Variation of Glucosinolate Contents in Clubroot-Resistant and -Susceptible Brassica napus Cultivars in Response to Virulence of Plasmodiophora brassicae
Abstract
1. Introduction
2. Results
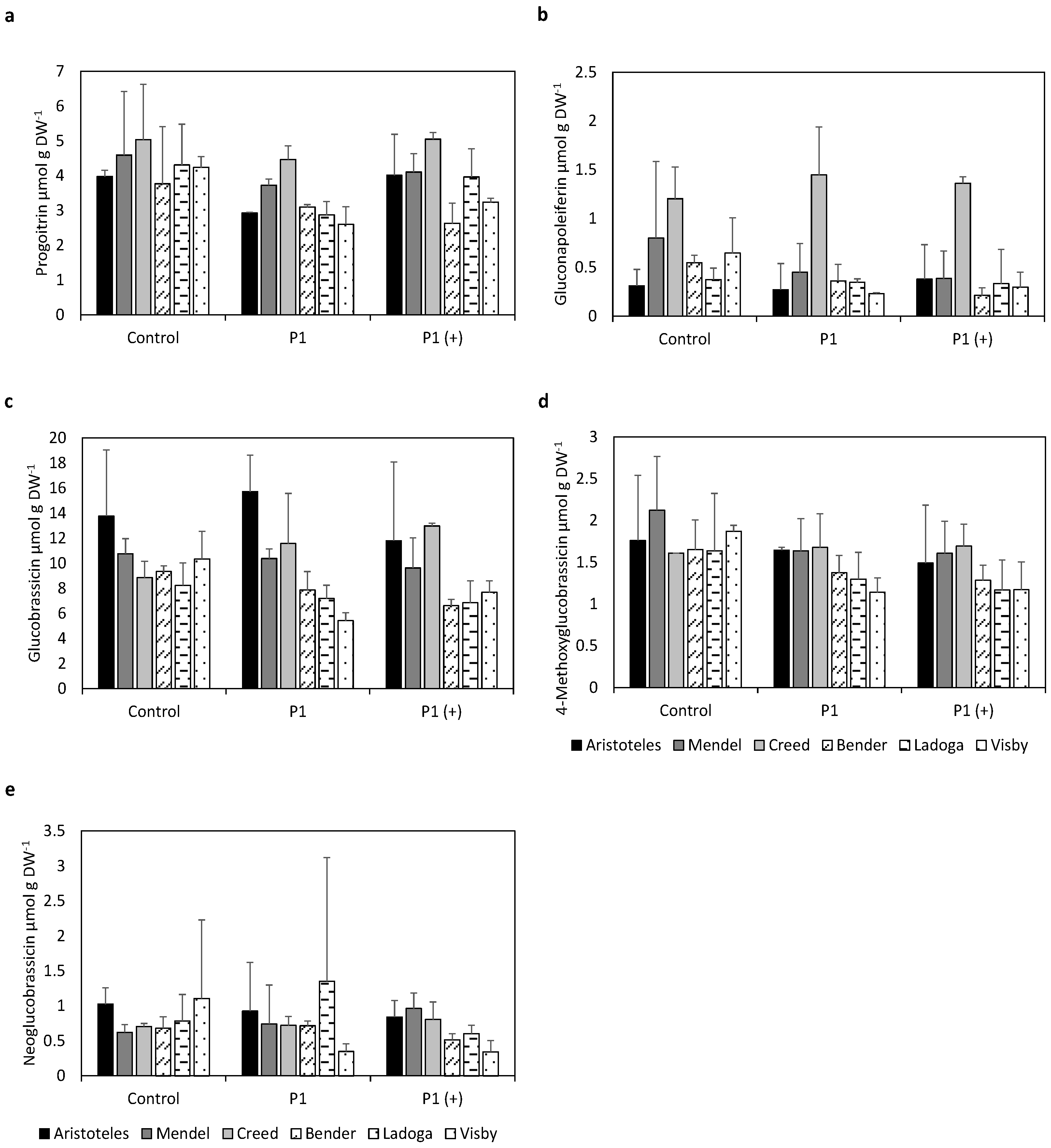
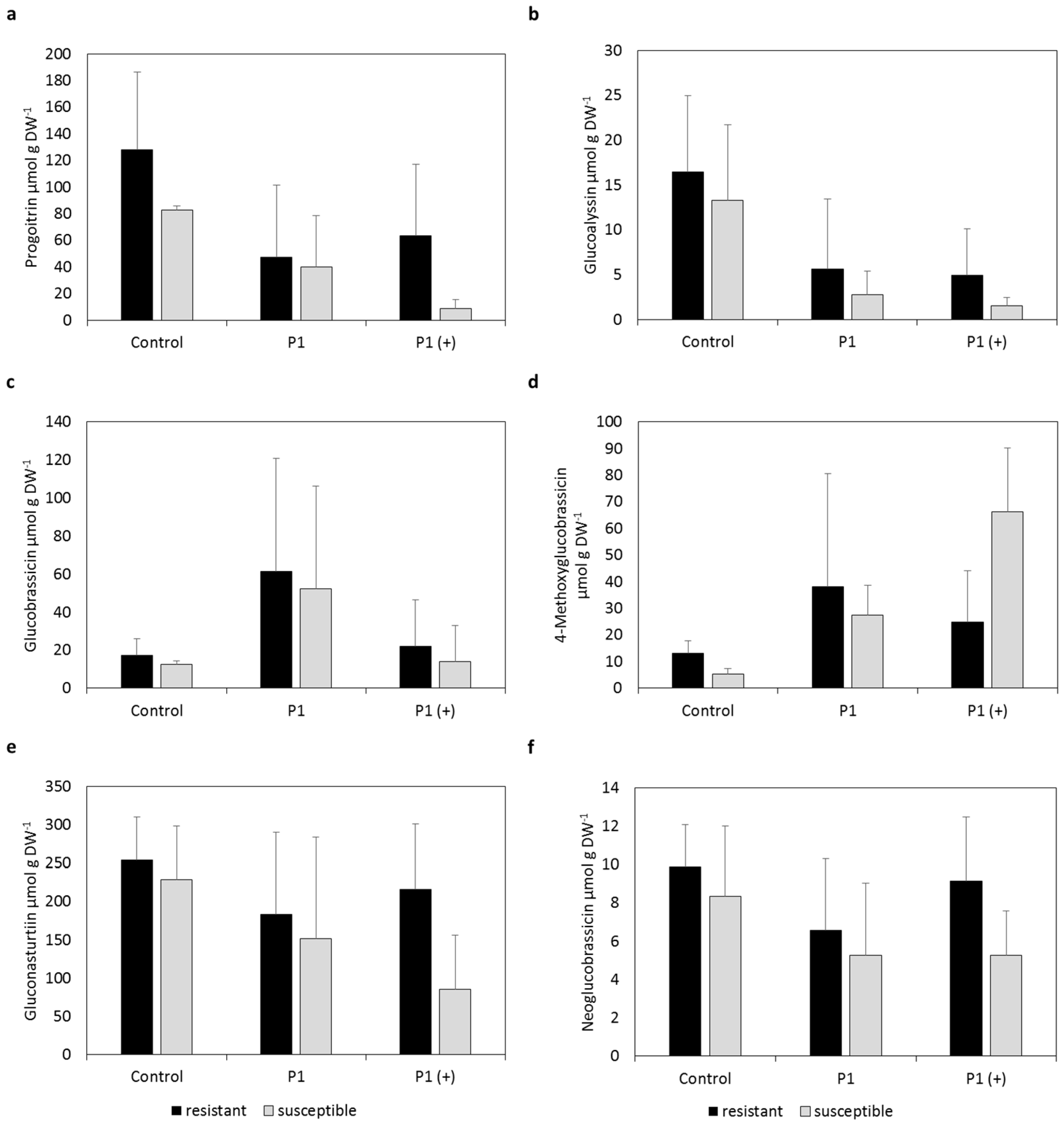
2.1. Individual Glucosinolate Profiles
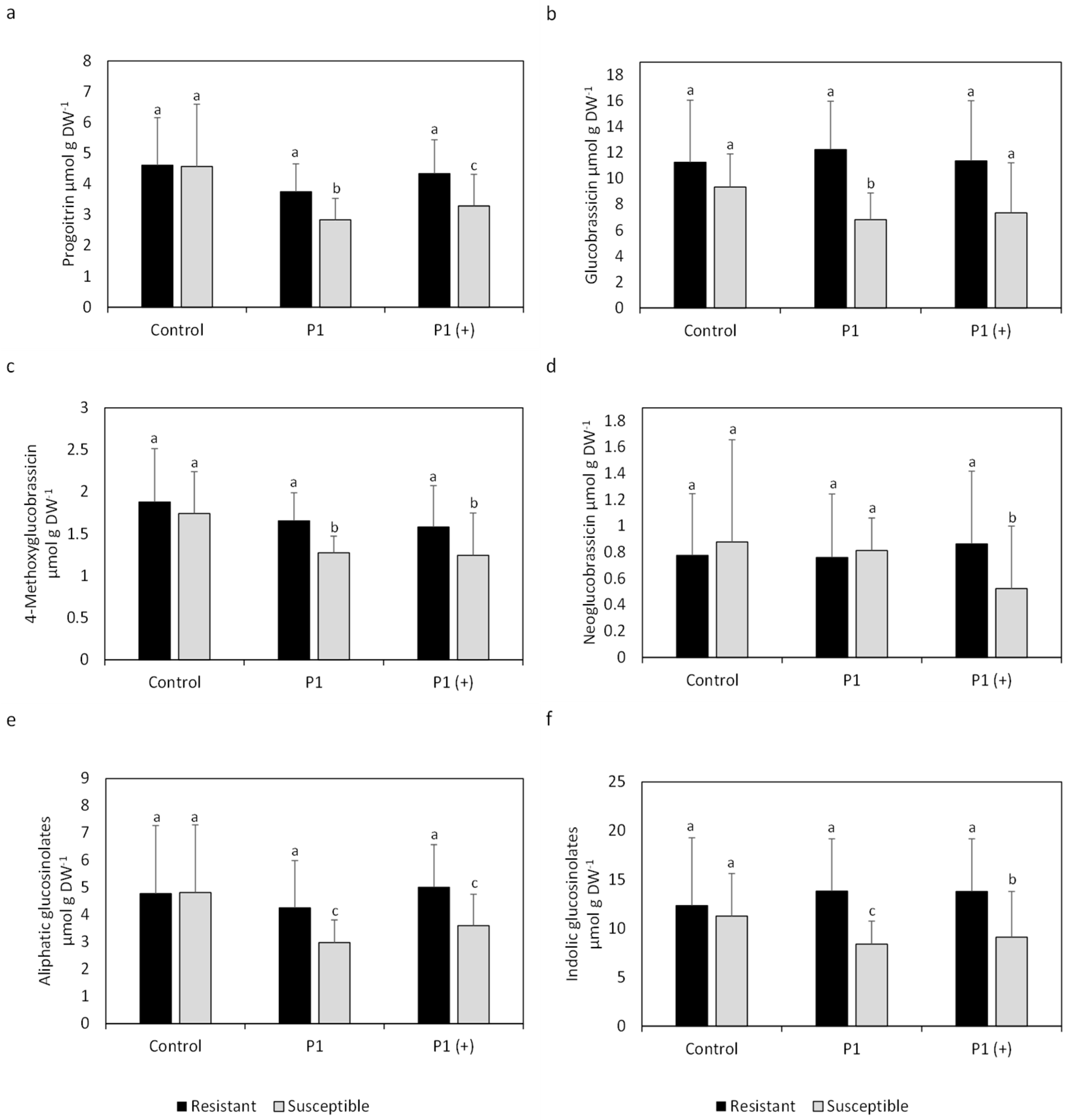
2.2. Mean Glucosinolate Profiles of Resistant and Susceptible Varieties
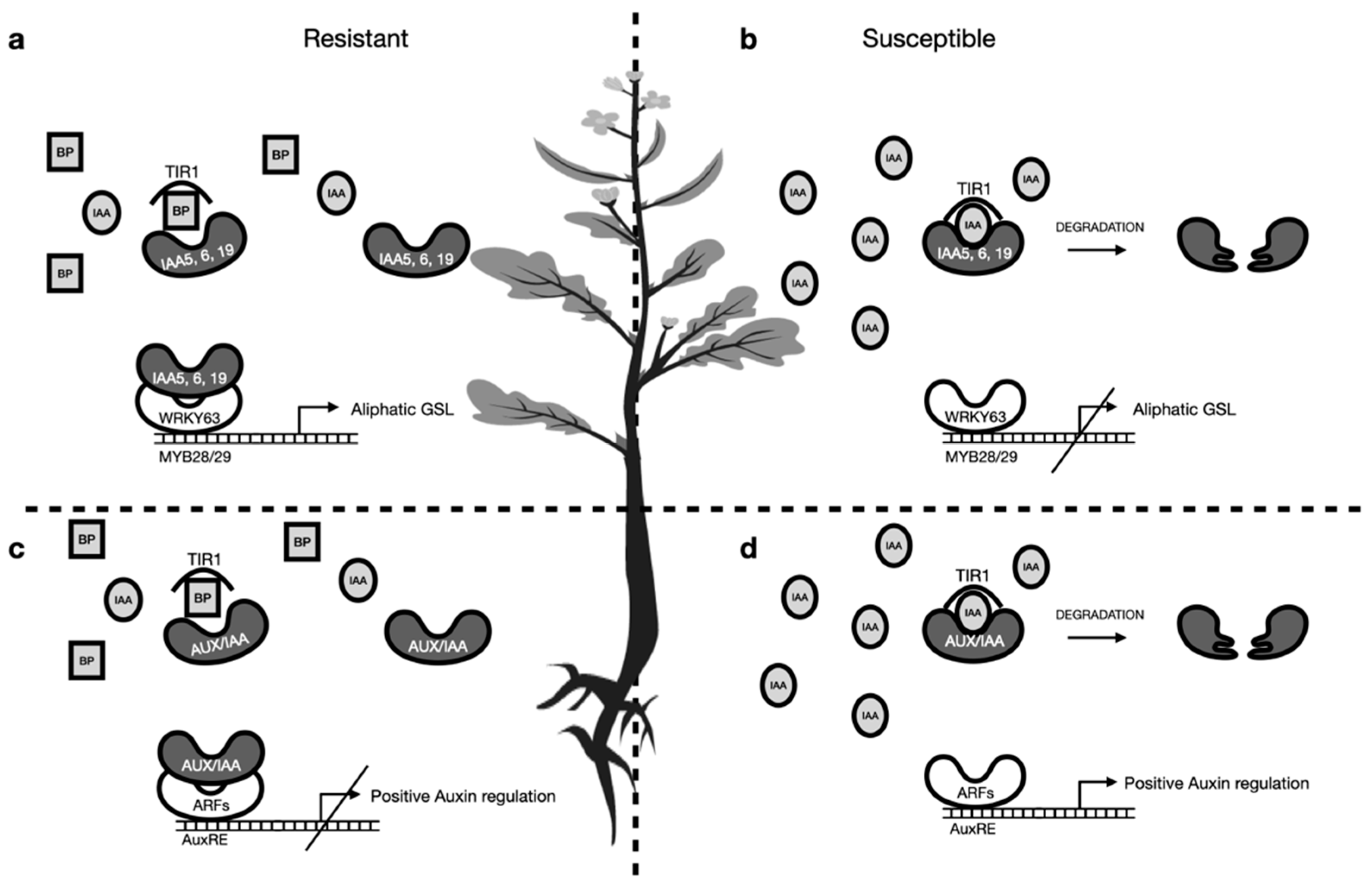
3. Discussion
3.1. Similarity of Glucosinolate Contents in Resistant Cultivars—A Coincidence?
3.2. Higher Contents of Indolic Glucosinolates—A Double-Edged Sword
3.3. Is There an Interplay Between Aliphatic and Indolic Glucosinolates?
3.4. Direct Effect of Breakdown Products on P. brassicae
4. Materials and Methods
4.1. Plant and Pathogen Materials
4.2. Plant Cultivation and Inoculation
4.3. Plant Sampling and Disease Assessment
4.4. Extraction of Glucosinolates
4.5. Liquid Chromatography Mass Spectrometry (LCMS) and Analysis of Glucosinolates
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallsgrove, R.M.; Doughty, K.J.; Bennett, R.N. Glucosinolates. In Plant Amino Acids: Biochemistry and Biotechnology; Singh, B., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1998; pp. 523–562. [Google Scholar]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The Chemical Diversity and Distribution of Glucosinolates and Isothiocyanates among Plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E. Glucosinolate Structures in Evolution. Phytochemistry 2012, 77, 16–45. [Google Scholar] [CrossRef] [PubMed]
