Occurrence of Legionella spp. in Man-Made Water Sources: Isolates Distribution and Phylogenetic Characterization in the Emilia-Romagna Region
Abstract
1. Introduction
2. Results
2.1. Legionella Strains Environmental Distribution
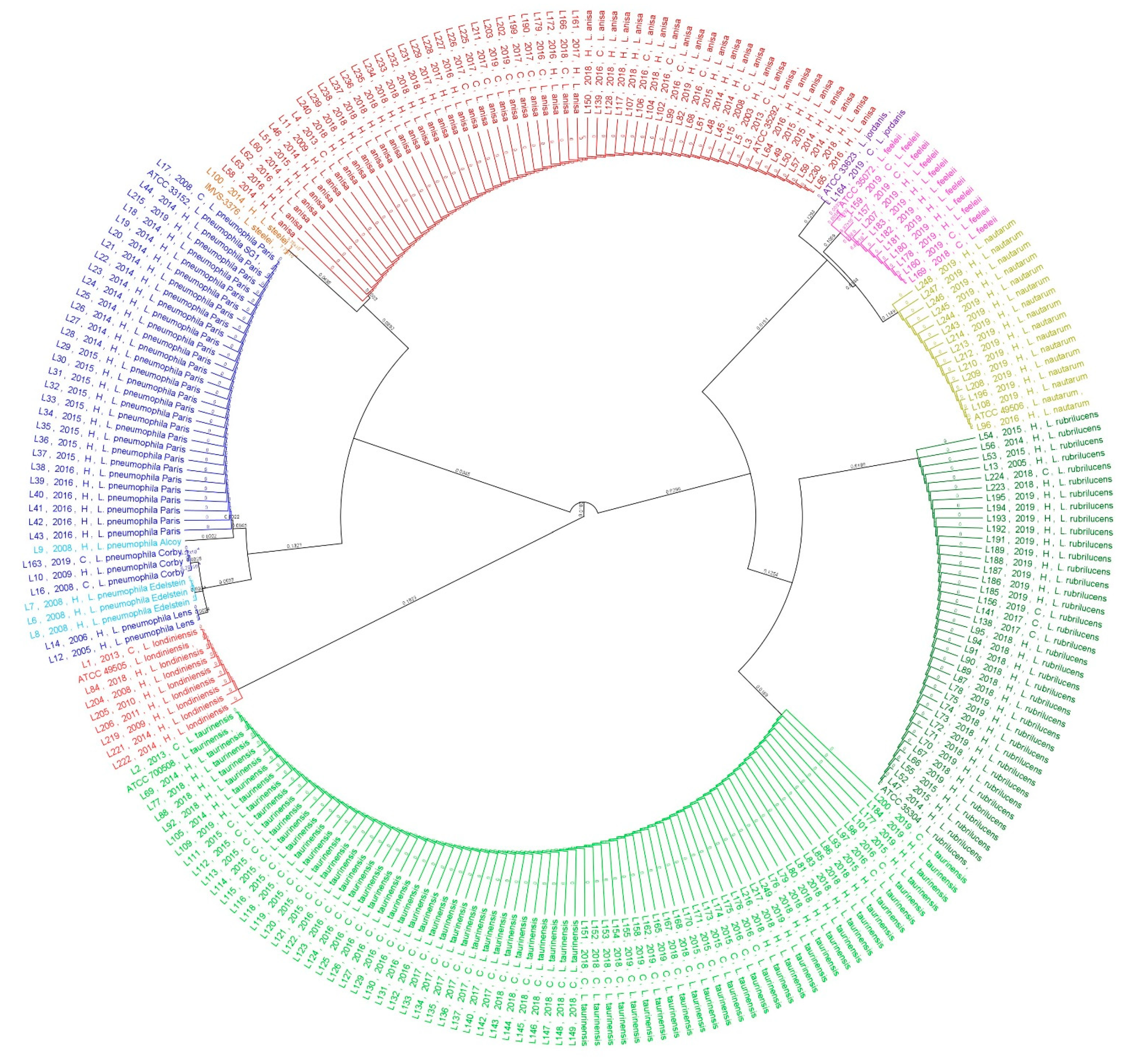
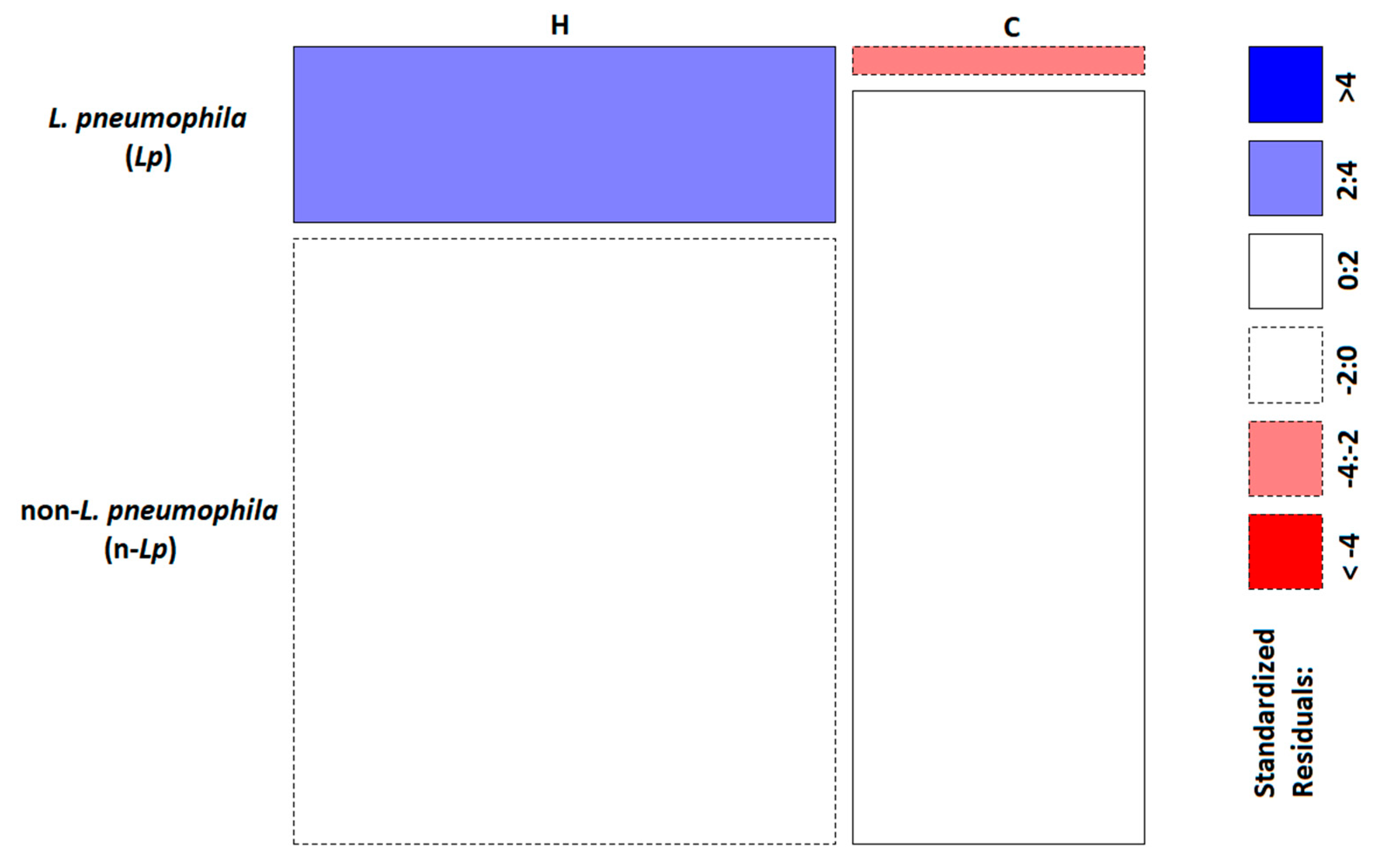
2.2. Phylogenetic and Statistical Relationship
3. Discussion
4. Materials and Methods
4.1. Samples Collection and Microbiological Analysis
4.2. Molecular Analysis: DNA Extraction, PCR, and Mip-Gene Sequencing
4.3. Phylogenetic Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diederen, B.M.W. Legionella spp. and Legionnaires’ disease. J. Infect. 2008, 56, 1–12. [Google Scholar] [CrossRef]
- LPSN Bacterio.net LPSN. List of prokaryotic Names with Standing in Nomenclature Legionella. Available online: https://www.bacterio.net/genus/legionella (accessed on 16 February 2021).
