Exposure to Major Vector-Borne Diseases in Dogs Subjected to Different Preventative Regimens in Endemic Areas of Italy
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
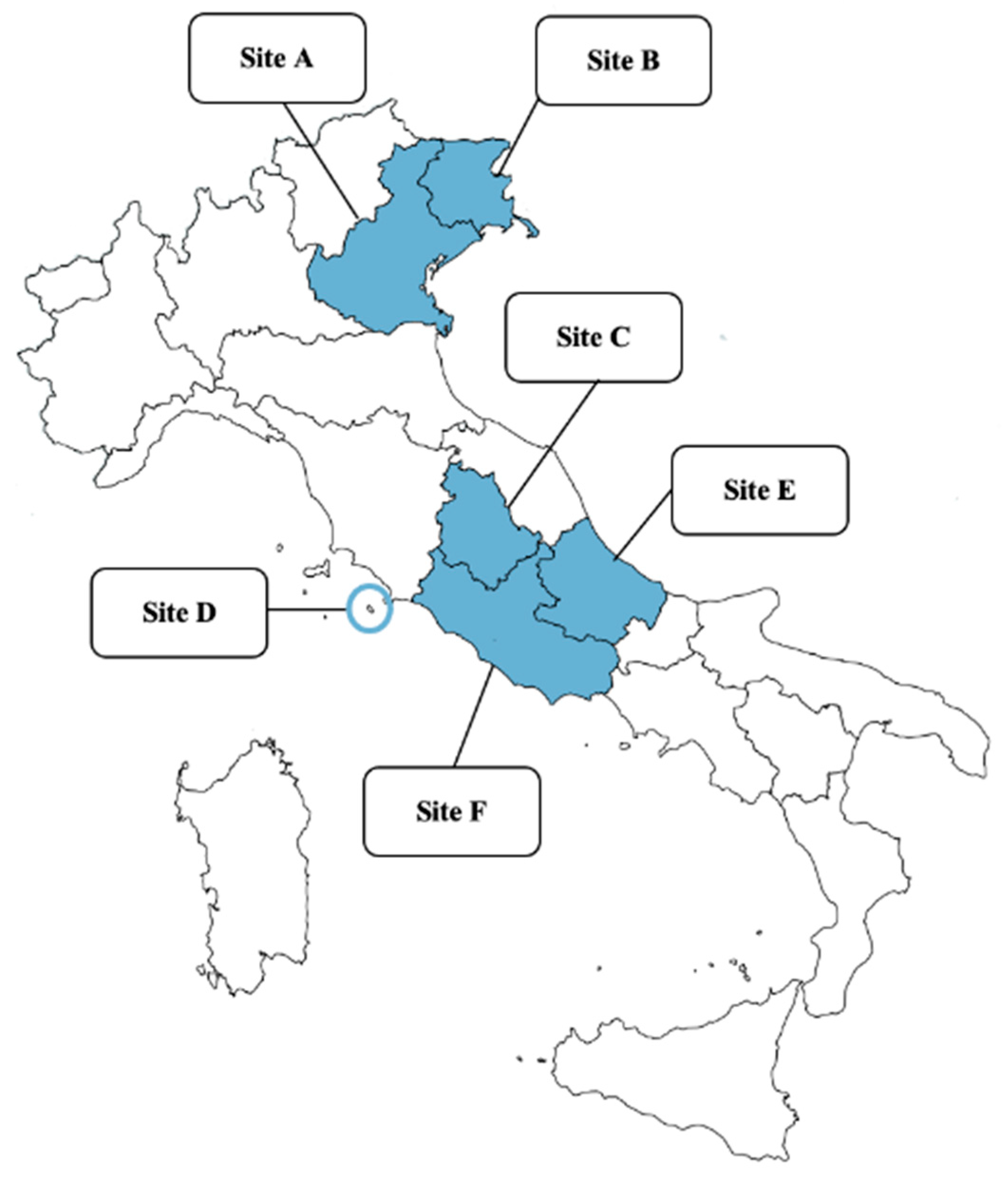
4.1. Study Design, Areas, and Sampling
4.2. Serology
4.3. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beugnet, F.; Marié, J.L. Emerging arthropod-borne diseases of companion animals in Europe. Vet. Parasitol. 2009, 163, 298–305. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Breitschwerdt, E.B. Managing canine vector-borne diseases of zoonotic concern: Part one. Trends Parasitol. 2009, 25, 157–163. [Google Scholar] [CrossRef]
- Self, S.C.W.; Liu, Y.; Nordone, S.K.; Yabsley, M.J.; Walden, H.S.; Lund, R.B.; Bowman, D.D.; Carpenter, C.; McMahan, C.S.; Gettings, J.R. Canine vector-borne disease: Mapping and the accuracy of forecasting using big data from the veterinary community. Anim. Health Res. Rev. 2019, 20, 47–60. [Google Scholar] [CrossRef]
- Jefferies, R.; Ryan, U.M.; Muhlnickel, C.J.; Irwin, P.J. Two species of canine Babesia in Australia: Detection and characterization by PCR. J. Parasitol. 2003, 89, 409–412. [Google Scholar] [CrossRef]
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and animal dirofilariasis: The emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, M.; Mhadhbi, M.; Rejeb, A.; Jaouadi, K.; Rouatbi, M.; Darghouth, M.A. Leishmaniosis (Leishmania infantum infection) in dogs. Rev. Sci. Technol. Off. Int. Epiz. 2015, 342, 613–626. [Google Scholar] [CrossRef]
- Singh, M.N.; Raina, O.K.; Sankar, M.; Rialch, A.; Tigga, M.N.; Kumar, G.R.; Banerjee, P.S. Molecular detection and genetic diversity of Babesia gibsoni in dogs in India. Infect. Genet. Evol. 2016, 41, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Sainz, Á.; Roura, X.; Estrada-Peña, A.; Miró, G. A review of canine babesiosis: The European perspective. Parasit. Vectors 2016, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Barash, N.R.; Thomas, B.; Birkenheuer, A.J.; Breitschwerdt, E.B.; Lemler, E.; Qurollo, B.A. Prevalence of Babesia spp. and clinical characteristics of Babesia vulpes infections in North American dogs. J. Vet. Intern. Med. 2019, 33, 2075–2081. [Google Scholar] [CrossRef]
- Guo, W.P.; Xie, G.C.; Li, D.; Su, M.; Jian, R.; Du, L.Y. Molecular detection and genetic characteristics of Babesia gibsoni in dogs in Shaanxi Province, China. Parasit. Vectors 2020, 13, 366. [Google Scholar] [CrossRef]
- McCall, J.W.; Genchi, C.; Kramer, L.H.; Guerrero, J.; Venco, L. Heartworm disease in animals and humans. Adv. Parasitol. 2008, 66, 193–285. [Google Scholar] [PubMed]
