1. Introduction
Francisella tularensis is an intracellular Gram-negative bacterium and the causative agent of the disease tularemia. Infection generally produces an acute febrile illness; the disease can manifest as a range of possible clinical presentations, largely dependent on the route of infection, dose, virulence of the bacterial strain, and host immune response [
1]. After exposure,
F. tularensis multiplies at the initial site of infection prior to spreading to the regional lymph nodes, liver, and spleen, and then to other organs [
2]. The most common form of disease is ulceroglandular, involving an ulcer at the site of entry and regional lymphadenopathy, which is commonly acquired by direct contact with infected animals or arthropod bites [
3]. Less commonly, variations of ulceroglandular disease are associated with different sites of entry and include oculoglandular and oropharyngeal disease. The oropharyngeal form results from ingesting contaminated food or water and, while rare, it has been reported with increasing frequency in Turkey and other European countries [
4,
5,
6]. Pulmonary disease (pneumonic tularemia) can result from clinical progression of the ulceroglandular forms or from direct inhalation of
F. tularensis organisms. Further clinical progression from any of the forms of tularemia can result in typhoidal tularemia leading to a state of septic shock, organ failure, and death of the host.
F. tularensis is highly successful at evading the immune system, principally by escaping the phagosome and replicating in the cytosol of eukaryotic cells, including macrophages and dendritic cells. Dissemination of the microbe to distant sites within the body occurs via trafficking through the regional lymph nodes into the bloodstream [
7].
Naturally occurring outbreaks of pneumonic tularemia still occur in the United States, as exemplified by outbreaks on Martha’s Vineyard, Massachusetts in the summer of 1978 and again in 2000 [
8,
9]. The number of cases averaged in the thousands early in the 20th century and has steadily declined since the 1950s, likely a result of increased urbanization of the population. Historically,
F. tularensis was studied by Japan (prior to 1945) and the former Soviet Union for its potential as a biological weapon, and it was included in the United States offensive biological weapons program until that program was discontinued in 1969 [
10,
11]. A WHO study in 1969 found that intentional release of aerosolized
F. tularensis in a densely populated area could cause 250,000 cases and 19,000 fatalities in a short timeframe [
12]. The organism is currently listed as a Tier 1 select agent by the Federal Select Agent & Toxins Program (FSAP), those for which there is the most concern about intentional use as a biological weapon [
13].
Models of tularemia disease have been evaluated in a variety of animal species, including mice, rabbits, rats, guinea pigs, and monkeys [
14]. In a previous study, we compared the LD
50 and disease outcome of inhalational tularemia in three nonhuman primate (NHP) species (cynomolgus and rhesus macaques, and African green monkeys) [
15,
16]. Thus, to more extensively characterize the disease progression and pathogenesis in a cynomolgus macaque model, we exposed groups of cynomolgus macaques to three different target doses (50 CFU, 500 CFU, and 5000 CFU) of aerosolized
F. tularensis SCHU S4. Based on these data, as well as more comprehensive analysis of studies from multiple facilities, the cynomolgus macaque inhalation model was selected as the nonhuman primate model of the human disease resulting from inhalation exposure to
F. tularensis. The resulting data from this study contributed to the knowledge base needed for use of this model for evaluation of vaccines and therapeutics for tularemia to satisfy the FDA Animal Rule.
3. Discussion
Respiratory or inhalational infection with aerosolized
F. tularensis in humans causes a rapid, severe bacterial pneumonia and sepsis which can be lethal with a minimum infectious dose of fewer than 10 CFU [
17]. Due to its high infectivity and relative ease of dissemination,
F. tularensis has been studied as a potential candidate for weaponization and categorized as an agent of biological terrorism [
2,
9,
10]. Development of medical countermeasures to lethal diseases such as inhalation tularemia is contingent on the characterization of animal models that effectively mimic the most severe form of disease in humans. The cynomolgus macaque has been established as the nonhuman primate model for pneumonic tularemia. To further characterize the disease in the cynomolgus macaque, groups of cynomolgus macaques were exposed to target doses of 50, 500 or 5000 CFU of aerosolized
F. tularensis. Survival was dose dependent with target doses of 500 CFU (134 to 749 CFU range) and 5000 CFU (1177 to 5860 CFU range) causing 100% lethality by Days 7 and 8, respectively. Target doses of 50 CFU (10 to 110 CFU range) resulted in survival of 2 out of 10 macaques (20%).
