytomegalovirus (MCMV) Infection Upregulates P38 MAP Kinase in Aortas of Apo E KO Mice: A Molecular Mechanism for MCMV-Induced Acceleration of Atherosclerosis. J. Cardiovasc. Trans. Res. 2013, 6, 54–64. [Google Scholar] [CrossRef]
- Rott, D.; Zhu, J.; Burnett, M.S.; Zhou, Y.; Zalles-Ganley, A.; Ogunmakinwa, J.; Epstein, S.E. Effects of MF-Tricyclic, a Selective Cyclooxygenase-2 Inhibitor, on Atherosclerosis Progression and Susceptibility to Cytomegalovirus Replication in Apolipoprotein-E Knockout Mice. J. Am. Coll. Cardiol. 2003, 41, 1812–1819. [Google Scholar] [CrossRef]
- Basman, C.; Parmar, Y.J.; Kronzon, I. Intracardiac Echocardiography for Structural Heart and Electrophysiological Interventions. Curr. Cardiol. Rep. 2017, 19, 102. [Google Scholar] [CrossRef]
- Cardin, R.D.; Schaefer, G.C.; Allen, J.R.; Davis-Poynter, N.J.; Farrell, H.E. The M33 Chemokine Receptor Homolog of Murine Cytomegalovirus Exhibits a Differential Tissue-Specific Role during In Vivo Replication and Latency. JVI 2009, 83, 7590–7601. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Sharma, P.; Agrawal, S.; Agrawal, D.K. Relevance of Mouse Models of Cardiac Fibrosis and Hypertrophy in Cardiac Research. Mol. Cell Biochem. 2017, 424, 123–145. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; de Boer, R.A. From Inflammation to Fibrosis—Molecular and Cellular Mechanisms of Myocardial Tissue Remodelling and Perspectives on Differential Treatment Opportunities. Curr. Heart Fail. Rep. 2017, 14, 235–250. [Google Scholar] [CrossRef]
- Glass, A.M.; Coombs, W.; Taffet, S.M. Spontaneous Cardiac Calcinosis in BALB/CByJ Mice. Comp. Med. 2013, 63, 9. [Google Scholar]
- Bonavita, C.M. Louisiana State University, Baton Rouge, LA, USA, Unpublished work. 2021.
- Almanan, M.; Raynor, J.; Sholl, A.; Wang, M.; Chougnet, C.; Cardin, R.D.; Hildeman, D.A. Tissue-Specific Control of Latent CMV Reactivation by Regulatory T Cells. PLoS Pathog. 2017, 13, e1006507. [Google Scholar] [CrossRef]
- Roizman, B.; Sears, A. An Inquiry Into The Mechanisms Of Herpes Simplex Virus Latency. Annu. Rev. Microbiol. 1987, 41, 543–571. [Google Scholar] [CrossRef]
- Hertel, L.; Mocarski, E.S. Global Analysis of Host Cell Gene Expression Late during Cytomegalovirus Infection Reveals Extensive Dysregulation of Cell Cycle Gene Expression and Induction of Pseudomitosis Independent of US28 Function. JVI 2004, 78, 11988–12011. [Google Scholar] [CrossRef]
- Farrell, H.E.; Abraham, A.M.; Cardin, R.D.; Sparre-Ulrich, A.H.; Rosenkilde, M.M.; Spiess, K.; Jensen, T.H.; Kledal, T.N.; Davis-Poynter, N. Partial Functional Complementation between Human and Mouse Cytomegalovirus Chemokine Receptor Homologues. J. Virol. 2011, 85, 6091–6095. [Google Scholar] [CrossRef]
- Fritz, N.M.; Stamminger, T.; Ramsperger-Gleixner, M.; Kuckhahn, A.V.; Müller, R.; Weyand, M.; Heim, C. Cytomegalovirus Chemokine Receptor M33 Knockout Reduces Chronic Allograft Rejection in a Murine Aortic Transplant Model. Transplant. Immunol. 2021, 64, 101359. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonavita, C.M.; Cardin, R.D. Don’t Go Breaking My Heart: MCMV as a Model for HCMV-Associated Cardiovascular Diseases. Pathogens 2021, 10, 619. https://doi.org/10.3390/pathogens10050619
Bonavita CM, Cardin RD. Don’t Go Breaking My Heart: MCMV as a Model for HCMV-Associated Cardiovascular Diseases. Pathogens. 2021; 10(5):619. https://doi.org/10.3390/pathogens10050619
Chicago/Turabian StyleBonavita, Cassandra M., and Rhonda D. Cardin. 2021. "Don’t Go Breaking My Heart: MCMV as a Model for HCMV-Associated Cardiovascular Diseases" Pathogens 10, no. 5: 619. https://doi.org/10.3390/pathogens10050619
APA StyleBonavita, C. M., & Cardin, R. D. (2021). Don’t Go Breaking My Heart: MCMV as a Model for HCMV-Associated Cardiovascular Diseases. Pathogens, 10(5), 619. https://doi.org/10.3390/pathogens10050619