- Fields, S.B.; Benson, R.F.; Besser, R.E. Legionella and Legionnaires’ Disease: 25 Years of Investigation. Clin. Microbiol. Rev. 2002, 15, 506–526. [Google Scholar] [CrossRef]
- Mercante, J.W.; Winchell, J.M. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin. Microbiol. Rev. 2015, 28, 95–133. [Google Scholar] [CrossRef]
- Mondino, S.; Schmidt, S.; Rolando, M.; Escoll, P.; Gomez-Valero, L.; Buchrieser, C. Legionnaires’ Disease: State of the Art Knowledge of Pathogenesis Mechanisms of Legionella. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 439–466. [Google Scholar] [CrossRef] [PubMed]
- Oliva, G.; Sahr, T.; Buchrieser, C. The life cycle of L. pneumophila: Cellular differentiation is linked to virulence and metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Cunha, B.A.; Burillo, A.; Bouza, E. Legionnaires’ disease. Lancet 2016, 387, 376–385. [Google Scholar] [CrossRef]
- Burillo, A.; Pedro-Botet, M.L.; Bouza, E. Microbiology and Epidemiology of Legionnaire’s Disease. Infect. Dis. Clin. North Am. 2017, 31, 7–27. [Google Scholar] [CrossRef]
- Muder, R.R.; Yu, V.L. Infection due to Legionella species other than L. pneumophila. Clin. Infect. Dis. 2002, 35, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.L.; Plouffe, J.F.; Pastoris, M.C.; Stout, J.E.; Schousboe, M.; Widmer, A.; Summersgill, J.; File, T.; Heath, C.M.; Paterson, D.L.; et al. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: An international collaborative survey. J. Infect. Dis. 2002, 186, 127–128. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control Legionnaires’ Disease. In ECDC. Annual Epidemiological Report for 2018; Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/en/publications-data/legionnaires-disease-annual-epidemiological-report-2018 (accessed on 16 February 2021).
- Rota, M.C.; Caporali, M.G.; Bella, A.; Scaturro, M.; Giannitelli, S.; Ricci, M.L. Rapporto annuale sulla legionellosi in Italia nel 2018; Notiziario Istituto Superiore di Sanità: Rome, Italy, 2019; Volume 32. [Google Scholar]
- Gomez-Valero, L.; Rusniok, C.; Buchrieser, C. Legionella pneumophila: Population genetics, phylogeny and genomics. Infect. Genet. Evol. 2009, 9, 727–739. [Google Scholar] [CrossRef]
- Potts, A.; Donaghy, M.; Marley, M.; Othieno, R.; Stevenson, J.; Hyland, J.; Pollock, K.G.; Lindsay, D.; Edwards, G.; Hanson, M.F.; et al. Cluster of Legionnaires’ disease cases caused by Legionella longbeachae serogroup 1, Scotland, August to September 2013. Eurosurveillance 2013, 18, 1–5. [Google Scholar] [CrossRef]
- Amemura-maekawa, J.; Kura, F.; Chida, K.; Ohya, H.; Kanatani, J.; Isobe, J.; Tanaka, S.; Nakajima, H.; Hiratsuka, T.; Yoshino, S.; et al. Legionella pneumophila and Other Legionella Species Isolated from Legionellosis Patients in Japan between 2008 and 2016. Appl. Environ. Microbiol. 2018, 84, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Byrne, B.; Mccolm, S.; Mcelmurry, S.; Swanson, M. Prevalence of Infection-Competent Serogroup 6 Legionella pneumophila within Premise Plumbing in Southeast Michigan. mBio Am. Soc. Microbil. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Beauté, J. Legionnaires’ disease in Europe, 2011 to 2015. Eurosurveillance 2017, 22, 1–8. [Google Scholar] [CrossRef]
- Italian Health Ministry, Guidelines for Prevention and Control of Legionellosis; Approvate in Conferenza Stato-Regioni Seduta del 7 maggio: Rome, Italy, 2015.
- Prevention, Control and Investigation, of Infections Caused by Legionella species; European Working Group for Legionella Infections: Solna, Stockholm, Sweden, 2017.