- Paltrinieri, S.; Solano-Gallego, L.; Fondati, A.; Lubas, G.; Gradoni, L.; Castagnaro, M.; Crotti, A.; Maroli, M.; Oliva, G.; Roura, X.; et al. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Strobl, A.; Künzel, F.; Tichy, A.; Leschnik, M. Complications and risk factors regarding the outcomes of canine babesiosis in Central Europe—A retrospective analysis of 240 cases. Acta Vet. Hung. 2020, 68, 160–168. [Google Scholar] [CrossRef]
- Sainz, Á.; Roura, X.; Miró, G.; Estrada-Peña, A.; Kohn, B.; Harrus, S.; Solano-Gallego, L. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit. Vectors 2015, 4, 75. [Google Scholar] [CrossRef]
- Diakou, A.; Di Cesare, A.; Morelli, S.; Colombo, M.; Halos, L.; Simonato, G.; Tamvakis, A.; Beugnet, F.; Paoletti, B.; Traversa, D. Endoparasites and vector-borne pathogens in dogs from Greek islands: Pathogen distribution and zoonotic implications. PLoS Negl. Trop. Dis. 2019, 13, e0007003. [Google Scholar] [CrossRef]
- Springer, A.; Glass, A.; Topp, A.K.; Strube, C. Zoonotic Tick-Borne Pathogens in Temperate and Cold Regions of Europe—A Review on the Prevalence in Domestic Animals. Front. Vet. Sci. 2020, 7, 604910. [Google Scholar] [CrossRef]
- Little, S.; Braff, J.; Place, J.; Buch, J.; Dewage, B.G.; Knupp, A.; Beall, M. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2013-2019. Parasit. Vectors 2021, 14, 10. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Mihalca, A.; Traub, R.; Lappin, M.; Baneth, G. Zoonotic parasites of sheltered and stray dogs in the era of the global economic and political crisis. Trends Parasitol. 2017, 33, 813–825. [Google Scholar] [CrossRef]
- Wright, I.; Jongejan, F.; Marcondes, M.; Peregrine, A.; Baneth, G.; Bourdeau, P.; Bowman, D.D.; Breitschwerdt, E.B.; Capelli, G.; Cardoso, L.; et al. Parasites and vector-borne diseases disseminated by rehomed dogs. Parasit. Vectors 2020, 13, 546. [Google Scholar] [CrossRef] [PubMed]
- Beugnet, F.; Chalvet-Monfray, K. Impact of climate change in the epidemiology of vector-borne diseases in domestic carnivores. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Lindsay, L.R. Effects of Climate and Climate Change on Vectors and Vector-Borne Diseases: Ticks Are Different. Trends Parasitol. 2016, 32, 646–656. [Google Scholar] [CrossRef]
- Díaz-Regañón, D.; Roura, X.; Suárez, M.L.; León, M.; Sainz, Á. Serological evaluation of selected vector-borne pathogens in owned dogs from northern Spain based on a multicenter study using a commercial test. Parasit. Vectors 2020, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, D.; Gizzarelli, M.; Ligda, P.; Foglia Manzillo, V.; Saratsi, K.; Montagnaro, S.; Schunack, B.; Boegel, A.; Pollmeier, M.; Oliva, G.; et al. Mapping the canine vector-borne disease risk in a Mediterranean area. Parasit. Vectors 2020, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Petruccelli, A.; Ferrara, G.; Iovane, G.; Schettini, R.; Ciarcia, R.; Caputo, V.; Pompameo, M.; Pagnini, U.; Montagnaro, S. Seroprevalence of Ehrlichia spp., Anaplasma spp., Borrelia burgdorferi sensu lato, and Dirofilaria immitis in Stray Dogs, from 2016 to 2019, in Southern Italy. Animals 2020, 11, 9. [Google Scholar] [CrossRef]