- Dixon, G.R. Clubroot (Plasmodiophora brassicae Woronin)—An Agricultural and Biological Challenge Worldwide. Can. J. Plant Pathol. 2014, 36, 5–18. [Google Scholar] [CrossRef]
- Diederichsen, E.; Beckmann, J.; Schondelmeier, J.; Dreyer, F. Genetics of Clubroot Resistance in Brassica napus ‘Mendel’. Acta Horticulturae 2006, 307–311. [Google Scholar] [CrossRef]
- Honig, F. Der Kohlkropferreger (Plasmodiophora brassicae Wor.): Eine Monographie. In Gartenbauwissen; Gustav Fischer Verlag: Jena, Germany, 1931; pp. 116–225. [Google Scholar]
- Řičařová, V.; Kaczmarek, J.; Strelkov, S.E.; Kazda, J.; Lueders, W.; Rysanek, P.; Manolii, V.; Jedryczka, M. Pathotypes of Plasmodiophora brassicae Causing Damage to Oilseed Rape in the Czech Republic and Poland. Eur. J. Plant Pathol. 2016, 145, 559–572. [Google Scholar] [CrossRef]
- Lüders, W. Analyses of Virulence of European Isolates of Clubroot (Plasmodiophora brassicae Wor.) and Mapping of Resistance Genes in Rapeseed (Brassica napus L.). Ph.D. Thesis, Justus-Liebig-Universität, Gießen, Germany, 2017. [Google Scholar] [CrossRef]
- Zamani-Noor, N. Variation in Pathotypes and Virulence of Plasmodiophora brassicae Populations in Germany. Plant Pathol. 2017, 66, 316–324. [Google Scholar] [CrossRef]
- Zamani-Noor, N.; Diederichsen, E.; Wallenhammar, A.-C.; Cordsen-Nielsen, G.; Orgeur, G.; Konradyova, V.; Dussart, F.; Smith, J.; Jedryczka, M. Epidemiology of Clubroot Disease and Pathogenic Variation among Isolates of Plasmodiophora brassicae from Oilseed Rape Growing in Europe. Can. J. Plant Pathol. 2019, 41, 491–492. [Google Scholar] [CrossRef]