- Blyth, C.C.; Adams, D.N.; Chen, S.C.A. Diagnostic and typing methods for investigating Legionella infection. N. S. W. Public Health Bull. 2009, 20, 157–161. [Google Scholar] [CrossRef]
- Kyritsi, M.A.; Mouchtouri, V.A.; Katsioulis, A.; Kostara, E.; Nakoulas, V.; Hatzinikou, M.; Hadjichristodoulou, C. Legionella Colonization of Hotel Water Systems in Touristic Places of Greece: Association with System Characteristics and Physicochemical Parameters. Int. J. Environ. Res. Public Health 2018, 15, 2707. [Google Scholar] [CrossRef]
- Yakunin, E.; Kostyal, E.; Agmon, V.; Grotto, I.; Valinsky, L.; Moran-Gilad, J. A snapshot of the prevalence and molecular diversity of Legionella pneumophila in the water systems of Israeli hotels. Pathogens 2020, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.E.; Yu, V.L.; Yee, Y.C.; Vaccarello, S.; Diven, W.; Lee, T.C. Legionella pneumophila in residential water supplies: Environmental surveillance with clinical assessment for Legionnaires’ disease. Epidemiol. Infect. 1992, 109, 49–57. [Google Scholar] [PubMed]
- Hamilton, K.A.; Hamilton, M.T.; Johnson, W.; Jjemba, P.; Bukhari, Z.; Lechevallier, M.; Haas, C.N.; Gurian, P.L. Risk-Based Critical Concentrations of Legionella pneumophila for Indoor Residential Water Uses. Environ. Sci. Technol. 2019, 53, 4528–4541. [Google Scholar] [CrossRef]
- Dabrera, G.; Naik, F.; Phin, N. Legionellosis incidents associated with spa pools, England, 2002–2018. Public Health 2020, 185, 232–234. [Google Scholar] [CrossRef]
- Zeybek, Z.; Türkmen, A. Investigation of the incidence of Legionella and free-living amoebae in swimming pool waters and biofilm specimens in istanbul by different methods. Mikrobiyol. Bul. 2020, 54, 50–65. [Google Scholar] [CrossRef]
- Benin, A.L.; Benson, R.F.; Arnold, K.E.; Fiore, A.E.; Cook, P.G.; Williams, L.K.; Fields, B.; Besser, R.E. An outbreak of travel-associated legionnaires disease and pontiac fever: The need for enhanced surveillance of travel-associated legionellosis in the United States. J. Infect. Dis. 2002, 185, 237–243. [Google Scholar] [CrossRef]
- Quaranta, G.; Vincenti, S.; Ferriero, A.M.; Boninti, F.; Sezzatini, R.; Turnaturi, C.; Gliubizzi, M.D.; Munafò, E.; Ceccarelli, G.; Causarano, C.; et al. Legionella on board trains: Effectiveness of environmental surveillance and decontamination. BMC Public Health 2012, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rota, M.C.; Bella, A.; Caporali, M.G.; Nicolau, A.; Drasar, V.; Ricci, M.L.; Scaturro, M.; Gumá, M.; Crespi, S. Travel-associated Legionnaires’ disease: Would changing cluster definition lead to the prevention of a larger number of cases? Epidemiol. Infect. 2018, 147. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, R.M.; Lanser, J.A.; Manning, P.A.; Heuzenroeder, M.W. Sequence-based classification scheme for the genus Legionella targeting the mip gene. J. Clin. Microbiol. 1998, 36, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Haroon, A.; Koide, M.; Higa, F.; Tateyama, M.; Fujita, J. Identification of Legionella pneumophila serogroups and other Legionella species by mip gene sequencing. J. Infect. Chemother. 2012, 18, 276–281. [Google Scholar] [CrossRef]