In humans, tularemia is an acute febrile illness. The disease manifestation is dependent on the route of infection, the dose, virulence of the strain, and host immune response. After an average incubation period of 3–5 days, there is an onset of symptoms including fever, chills, cough, headache, general malaise and in some cases diarrhea [
18,
19]. In this study, fever began in cynomolgus macaques generally between 2–3 after aerosol exposure to
F. tularensis. Onset of other clinical changes noted (heart rate, blood pressure, changes in behavior, hematological changes) all coincided with fever onset. This data is strikingly similar to what has been reported in rats and rabbits, particularly with regard to fever onset and other clinical signs [
20,
21].
The generally mild increase in heart rate seen in most macaques was quite intriguing. While heart rate was elevated compared to baseline in 12 of 13 macaques, the heart rate in the febrile period was still within the normal range for an unrestrained adult cynomolgus macaque [
22]. In other severe zoonotic infections in nonhuman primate models, heart rate increases between 40–60% in the febrile period (D.S. Reed, manuscript in preparation). This contrasts sharply with the median 20% increase seen here, and notably six of the thirteen macaques had a less than 20% increase in heart rate. Leibermeister’s rule stipulates an 8 bpm increase in heart rate for every 1oC elevation in body temperature [
23]. This allowed us to calculate a predicted increase in heart rate during the febrile period; while 8 of 12 had an increase in heart rate greater than predicted, for four macaques (33%) heart rate was lower than predicted. Intriguingly, Faget’s sign (fever paired with bradycardia) has been reported in 42% of human tularemia patients [
24]. Faget’s has also been reported in yellow fever, typhoid, brucellosis, and Legionnaire’s disease. This finding lends further credence to the cynomolgus macaque as a relevant model of human tularemia and would further suggest that this model could be used to gain a better understanding of the underlying pathophysiology associated with Faget’s sign.
Hematology and clinical chemistry parameters in humans infected with
F. tularensis have not shown any consistent remarkable findings. A review of 88 cases of tularemia from 1949 through 1979 reported increased white blood cell counts ranging from 5000 to 22,000 cells/mm
3 (median 10,400 cells/mm
3) upon hospital admission. An additional review of over 300 of cases reported that 50% presented with WBC counts between 3000 and 24,000 cells/mm
3 however the other half showed marked increases in leukocytes as great as 56,000 cells/mm
3 [
22]. Similarly, in this study, there was a clear increase in WBC by Day 3 followed by a sharp decrease between Days 4 and 6 for all macaques; however, no group fell significantly outside of the normal range. This decrease was attributable to a loss in lymphocytes. A similar loss in platelets was also seen on Day 3. These changes in CBC match very well with what has been reported in both rats and rabbits. Increases in liver enzymes AST, ALT, and ALKP, as seen in this study, have been previously reported in human tularemia cases where of 58 patients tested, 58% had elevations in at least one of these three enzymes.