- Some, A.; Manzanares, M.J.; Laurens, F.; Baron, F.; Thomas, G.; Rouxel, F. Variation for Virulence on Brassica Napus L. amongst Plasmodiophora brassicae. Collections from France and Derived Single-Spore Isolates. Plant Pathol. 1996, 45, 432–439. [Google Scholar] [CrossRef]
- Buczacki, S.T.; Toxopeus, H.; Mattusch, P.; Johnston, T.D.; Dixon, G.R.; Hobolth, L.A. Study of Physiologic Specialization in Plasmodiophora brassicae: Proposals for Attempted Rationalization through an International Approach. Transact. British Mycolog. Soc. 1975, 65, 295–303. [Google Scholar] [CrossRef]
- Korbas, M.; Jajor, E.; Budka, A. Clubroot (Plasmodiophora brassicae)–A Threat for Oilseed Rape. J. Plant Prot. Res. 2009, 49. [Google Scholar] [CrossRef]
- Siemens, J.; Glawischnig, E.; Ludwig-Müller, J. Indole Glucosinolates and Camalexin Do Not Influence the Development of the Clubroot Disease in Arabidopsis thaliana: Cyp79b2/B3 Double Mutant and Clubroot. J. Phytopathol. 2008, 156, 332–337. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Schubert, B.; Pieper, K.; Ihmig, S.; Hilgenberg, W. Glucosinolate Content in Susceptible and Resistant Chinese Cabbage Varieties during Development of Clubroot Disease. Phytochemistry 1997, 44, 407–414. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Bennett, R.N.; Kiddle, G.; Ihmig, S.; Ruppel, M.; Hilgenberg, W. The Host Range of Plasmodiophora brassicae and Its Relationship to Endogenous Glucosinolate Content. New Phytologist 1999, 141, 443–458. [Google Scholar] [CrossRef]