- Diakou, A.; Di Cesare, A.; Accettura, P.M.; Barros, L.; Iorio, R.; Paoletti, B.; Frangipane di Regalbono, A.; Halos, L.; Beugnet, F.; Traversa, D. Intestinal parasites and vector-borne pathogens in stray and free-roaming cats living in continental and insular Greece. PLoS Negl. Trop. Dis. 2017, 11, e0005335. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Crisi, P.E.; Di Cesare, A.; De Santis, F.; Barlaam, A.; Santoprete, G.; Parrinello, C.; Palermo, S.; Mancini, P.; Traversa, D. Exposure of client-owned cats to zoonotic vector-borne pathogens: Clinic-pathological alterations and infection risk analysis. Comp. Immunol. Microbiol. Infect. Dis. 2019, 66, 101344. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.; Lopes, A.P.; Martins, C.; Fino, R.; Paixão, C.; Damil, L.; Lima, C.; Alho, A.M.; Schallig, H.D.F.H.; Dubey, J.P.; et al. Survey of Dirofilaria immitis antigen and antibodies to Leishmania infantum and Toxoplasma gondii in cats from Madeira Island, Portugal. Parasit. Vectors 2020, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Otranto, D. Best Practices for Preventing Vector-Borne Diseases in Dogs and Humans. Trends Parasitol. 2016, 32, 43–55. [Google Scholar] [CrossRef]
- Lavan, R.P.; Tunceli, K.; Zhang, D.; Normile, D.; Armstrong, R. Assessment of dog owner adherence to veterinarians’ flea and tick prevention recommendations in the United States using a cross-sectional survey. Parasit. Vectors 2017, 10, 284. [Google Scholar] [CrossRef]
- Lavan, R.; Armstrong, R.; Tunceli, K.; Normile, D. Dog owner flea/tick medication purchases in the USA. Parasit. Vectors 2018, 11, 581. [Google Scholar] [CrossRef]
- Morelli, S.; Colombo, M.; Dimzas, D.; Barlaam, A.; Traversa, D.; Di Cesare, A.; Russi, I.; Spoletini, R.; Paoletti, B.; Diakou, A. Leishmania infantum Seroprevalence in Cats From Touristic Areas of Italy and Greece. Front. Vet. Sci. 2020, 7, 616566. [Google Scholar] [CrossRef]
- Morelli, S.; Diakou, A.; Di Cesare, A.; Schnyder, M.; Colombo, M.; Strube, C.; Dimzas, D.; Latino, R.; Traversa, D. Feline lungworms in Greece: Copromicroscopic, molecular and serological study. Parasitol. Res. 2020, 119, 2877–2883. [Google Scholar] [CrossRef]
- Di Cesare, A.; Morelli, S.; Colombo, M.; Simonato, G.; Veronesi, F.; Marcer, F.; Diakou, A.; D’Angelosante, R.; Pantchev, N.; Psaralexi, E.; et al. Is Angiostrongylosis a Realistic Threat for Domestic Cats? Front. Vet. Sci. 2020, 7, 195. [Google Scholar] [CrossRef]