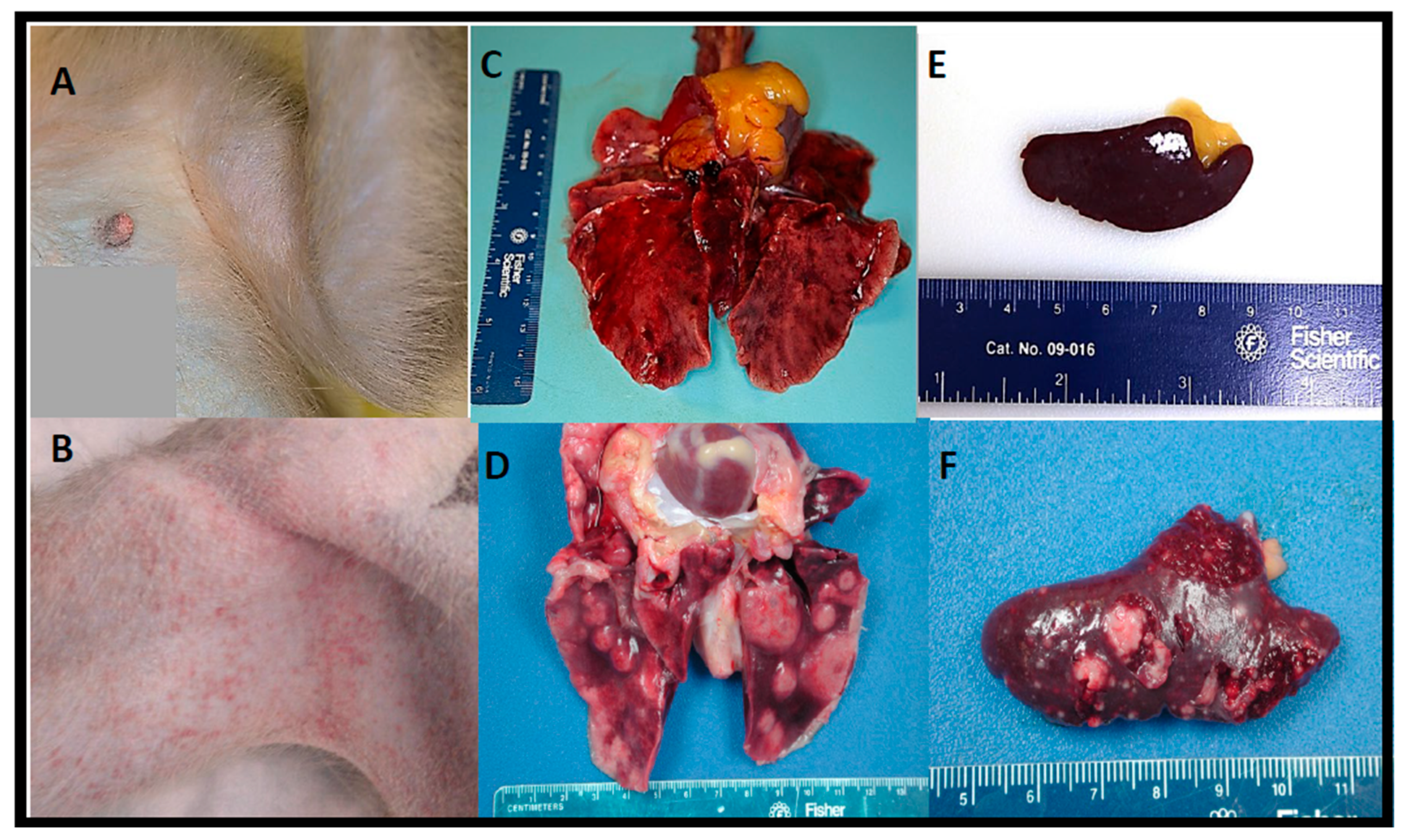
As previously seen in lethal human cases of tularemia [
25], in this study, the lung, lymph nodes (particularly the mediastinal and tracheobronchial lymph nodes), spleen, liver, and bone marrow were the most severely affected tissues during
F. tularensis infection regardless of exposure dose. Although varying in severity, multifocal and coalescing, necrotizing and pyogranulomatous pneumonia and abscessation with (or without) extensive edema, fibrinous pleuritis, and pleural fibrosis were consistent pathological findings in almost all macaques and corresponded with the intrathoracic histopathologic changes. Additionally, necrotizing mediastinal lymphadenitis, splenitis, myelitis, and hepatitis were consistently observed regardless of the inhaled
F. tularensis dose. The random focal necrotizing lesions in the liver were discrete but small unlike the larger coalescing lesions seen in the lung and spleen.
Here we demonstrated that aerosolized F. tularensis infection in cynomolgus macaques causes similar disease progression, pathogenesis, and clinical pathology profiles as compared to respiratory tularemia in humans. Therefore, the cynomolgus macaque model of pneumonic tularemia is an appropriate animal model for testing medical countermeasures for treatment of tularemia.
4. Materials and Methods
4.1. Animals and Ethical Statement
Twenty-eight healthy, adult female and male cynomolgus macaques (Macaca fascicularis) of primarily Chinese descent weighing between 3.6 kg and 7.4 kg were obtained from USAMRIID NHP colony. The age of the animals ranged from 3 years to 7 years. Animals were experimentally naïve with the exception of four animals that were either previously used as negative controls or survived a previous challenge with Francisella tularensis SCHU S4 and had a calculated exposure dose of 0 CFU with no signs of the disease and no antibodies against F. tularensis. Animals were in good physical condition, lacked physical malformations, and were free of clinical signs of infectious, contagious, or communicable diseases or parasites. All animals exposed to F. tularensis were handled in a BSL-3 containment laboratory at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID). Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals, and adhered principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996. The facility where this research was conducted (USAMRIID) is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Research was conducted under a protocol approved by the Institutional Animal Care and Use Committee (IACUC) at USAMRIID. All animals were examined and evaluated twice per day by study personnel. Early endpoint criteria, as specified by the score parameters within the “Post-exposure observations” section of these methods, were used to determine when animals should be humanely euthanized.