- Vik, D.; Mitarai, N.; Wulff, N.; Halkier, B.A.; Burow, M. Dynamic Modeling of Indole Glucosinolate Hydrolysis and Its Impact on Auxin Signaling. Front. Plant Sci. 2018, 9, 550. [Google Scholar] [CrossRef]
- Jahn, L.; Mucha, S.; Bergmann, S.; Horn, C.; Staswick, P.; Steffens, B.; Siemens, J.; Ludwig-Müller, J. The Clubroot Pathogen (Plasmodiophora brassicae) Influences Auxin Signaling to Regulate Auxin Homeostasis in Arabidopsis. Plants 2013, 2, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, K.; Niwa, T.; Kato, T.; Ohara, T.; Kakizaki, T.; Matsumoto, S. The Tandem Repeated Organization of NB-LRR Genes in the Clubroot-Resistant CRb Locus in Brassica rapa L. Mol. Genet. Genomic. 2017, 292, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Fredua-Agyeman, R.; Rahman, H. Mapping of the Clubroot Disease Resistance in Spring Brassica napus Canola Introgressed from European Winter Canola Cv. ‘Mendel.’. Euphytica 2016, 211, 201–213. [Google Scholar] [CrossRef]
- McHale, L.; Tan, X.; Koehl, P.; Michelmore, R.W. Plant NBS-LRR Proteins: Adaptable Guards. Genome Biol. 2006, 7, 1–11. [Google Scholar] [CrossRef]
- Chhajed, S.; Misra, B.B.; Tello, N.; Chen, S. Chemodiversity of the glucosinolate-myrosinase system at the single cell type resolution. Front. Plant Sci. 2019, 10, 618. [Google Scholar] [CrossRef]