- Panarese, R.; Iatta, R.; Latrofa, M.S.; Zatelli, A.; Ćupina, A.I.; Montarsi, F.; Pombi, M.; Mendoza-Roldan, J.A.; Beugnet, F.; Otranto, D. Hyperendemic Dirofilaria immitis infection in a sheltered dog population: An expanding threat in the Mediterranean region. Int. J. Parasitol. 2020, 50, 555–559. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Morchón, R.; Costa-Rodríguez, N.; Matos, J.I.; Falcón-Cordón, Y.; Carretón, E. Current Distribution of Selected Vector-Borne Diseases in Dogs in Spain. Front. Vet. Sci. 2020, 7, 564429. [Google Scholar] [CrossRef]
- Piantedosi, D.; Neola, B.; D’Alessio, N.; Di Prisco, F.; Santoro, M.; Pacifico, L.; Sgroi, G.; Auletta, L.; Buch, J.; Chandrashekar, R.; et al. Seroprevalence and risk factors associated with Ehrlichia canis, Anaplasma spp., Borrelia burgdorferi sensu lato, and D. immitis in hunting dogs from southern Italy. Parasitol. Res. 2017, 116, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
- Veneziano, V.; Piantedosi, D.; Ferrari, N.; Neola, B.; Santoro, M.; Pacifico, L.; Sgroi, G.; D’Alessio, N.; Panico, T.; Leutenegger, C.M.; et al. Distribution and risk factors associated with Babesia spp. infection in hunting dogs from Southern Italy. Ticks Tick Borne Dis. 2018, 9, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Gizzarelli, M.; Foglia Manzillo, V.; Ciuca, L.; Morgoglione, M.E.; El Houda Ben Fayala, N.; Cringoli, G.; Oliva, G.; Rinaldi, L.; Maurelli, M.P. Simultaneous Detection of Parasitic Vector Borne Diseases: A Robust Cross-Sectional Survey in Hunting, Stray and Sheep Dogs in a Mediterranean Area. Front. Vet. Sci. 2019, 6, 288. [Google Scholar] [CrossRef]
- Migliore, S.; Gargano, V.; De Maria, C.; Gambino, D.; Gentile, A.; Vitale Badaco, V.; Schirò, G.; Mira, F.; Galluzzo, P.; Vicari, D.; et al. A Cross Sectional Study on Serological Prevalence of Ehrlichia canis and Rickettsia conorii in Different Canine Population of Sicily (South-Italy) during 2017–2019. Animals 2020, 10, 2444. [Google Scholar] [CrossRef] [PubMed]
- Day, M. Cats are not small dogs: Is there an immunological explanation for why cats are less affected by arthropod-borne disease than dogs? Parasit. Vectors 2016, 9, 507. [Google Scholar] [CrossRef]
- Olivieri, E.; Zanzani, S.A.; Latrofa, M.S.; Lia, R.P.; Dantas-Torres, F.; Otranto, D.; Manfredi, M.T. The southernmost foci of Dermacentor reticulatus in Italy and associated Babesia canis infection in dogs. Parasit. Vectors 2016, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Di Cesare, A.; Simonato, G.; Cassini, R.; Merola, C.; Diakou, A.; Halos, L.; Beugnet, F.; Frangipane di Regalbono, A. Zoonotic intestinal parasites and vector-borne pathogens in Italian shelter and kennel dogs. Comp. Immunol. Microbiol. Infect. Dis. 2017, 51, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Gaudart, J.; Bitam, I.; Huynh, T.P.; Raoult, D.; Parola, P. Why are there so few Rickettsia conorii-infected Rhipicephalus sanguineus ticks in the wild? PLoS Negl. Trop. Dis. 2012, 6, e1697. [Google Scholar] [CrossRef] [PubMed]