4.2. Bacteria
F. tularensis SCHU S4 strain (Biodefense and Emerging Infections Research Resources Repository (BEI Resources) Cat. No. NR-10492) was provided by the National Institute of Allergy and Infectious Diseases. A 25-mL flask of Mueller Hinton II (MHII) liquid medium containing 2% IsoVitaleX enrichment was inoculated with 10 µL of a SCHU S4 seed stock. After 23 h of incubation at 37 °C with shaking at 200 rpm, the optical density (OD660 nm) was measured. Logarithmic growth cultures were at the mid-logarithmic phase of growth and had an optical density (OD660 nm) of 0.15 to 0.85. Microorganisms were diluted to the desired nebulizer concentration in MHII medium.
4.3. Aerosol Exposures
Each macaque was anesthetized by intramuscular (i.m.) injection of tiletamine/zolazepam (3 mg/kg) and challenged by aerosol as previously described [
26]. Briefly, the respiratory function of each of the macaques was measured using whole-body plethysmography (Buxco Systems, Sharon, CT) before aerosol challenge. Aerosol procedures were conducted using a 32-L, airtight Lexan chamber assembled in a head-only configuration for individual macaque exposures in a class III biological safety cabinet located inside a BSL-3 suite. All airflows, environmental monitoring, and system balancing were configured, controlled, and recorded through the institute standard automated bioaerosol exposure system (ABES)(Biaera Technologies, LLC, Hagerstown, MD [
27]. Integrated air samples were obtained for each individual exposure using an all-glass impinger (AGI) drawing 6 L/min from a port centered on the chamber and opposite to the animal’s head. Aerosols were generated with a three-jet Collison nebulizer running at 7.5 L/min, 25–30 psi. The amounts of live bacteria in AGI samples were analyzed on the same day as the aerosol exposure by performing a bacterial plating assay on Modified Thayer–Martin (MTM) agar plates to determine the colony forming units (CFU)/mL.
4.4. Telemetry
Macaques were implanted with a radiotelemetry device [Data Sciences International (DSI), St. Paul, MN] at least 14 days before aerosol exposure. Body temperatures, electrocardiography, and blood pressure were recorded once per hour by the DataQuest A.R.T. 4.1 system (DSI). Fever was defined as body temperature greater than two standard deviations above baseline temperature as determined by autoregressive integrated moving average (ARIMA) modeling. Due to telemetry equipment failure, two animals in the high target dose group had shorter baselines starting Day -1.5 and Day -0.5; for these animals, temperature recordings were initiated at approximately 12:00 PM on respective days.
4.5. Post-Exposure Observations and Clinical Scoring
Macaques were observed at least twice a day after aerosol exposure and scored for clinical signs of disease prior to, and while under anesthesia. The scoring parameters were as follows, appearance (0: normal with coat smooth, eyes/nose clear; 1: reduced grooming; 2: dull/rough coat, ocular/nasal discharge; 3: absence of grooming, or piloerection, or posture change), appetite (0: normal; 1: decreased biscuit intake but still eats enrichment × 24 h; 2: will only eat food enrichment × 48 h; 3: anorexic × 48 h), clinical signs (0: normal temperature and respiration/min; 1: Slight change in temperature/respiration (±1 °C out of normal range/< 30% ± n respiration range); 2: Temp: ±2 °C; respiration: ±30% (above 51 or below 27); intermittent tremors; intermittent convulsions <10 min duration; transient prostration <1 h; 3: Temp: ±3 °C; respiration: ±50% (above 59 or below 19); continuous tremors or convulsions; persistent prostration ≥1 h), natural behavior (0: normal; 1: minor changes, less interaction with staff; 2: little interaction, less mobile and alert, isolated; 3: no interaction, vocalization, self-mutilation, restless, or still), provoked behavior (0: normal; 1: subdued but normal when stimulated, 2: subdued even when stimulated, 3: unresponsive when stimulated, weak, pre-comatose). An extra point for each score of 3 was added to the cumulative clinical score. The early endpoint criteria for humane euthanasia, indicative of very poor health status, were cumulative clinical scores of 16–20 (maximum score), and/or a sudden drop of >3 °C from baseline body temperature, continuous tremors or convulsions lasting ≥10 min duration, persistent prostration lasting ≥2 h, comatose or loss of consciousness. In some cases, such as accelerated dropping in body temperature without anesthesia, severe respiratory distress or continuous tremors, where the scoring range did not reflect the severity of the disease, the decision for euthanasia was made by the study director after consultation and verbal confirmation with the attending veterinarians.