- Ratzow, S.; Gaia, V.; Helbig, J.H.; Fry, N.K.; Lück, P.C. Addition of neuA, the gene encoding N-acylneuraminate cytidylyl transferase, increases the discriminatory ability of the consensus sequence-based scheme for typing Legionella pneumophila serogroup 1 strains. J. Clin. Microbiol. 2007, 45, 1965–1968. [Google Scholar] [CrossRef]
- Fry, N.K.; Afshar, B.; Bellamy, W.; Underwood, A.P.; Ratcliff, R.M.; Harrison, T.G.; Bangsborg, J.; Blanco, S.; Etienne, J.; Fendukly, F.; et al. Identification of Legionella spp. by 19 European reference laboratories: Results of the European Working Group for Legionella Infections External Quality Assessment Scheme using DNA sequencing of the macrophageinfectivity potentiator gene an. Clin. Microbiol. Infect. 2007, 13, 1119–1124. [Google Scholar] [CrossRef]
- Gaia, V.; Fry, N.K.; Harrison, T.G.; Peduzzi, R. Sequence-Based Typing of Legionella pneumophila Serogroup 1 Offers the Potential for True Portability in Legionellosis Outbreak Investigation. Society 2003, 41, 2932–2939. [Google Scholar] [CrossRef] [PubMed]
- Gaia, V.; Fry, N.K.; Afshar, B.; Luck, P.C.; Meugnier, H.; Etienne, J.; Peduzzi, R.; Harrison, T.G. Consensus Sequence-Based Scheme for Epidemiological Typing of Clinical and Environmental Isolates of Legionella pneumophila. J. Clin. Microbiol. 2005, 43, 2047–2052. [Google Scholar] [CrossRef]
- Veríssimo, A.; Morais, P.V.; Diogo, A.; Gomes, C.; Da Costa, M.S. Characterization of Legionella species by numerical analysis of whole-cell protein electrophoresis. Int. J. Syst. Bacteriol. 1996, 46, 41–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orsini, M.; Cristino, S.; Grottola, A.; Romano-Spica, V. Bacteria misagglutination in Legionella surveillance programmes. J. Hosp. Infect. 2011, 79, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Prussin, A.J.; Schwake, D.O.; Marr, L.C. Ten questions concerning the aerosolization and transmission of Legionella in the built environment. Build. Environ. 2017, 123, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Bartram, J.; Chartlier, Y.; Lee, J.V.; Pond, K.; Surman-Lee, S. Legionella and the Prevention of Legionellosis; World Health Organization: Geneva, Switzerland, 2007. Reviewed by Joseph E. Mcdade. Emerg. Infect. Dis. 2008, 14, 1006. [Google Scholar]
- Ducret, A.; Chabalier, M.; Dukan, S. Characterization and resuscitation of “non-culturable” cells of Legionella pneumophila. BMC Microbiol. 2014, 14. [Google Scholar] [CrossRef]