- Nakano, R.T.; Piślewska-Bednarek, M.; Yamada, K.; Edger, P.P.; Miyahara, M.; Kondo, M.; Böttcher, C.; Mori, M.; Nishimura, M.; Schulze-Lefert, P.; et al. PYK10 Myrosinase Reveals a Functional Coordination between Endoplasmic Reticulum Bodies and Glucosinolates in Arabidopsis thaliana. Plant J. 2017, 89, 204–220. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Liu, Y.; Miao, H.; Cai, C.; Shao, Z.; Guo, R.; Sun, B.; Jia, C.; Zhang, L.; et al. Classic Myrosinase-Dependent Degradation of Indole Glucosinolate Attenuates Fumonisin B1-Induced Programmed Cell Death in Arabidopsis. Plant J. 2015, 81, 920–933. [Google Scholar] [CrossRef]
- Lehmann, T.; Janowitz, T.; Sánchez-Parra, B.; Alonso, M.-M.P.; Trompetter, I.; Piotrowski, M.; Pollmann, S. Arabidopsis NITRILASE 1 Contributes to the Regulation of Root Growth and Development through Modulation of Auxin Biosynthesis in Seedlings. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E.; Sørensen, H. Initial and Final Products, Nitriles, and Ascorbigens Produced in Myrosinase-Catalyzed Hydrolysis of Indole Glucosinolates. J. Agricult. Food Chem. 1998, 46, 1563–1571. [Google Scholar] [CrossRef]
- Katz, E.; Nisani, S.; Yadav, B.S.; Woldemariam, M.G.; Shai, B.; Obolski, U.; Ehrlich, M.; Shani, E.; Jander, G.; Chamovitz, D.A. The Glucosinolate Breakdown Product Indole-3-carbinol Acts as an Auxin Antagonist in Roots of Arabidopsis thaliana. Plant J. 2015, 82, 547–555. [Google Scholar] [CrossRef]
- Andersen, T.G.; Nour-Eldin, H.H.; Fuller, V.L.; Olsen, C.E.; Burow, M.; Halkier, B.A. Integration of Biosynthesis and Long-Distance Transport Establish Organ-Specific Glucosinolate Profiles in Vegetative Arabidopsis. Plant Cell 2013, 25, 3133–3145. [Google Scholar] [CrossRef]