4.6. Clinical Laboratory Evaluation
Blood samples were collected from central venous catheters (CVC) inserted in the internal jugular vein beginning fourteen days before, one day before, Days 1–21, and Day 28 after exposure. If collection from the CVC was not possible, blood samples were collected from the femoral vein of macaques anesthetized with 3 mg/kg intramuscular tiletamine/zolazepam. Samples collected one day prior to exposure served as a reference baseline for each animal. Complete blood counts (CBCs) and blood chemistries were analyzed with Beckman Coulter hematology and VITROS 250 chemistry analyzers, respectively.
4.7. Postmortem Examination (Necropsy)
A full postmortem examination was performed under direct supervision of a pathologist board certified by the American College of Veterinary Pathologist in a BSL-3 necropsy facility. Tissues samples from all major organ systems (respiratory, gastrointestinal, genitourinary, hematopoietic, nervous, endocrine, skin and mucous membranes) were collected from each animal for histopathological and, in some cases, immunohistochemical examination. Tissue samples were frozen at −70 °C until processed for bacterial assays. All other tissues were immersion-fixed in 10% neutral buffered formalin for a minimum of 21 days prior to removal from biocontainment.
4.8. Histology and Immunohistochemistry
Formalin-fixed tissues for microscopic examination were trimmed, processed, and embedded in paraffin according to established protocols [
28]. Embedded tissues were sectioned at 5–6 µm on a rotary microtome, mounted on glass slides, and stained with hematoxylin and eosin (H&E). Tissues evaluated by immunohistochemistry were stained for
F. tularensis lipopolysaccharide (LPS) (Meridian Life Science, Inc., Cincinnati, OH, USA) using a mouse monoclonal antibody (USAMRIID immuno #927) and an immunoperoxidase assay system (EnVision System, DAKO Corp., Carpinteria, CA, USA). Unstained tissue sections were deparaffinized, rehydrated, subjected to methanol-hydrogen peroxide block, rinsed, and pretreated with proteinase K for 6 min at room temperature. A serum-free protein block (DAKO) plus 5% normal goat serum was applied for 30 min. The monoclonal antibody was then applied to the tissue at a dilution of 1:1200 and incubated at room temperature for 60 min. The tissue was exposed to the EnVision horseradish peroxidase labeled polymer for 30 min at room temperature. All sections were exposed to 3,3′-diaminobenzidine (DAB) permanent chromogen for 5 ± 1 min, rinsed, counter-stained with hematoxylin, dehydrated, and cover-slipped with Permount. Normal uninfected lung tissue served as the negative control; the positive control tissue was lung tissue from a known
F. tularensis-infected monkey. Normal mouse IgG was used as the negative serum control for the control slides.
4.9. Statistical Analysis
Survival curves were calculated by the Kaplan–Meier product-limit method. Body temperatures were recorded by telemetry once per hour, beginning at least 12 h and up to 3 days prior to exposure and continuing up to 28 days post-exposure. Pre-exposure temperature data taken between two to five days pre-exposure was used to develop a baseline period to fit an ARIMA model. Forecasted values for the post-exposure periods were based on the baseline extrapolated forward in time using SAS ETS (version 9.3). For body temperature, residual changes greater than two standard deviations were used to compute fever duration (number of hours of significant temperature elevation), fever hours (sum of the significant temperature elevations), and average fever elevation (fever hours divided by fever duration in hours). The number of surviving animals in each group was statistically insufficient at later time points; therefore, only post-exposure data through Day 8 was used for analysis. T-tests with stepdown bootstrap adjustment were utilized to compare mean temperature variables between groups. ANOVA at each individual time point was completed with post hoc Tukey’s tests for pairwise comparisons for respiratory rates, CBCs and chemistries for time points Day 0 through Day 8. Beyond time point Day 8, there were insufficient samples for comparison. For analysis of ECG and blood pressure data, data were imported into Ponemah 6.5 (DSI) and analyzed using MatLab 2020a and GraphPad software.