- Snellgrove, A.N.; Krapiunaya, I.; Ford, S.L.; Stanley, H.M.; Wickson, A.G.; Hartzer, K.L.; Levin, M.L. Vector competence of Rhipicephalus sanguineus sensu stricto for Anaplasma platys. Ticks Tick Borne Dis. 2020, 11, 101517. [Google Scholar] [CrossRef] [PubMed]
- Maurelli, M.P.; Pepe, P.; Colombo, L.; Armstrong, R.; Battisti, E.; Morgoglione, M.E.; Counturis, D.; Rinaldi, L.; Cringoli, G.; Ferroglio, E.; et al. A national survey of Ixodidae ticks on privately owned dogs in Italy. Parasit. Vectors 2018, 11, 420. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Pfeffer, M.; Chitimia-Dobler, L.; Didyk, Y.M.; Leverenz, S.; Dautel, H.; Kahl, O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016, 7, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Dantas-Torres, F. Canine and feline vector-borne diseases in Italy: Current situation and perspectives. Parasit. Vectors 2010, 3, 2. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Trotta, M.; Carli, E.; Carcy, B.; Caldin, M.; Furlanello, T. Babesia canis canis and Babesia canis vogeli clinicopathological findings and DNA detection by means of PCR-RFLP in blood from Italian dogs suspected of tick-borne disease. Vet. Parasitol. 2008, 157, 211–221. [Google Scholar] [CrossRef]
- Yamane, I.; Thomford, J.W.; Gardner, I.A.; Dubey, J.P.; Levy, M.; Conrad, P.A. Evaluation of the indirect fluorescent antibody test for diagnosis of Babesia gibsoni infections in dogs. Am. J. Vet. Res. 1993, 54, 1579–1584. [Google Scholar]
- Ehrmann, S.; Liira, J.; Gärtner, S.; Hansen, K.; Brunet, J.; Cousins, S.A.O.; Deconchat, M.; Decocq, G.; De Frenne, P.; De Smedt, P.; et al. Environmental drivers of Ixodes ricinus abundance in forest fragments of rural European landscapes. BMC Ecol. 2017, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V. Serological Survey of Ehrlichia canis and Anaplasma phagocytophilum in Dogs from Central Italy: An Update (2013–2017). Pathogens 2019, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Sauda, F.; Malandrucco, L.; Macrì, G.; Scarpulla, M.; De Liberato, C.; Terracciano, G.; Fichi, G.; Berrilli, F.; Perrucci, S. Leishmania infantum, Dirofilaria spp. and other endoparasite infections in kennel dogs in central Italy. Parasite 2018, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Rombolà, P.; Barlozzari, G.; Carvelli, A.; Scarpulla, M.; Iacoponi, F.; Macrì, G. Seroprevalence and risk factors associated with exposure to Leishmania infantum in dogs, in an endemic Mediterranean region. PLoS ONE 2021, 16, e0244923. [Google Scholar] [CrossRef] [PubMed]
- De Massis, F.; Ippoliti, C.; Simona, I.; Tittarelli, M.; Pelini, S.; Giansante, D.; Ciarrocchi, A. Canine Leishmaniasis: Serological Results in Private and Kennel Dogs Tested over a Six-Year Period (2009–2014) in Abruzzo and Molise Regions, Italy. Microorganisms 2020, 8, 1915. [Google Scholar] [CrossRef]