- Hwang, S.F.; Ahmed, H.U.; Zhou, Q.; Strelkov, S.E.; Gossen, B.D.; Peng, G.; Turnbull, G.D. Assessment of the Impact of Resistant and Susceptible Canola on Plasmodiophora brassicae Inoculum Potential: Volunteer Canola, Inoculum Density and Clubroot. Plant Pathol. 2012, 61, 945–952. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ye, W.; Hossain, M.A.; Okuma, E.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Glucosinolate Degradation Products, Isothiocyanates, Nitriles, and Thiocyanates, Induce Stomatal Closure Accompanied by Peroxidase-Mediated Reactive Oxygen Species Production in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2013, 77, 977–983. [Google Scholar] [CrossRef]
- Sobahan, M.A.; Akter, N.; Okuma, E.; Uraji, M.; Ye, W.; Mori, I.C.; Nakamura, Y.; Murata, Y. Allyl Isothiocyanate Induces Stomatal Closure in Vicia faba. Biosci. Biotechnol. Biochem. 2015, 79, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Salehin, M.; Li, B.; Tang, M.; Katz, E.; Song, L.; Ecker, J.R.; Kliebenstein, D.J.; Estelle, M. Auxin-Sensitive Aux/IAA Proteins Mediate Drought Tolerance in Arabidopsis by Regulating Glucosinolate Levels. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Wagner, G.; Laperche, A.; Lariagon, C.; Marnet, N.; Renault, D.; Guitton, Y.; Bouchereau, A.; Delourme, R.; Manzanares-Dauleux, M.J.; Gravot, A. Resolution of Quantitative Resistance to Clubroot into QTL-Specific Metabolic Modules. J. Exp. Bot. 2019, 70, 5375–5390. [Google Scholar] [CrossRef] [PubMed]