- Oliver, J.D. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol. Rev. 2010, 34, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, A.K.T. Determination of viable legionellae in engineered water systems: Do we find what we are looking for? Water Res. 2016, 93, 276–288. [Google Scholar] [CrossRef]
- Lee, H.K.; Shim, J.I.; Kim, H.E.; Yu, J.Y.; Kang, Y.H. Distribution of Legionella species from environmental water sources of public facilities and genetic diversity of L. pneumophila serogroup 1 in South Korea. Appl. Environ. Microbiol. 2010, 76, 6547–6554. [Google Scholar] [CrossRef]
- Fallon, R.J.; Stack, B.H.R. Legionnaires’ disease due to Legionella anisa. J. Infect. 1990, 20, 227–229. [Google Scholar] [CrossRef]
- Fenstersheib, M.D.; Miller, M.; Diggins, C.; Liska, S.; Detwiler, L.; Werner, S.B.; Lindquist, D.; Thacker, W.L.; Benson, R.F. Outbreak of Pontiac fever due to Legionella anisa. Lancet 1990, 336, 35–37. [Google Scholar] [CrossRef]
- McNally, C.; Hackman, B.; Fields, B.S.; Plouffe, J.F. Potential importance of Legionella species as etiologies in community acquired pneumonia (CAP). Diagn. Microbiol. Infect. Dis. 2000, 38, 79–82. [Google Scholar] [CrossRef]
- Lin, Y.E.; Stout, J.E.; Yu, V.L. Controlling Legionella in Hospital Drinking Water: An Evidence-Based Review of Disinfection Methods. Infect. Control Hosp. Epidemiol. 2011, 32, 166–173. [Google Scholar] [CrossRef]
- Rhoads, W.J.; Pruden, A.; Edwards, M.A. Anticipating Challenges with In-Building Disinfection for Control of Opportunistic Pathogens. Water Environ. Res. 2014, 86, 540–549. [Google Scholar] [CrossRef]
- García, M.T.; Baladrón, B.; Gil, V.; Tarancon, M.L.; Vilasau, A.; Ibañez, A.; Elola, C.; Pelaz, C. Persistence of chlorine-sensitive Legionella pneumophila in hyperchlorinated installations. J. Appl. Microbiol. 2008, 105, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Perola, O.; Kauppinen, J.; Kusnetsov, J.; Kärkkäinen, U.M.; Lück, P.C.; Katila, M.L. Persistent Legionella pneumophila colonization of a hospital water supply: Efficacy of control methods and a molecular epidemiological analysis. Apmis 2005, 113, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Huang, C.; Lin, J.; Wu, W.; Ashbolt, N.J.; Liu, W.T.; Nguyen, T.H. Effect of Disinfectant Exposure on Legionella pneumophila Associated with Simulated Drinking Water Biofilms: Release, Inactivation, and Infectivity. Environ. Sci. Technol. 2017, 51, 2087–2095. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Khan, M.A.; Zhu, F. Popular molecular markers in bacteria. Mol. Genet. Microbiol. Virol. 2012, 27, 103–107. [Google Scholar] [CrossRef]