- Kostalova, T.; Lestinova, T.; Maia, C.; Sumova, P.; Vlkova, M.; Willen, L.; Polanska, N.; Fiorentino, E.; Scalone, A.; Oliva, G.; et al. The recombinant protein rSP03B is a valid antigen for screening dog exposure to Phlebotomus perniciosus across foci of canine leishmaniasis. Med. Vet. Entomol. 2017, 31, 88–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morosetti, G.; Bongiorno, G.; Beran, B.; Scalone, A.; Moser, J.; Gramiccia, M.; Gradoni, L.; Maroli, M. Risk assessment for canine leishmaniasis spreading in the north of Italy. Geospat. Health 2009, 4, 115–127. [Google Scholar] [CrossRef]
- Signorini, M.; Cassini, R.; Drigo, M.; Frangipane di Regalbono, A.; Pietrobelli, M.; Montarsi, F.; Stensgaard, A.S. Ecological niche model of Phlebotomus perniciosus, the main vector of canine leishmaniasis in north-eastern Italy. Geospat. Health 2014, 9, 193–201. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.; Benelli, G.; Panarese, R.; Iatta, R.; Furlanello, T.; Beugnet, F.; Zatelli, A.; Otranto, D. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009-2019: Changing distribution patterns. Parasit. Vectors 2020, 13, 193. [Google Scholar] [CrossRef]
- Michelutti, A.; Toniolo, F.; Bertola, M.; Grillini, M.; Simonato, G.; Ravagnan, S.; Montarsi, F. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasit. Vectors 2021, 14, 164. [Google Scholar] [CrossRef]
- Santoro, M.; Miletti, G.; Vangone, L.; Spadari, L.; Reccia, S.; Fusco, G. Heartworm disease (Dirofilaria immitis) in two roaming dogs from the urban area of Castel Volturno, Southern Italy. Front. Vet. Sci. 2019, 6, 270. [Google Scholar] [CrossRef]
- Piergili-Fioretti, D.; Diaferia, M.; Grelloni, V.; Maresca, C. Canine filariosis in Umbria: An update of the occurrence one year after the first observation of autochthonous foci. Parassitologia 2003, 45, 79–83. [Google Scholar]
- Macchioni, F.; Sed, G.; Cecchi, F. Canine filarial infections in an area of Central Italy (Tuscany-Latium border) historically free from the disease. Vet. Parasitol. Reg. Stud. Rep. 2020, 20, 100404. [Google Scholar] [CrossRef]
- Traversa, D.; Aste, G.; Milillo, P.; Capelli, G.; Pampurini, F.; Tunesi, C.; Santori, D.; Paoletti, B.; Boari, A. Autochthonous foci of canine and feline infections by Dirofilaria immitis and Dirofilaria repens in central Italy. Vet. Parasitol. 2010, 169, 128–132. [Google Scholar] [CrossRef]
- Baneth, G.; Thamsborg, S.M.; Otranto, D.; Guillot, J.; Blaga, R.; Deplazes, P.; Solano-Gallego, L. Major Parasitic Zoonoses Associated with Dogs and Cats in Europe. J. Comp. Pathol. 2016, 155, S54–S74. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D. Arthropod-borne pathogens of dogs and cats: From pathways and times of transmission to disease control. Vet. Parasitol. 2018, 251, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Elsheikha, E. Flea and tick control: Innovative approaches to owner compliance. Vet. Times 2016, 46, 6–11. [Google Scholar]