- Strelkov, S.E.; Tewari, J.P.; Smith-Degenhardt, E. Characterization of Plasmodiophora brassicae Populations from Alberta, Canada. Can. J. Plant Pathol. 2006, 28, 467–474. [Google Scholar] [CrossRef]
- Hornbacher, J.; Rumlow, A.; Pallmann, P.; Turcios, A.E.; Riemenschneider, A.; Papenbrock, J. The Levels of Sulfur-Containing Metabolites in Brassica napus Are Not Influenced by the Circadian Clock but Diurnally. J. Plant Biol. 2019, 62, 359–373. [Google Scholar] [CrossRef]
- Thies, W. Detection and Utilization of a Glucosinolate Sulfohydrolase in the Edible Snail, Helix pomatia. Naturwissenschaften 1979, 66, 364–365. [Google Scholar] [CrossRef]
| Cultivar | Clubroot Resistance | P. brassicae P1 1 | P. brassicae P1 (+) 1 | ||
|---|---|---|---|---|---|
| DI 2 ± SD | DSI 2 ± SD | DI 2 ± SD | DSI 2 ± SD | ||
| Aristoteles | resistant | 0.0 ± 0.0 | 0.0 ± 0.0 | 44.0 ± 3.0 | 23.3 ± 1.6 |
| Creed | resistant | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Mendel | resistant | 15.3 ± 12.6 | 9.9 ± 6.8 | 60.0 ± 8.3 | 30.5 ± 3.6 |
| Bender | susceptible | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 98.8 ± 1.7 |
| Ladoga | susceptible | 100.0 ± 0.0 | 98.2 ± 2.5 | 97.9 ± 3.0 | 91.3 ± 12.6 |
| Visby | susceptible | 100.0 ± 0.0 | 98.8 ± 1.9 | 100.0 ± 0.0 | 100.0 ± 0.0 |
| Cultivar | Seed Source | Clubroot Resistance |
|---|---|---|
| Aristoteles | Limagrain GmbH | resistance: single dominant gene (based on ‘Mendel’ resistance) |
| Creed | Norddeutsche Pflanzenzucht | resistance: polygenic resistance (internal communication with the company) |