- Kozak-Muiznieks, N.A.; Morrison, S.S.; Mercante, J.W.; Ishaq, M.K.; Johnson, T.; Caravas, J.; Lucas, C.E.; Brown, E.; Raphael, B.H.; Winchell, J.M. Comparative genome analysis reveals a complex population structure of Legionella pneumophila subspecies. Infect. Genet. Evol. 2018, 59, 172–185. [Google Scholar] [CrossRef]
- David, S.; Mentasti, M.; Tewolde, R.; Aslett, M.; Harris, S.R.; Afshar, B.; Underwood, A.; Fry, N.K.; Parkhill, J.; Harrison, T.G. Evaluation of an optimal epidemiological typing scheme for Legionella pneumophila with whole-genome sequence data using validation guidelines. J. Clin. Microbiol. 2016, 54, 2135–2148. [Google Scholar] [CrossRef] [PubMed]
- Buse, H.Y.; Morris, B.J.; Gomez-Alvarez, V.; Szabo, J.G.; Hall, J.S. Legionella diversity and spatiotemporal variation in the occurrence of opportunistic pathogens within a large building water system. Pathogens 2020, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Lehtola, M.J.; Laxander, M.; Miettinen, I.T.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. The effects of changing water flow velocity on the formation of biofilms and water quality in pilot distribution system consisting of copper or polyethylene pipes. Water Res. 2006, 40, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Douterelo, I.; Sharpe, R.L.; Boxall, J.B. Influence of hydraulic regimes on bacterial community structure and composition in an experimental drinking water distribution system. Water Res. 2013, 47, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of water intended for human consumption, Official Journal of the European Union, Bruxelles, Belgium. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32020L2184 (accessed on 16 February 2021).
- El-Sayed, A.; Kamel, M. Climatic changes and their role in emergence and re-emergence of diseases. Environ. Sci. Pollut. Res. 2020, 27, 22336–22352. [Google Scholar] [CrossRef]
- EN ISO 19458:2006. In Water Quality—Sampling for Microbiological Analysis, 1st ed.; Geneva, Switzerland, September 2006; Available online: https://www.iso.org/obp/ui/#iso:std:iso:19458:ed-1:v1:en (accessed on 16 February 2021).
- ISO 11731:2017. In Water Quality—Enumeration of Legionella, 2nd ed.; Geneva, Switzerland, May 2017; Available online: https://www.iso.org/obp/ui/#iso:std:iso:11731:ed-2:v1:en (accessed on 16 February 2021).
- Mentasti, M.; Fry, N.K.; Afshar, B.; Palepou-Foxley, C.; Naik, F.C.; Harrison, T.G. Application of Legionella pneumophila-specific quantitative real-time PCR combined with direct amplification and sequence-based typing in the diagnosis and epidemiological investigation of Legionnaires’ disease. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2017–2028. [Google Scholar] [CrossRef]
- Fry, N.K.; Alexiou-Daniel, S.; Bangsborg, J.M.; Bernander, S.; Castellani Pastoris, M.; Etienne, J.; Forsblom, B.; Gaia, V.; Helbig, J.H.; Lindsay, D.; et al. A multicenter evaluation of genotypic methods for the epidemiologic typing of Legionella pneumophila serogroup 1: Results of a pan-European study. Clin. Microbiol. Infect. 1999, 5, 462–477. [Google Scholar] [CrossRef][Green Version]