- Elsheikha, H. Ectoparasites: Preventive plans and innovations in treatment. Vet. Times 2017, 47, 6–12. [Google Scholar]
- Jin, H.; Wei, F.; Liu, Q.; Qian, J. Epidemiology and control of human granulocytic anaplasmosis: A systematic review. Vector Borne Zoonotic Dis. 2012, 12, 269–274. [Google Scholar] [CrossRef]
- Portillo, A.; Santibáñez, S.; García-Álvarez, L.; Palomar, A.M.; Oteo, J.A. Rickettsioses in Europe. Microbes Infect. 2015, 17, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Littman, M.P.; Gerber, B.; Goldstein, R.E.; Labato, M.A.; Lappin, M.R.; Moore, G.E. ACVIM consensus update on Lyme borreliosis in dogs and cats. J. Vet. Intern. Med. 2018, 32, 887–903. [Google Scholar] [CrossRef]
- Miró, G.; López-Vélez, R. Clinical management of canine leishmaniosis versus human leishmaniasis due to Leishmania infantum: Putting “One Health” principles into practice. Vet. Parasitol. 2018, 254, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Xhaxhiu, D.; Kusi, I.; Rapti, D.; Kondi, E.; Postoli, R.; Rinaldi, L.; Dimitrova, Z.M.; Visser, M.; Knaus, M.; Rehbein, S. Principal intestinal parasites of dogs in Tirana, Albania. Parasitol. Res. 2011, 108, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Dado, D.; Izquierdo, F.; Vera, O.; Montoya, A.; Mateo, M.; Fenoy, S.; Galván, A.L.; García, S.; García, A.; Aránguez, E.; et al. Detection of zoonotic intestinal parasites in public parks of Spain. Potential epidemiological role of microsporidia. Zoonoses Public Health 2012, 59, 23–28. [Google Scholar] [CrossRef]
- Otero, D.; Alho, A.M.; Nijsse, R.; Roelfsema, J.; Overgaauw, P.; de Carvalho, L.M. Environmental contamination with Toxocara spp. eggs in public parks and playground sandpits of Greater Lisbon, Portugal. J. Infect. Public Health 2018, 11, 94–98. [Google Scholar] [CrossRef]
- Simonato, G.; Cassini, R.; Morelli, S.; Di Cesare, A.; La Torre, F.; Marcer, F.; Traversa, D.; Pietrobelli, M.; Frangipane di Regalbono, A. Contamination of Italian parks with canine helminth eggs and health risk perception of the public. Prev. Vet. Med. 2019, 172, 104788. [Google Scholar] [CrossRef] [PubMed]
| SNAP 4DX | IFAT | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Site | An n/tot (%) | Eh n/tot (%) | Bb n/tot (%) | Di n/tot (%) | Li n/tot (%) | Rc n/tot (%) | Bc n/tot (%) | Mixed n/tot ** (%) | Total n/tot * (%) |
| A | 7/33 (21.2) | - - | 3/33 (9.1) | - - | 1/33 (3.0) | 19/33 (57.6) | - - | 7/33 (21.2) | 22/33 (66.7) |
| B | 1/34 (2.9) | - - | 1/34 (2.9) | 2/34 (5.9) | - - | 22/34 (64.7) | - - | 3/34 (8.8) | 23/34 (67.6) |
| C | 1/45 (2.2) | - - | - - | 1/45 (2.2) | 3/45 (6.7) | 5/45 (11.1) | 2/45 (4.4) | 2/45 (4.4) | 10/45 (22.2) |
| D | 3/42 (7.1) | - - | - - | 1/42 (2.4) | 1/42 (2.4) | 13/42 (31.0) | - - | 1/42 (2.4) | 17/42 (40.5) |