| Mendel | Norddeutsche Pflanzenzucht | resistance: single dominant gene-based resistance [5] |
| Bender | Deutsche Saatveredelung AG | susceptible |
| Ladoga | Limagrain GmbH | susceptible |
| Visby | Norddeutsche Pflanzenzucht | susceptible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamani-Noor, N.; Hornbacher, J.; Comel, C.J.; Papenbrock, J. Variation of Glucosinolate Contents in Clubroot-Resistant and -Susceptible Brassica napus Cultivars in Response to Virulence of Plasmodiophora brassicae. Pathogens 2021, 10, 563. https://doi.org/10.3390/pathogens10050563
Zamani-Noor N, Hornbacher J, Comel CJ, Papenbrock J. Variation of Glucosinolate Contents in Clubroot-Resistant and -Susceptible Brassica napus Cultivars in Response to Virulence of Plasmodiophora brassicae. Pathogens. 2021; 10(5):563. https://doi.org/10.3390/pathogens10050563
Chicago/Turabian StyleZamani-Noor, Nazanin, Johann Hornbacher, Christel Joy Comel, and Jutta Papenbrock. 2021. "Variation of Glucosinolate Contents in Clubroot-Resistant and -Susceptible Brassica napus Cultivars in Response to Virulence of Plasmodiophora brassicae" Pathogens 10, no. 5: 563. https://doi.org/10.3390/pathogens10050563
APA StyleZamani-Noor, N., Hornbacher, J., Comel, C. J., & Papenbrock, J. (2021). Variation of Glucosinolate Contents in Clubroot-Resistant and -Susceptible Brassica napus Cultivars in Response to Virulence of Plasmodiophora brassicae. Pathogens, 10(5), 563. https://doi.org/10.3390/pathogens10050563