- Boratyn, G.M.; Camacho, C.; Cooper, P.S.; Coulouris, G.; Fong, A.; Ma, N.; Madden, T.L.; Matten, W.T.; McGinnis, S.D.; Merezhuk, Y.; et al. BLAST: A more efficient report with usability improvements. Nucleic Acids Res. 2013, 41, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Michener, C.D.; Sokal, R.R. A Quantitative Approach to a Problem in Classification. Evolution 1957, 11, 130–162. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Hutcheson, K. A test for comparing diversities based on the shannon formula. J. Theor. Biol. 1970, 29, 151–154. [Google Scholar] [CrossRef]
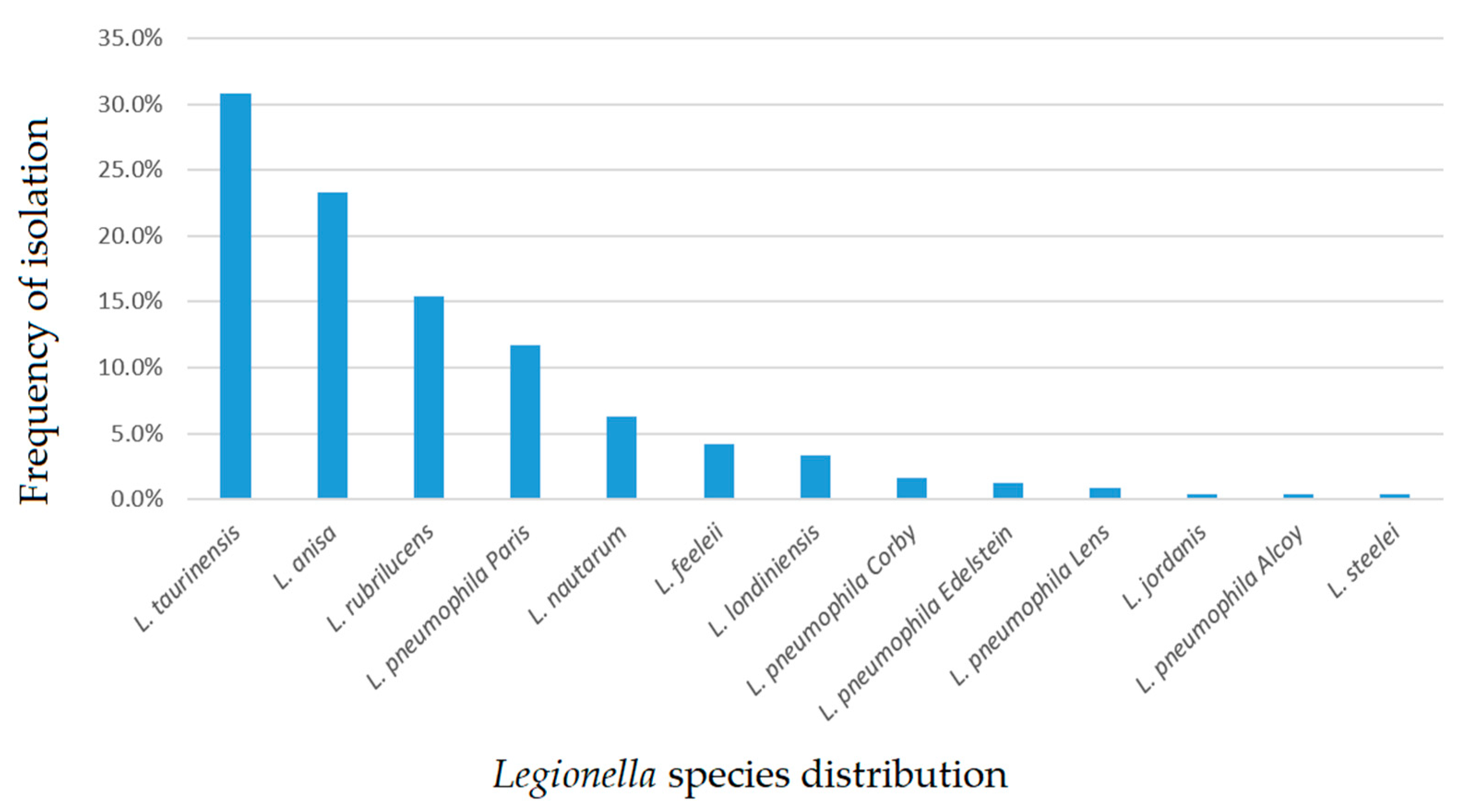
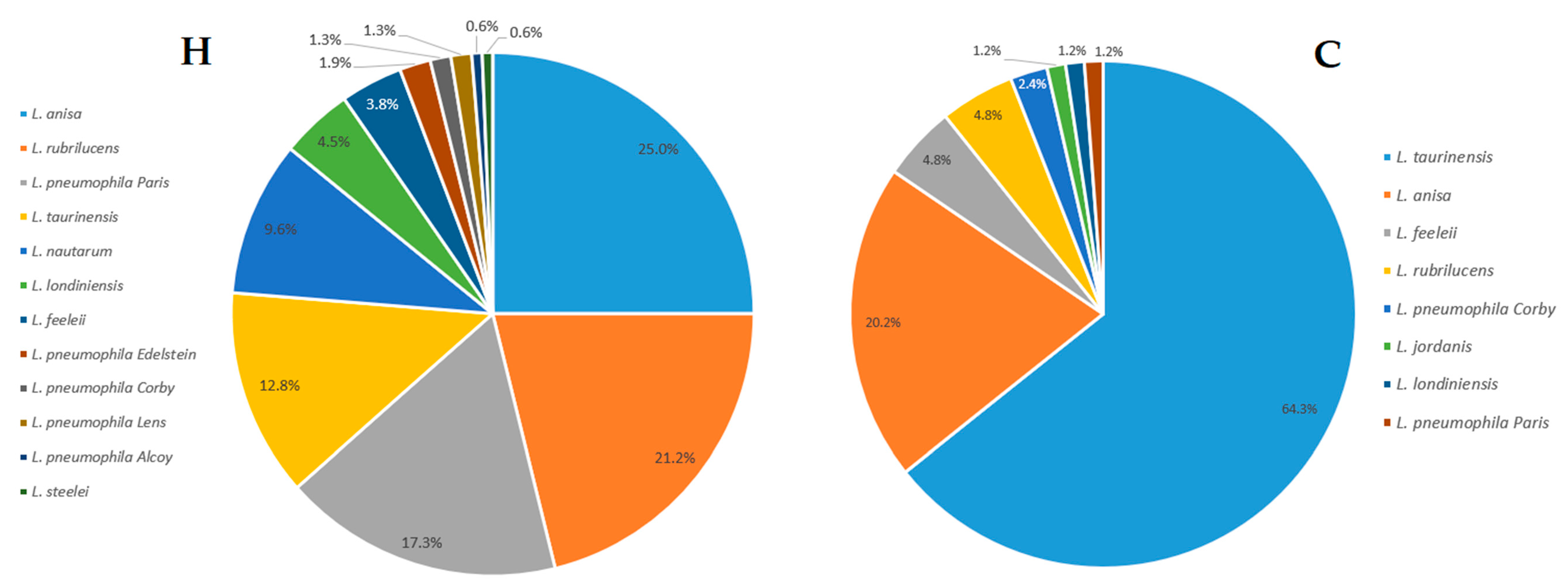

| Legionella Population | Hospital Category (H) No. of Isolates (n) | Community Category (C) No. of Isolates (n) |
|---|---|---|
| L. anisa | 39 | 17 |
| L. feeleii | 6 | 4 |
| L. jordanis | 0 | 1 |
| L. londiniensis | 7 | 1 |
| L. nautarum | 15 | 0 |
| L. pneumophila (Lp) Alcoy | 1 | 0 |
| Lp Corby | 2 | 2 |
| Lp Edelstein | 3 | 0 |
| Lp Lens | 2 | 0 |
| Lp Paris | 27 | 1 |
| L. rubrilucens | 33 | 4 |
| L. steelei | 1 | 0 |
| L. taurinensis | 20 | 54 |
| Shannon’s index (H’) | 1.98 | 1.14 |
| Hutcheson t-test | p-value (p) = 0.00017 * | |
| Environmental Category | L. pneumophila (Lp) | Non-L. pneumophila (n-Lp) |
|---|---|---|
| Hospitals (H) | 35 | 121 |
| Communities (C) | 3 | 81 |
| χ2 test | p-value (p) = 0.00028 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzotta, M.; Salaris, S.; Pascale, M.R.; Girolamini, L.; Cristino, S. Occurrence of Legionella spp. in Man-Made Water Sources: Isolates Distribution and Phylogenetic Characterization in the Emilia-Romagna Region. Pathogens 2021, 10, 552. https://doi.org/10.3390/pathogens10050552
Mazzotta M, Salaris S, Pascale MR, Girolamini L, Cristino S. Occurrence of Legionella spp. in Man-Made Water Sources: Isolates Distribution and Phylogenetic Characterization in the Emilia-Romagna Region. Pathogens. 2021; 10(5):552. https://doi.org/10.3390/pathogens10050552
Chicago/Turabian StyleMazzotta, Marta, Silvano Salaris, Maria Rosaria Pascale, Luna Girolamini, and Sandra Cristino. 2021. "Occurrence of Legionella spp. in Man-Made Water Sources: Isolates Distribution and Phylogenetic Characterization in the Emilia-Romagna Region" Pathogens 10, no. 5: 552. https://doi.org/10.3390/pathogens10050552
APA StyleMazzotta, M., Salaris, S., Pascale, M. R., Girolamini, L., & Cristino, S. (2021). Occurrence of Legionella spp. in Man-Made Water Sources: Isolates Distribution and Phylogenetic Characterization in the Emilia-Romagna Region. Pathogens, 10(5), 552. https://doi.org/10.3390/pathogens10050552