| E | 4/48 (8.3) | 1/48 (2.1) | - - | - - | 4/48 (8.3) | 13/48 (27.1) | 5/48 (10.4) | 4/48 (8.3) | 21/48 (43.8) |
| F | 6/40 (15.0) | - - | - - | - - | 2/40 (5.0) | 26/40 (65.0) | 18/40 (45.0) | 17/40 (42.5) | 31/40 (77.5) |
| Total n/tot (%) | 22/242 (9.1) | 1/242 (0.4) | 4/242 (1.7) | 4/242 (1.7) | 11/242 (4.5) | 98/242 (40.5) | 25/242 (10.3) | 34/242 (14.0) | 124/242 (51.2) |
| SNAP 4DX | IFAT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ectoparasite infestations | n/tot (%) | An | Eh | Bb | Di | Li | Rc | Bc | Mixed | Total |
| n/tot (%) | n/tot (%) | n/tot (%) | n/tot (%) | n/tot (%) | n/tot (%) | n/tot (%) | n/tot ** (%) | n/tot * (%) | ||
| Flea (current) | 30 (12.4%) | 2/30 | - | - | - | 2/30 | 13/30 | 8/30 | 8/30 | 16/30 |
| (6.7) | - | - | - | (6.7) | (43.3) | (26.7) | (26.7) | (53.3) | ||
| Flea (past) | 73 (30.2%) | 11/73 | - | - | 1/73 | 5/73 | 33/73 | 16/73 | 18/73 | 40/73 |
| (15.0) | - | - | (1.3) | (6.8) | (45.2) | (21.9) | (24.6) | (54.8) | ||
| Ticks (current) | 3 (1.2%) | - | - | - | - | - | 3/3 | 1/3 | 1/3 | 3/3 |
| - | - | - | - | - | (100) | (33.3) | (33.30 | (100) | ||
| Ticks (past) | 128 (52.9%) | 18/128 | - | 3/128 | 4/128 | 4/128 | 68/128 | 16/128 | 29/128 | 79/128 |
| (14.1) | - | (2.3) | (3.1) | (3.1) | (53.1) | (12.5) | (22.7) | (61.7) | ||
| SNAP 4DX | IFAT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Formulations n (%) | Treatment Schedule | n/tot (%) | An n/tot (%) | Eh n/tot (%) | Bb n/tot (%) | Di n/tot (%) | Li n/tot (%) | Rc n/tot (%) | Bc n/tot (%) | Total n/tot * (%) |
| Pyrethroids 120 (49.6) | Throughout the year | 46/120 (38.3) | 1/46 (2.8) | - | - | - | 3/46 (6.5) | 10/46 (21.7) | 4/46 (8.7) | 16/46 (34.8) |
| From spring to autumn | 44/120 (36.6) | 1/44 (2.3) | - | - | 1/44 (2.3) | 1/44 (2.3) | 13/44 (29.5) | 3/44 (6.8) | 19/44 (43.2) | |
| Only in summer | 13/120 (10.8) | 1/13 (7.7) | - | 1/13 (7.7) | - | - | 4/13 (30.8) | - | 6/13 (46.2) | |
| Irregular | 17/120 (14.2) | 2/17 (11.8) | - | 2/17 (11.8) | 1/17 (5.9) | 1/17 (5.9) | 7/17 (41.2) | - | 11/17 (64.7) | |
| Isoxazolines 36 (14.9) | Throughout the year | 15/36 (41.7) | 4/15 (26.7) | 1/15 (6.6) | - | - | 2/15 (13.3) | 8/15 (53.3) | - | 10/15 (66.7) |
| From spring to autumn | 2/36 (5.6) | - | - | - | - | - | 1/2 (50.0) | - | 1/2 (50.0) | |
| Only in summer | 1/36 (2.8) | - | - | - | - | - | - | - | - | |
| Irregular | 18/36 (50) | 6/18 (33.3) | - | - | 1/18 (5.5) | - | 11/18 (61.1) | - | 11/18 (61.1) | |
| Fenilpirazoles 27 (11.1) | From spring to autumn | 2/27 (7.4) | - | - | - | - | - | 1/2 (50.0) | - | 1/2 (50.0) |
| Only in summer | 5/27 (18.5) | 1/5 (20.0) | - | - | - | - | 3/5 (60.0) | - | 3/5 (60.0) | |
| Irregular | 20/27 (74.0) | - | - | - | - | 1/20 (5.0) | 8/20 (40.0) | 2/20 (10.0) | 11/20 (55.0) | |
| Organophosphorus insecticides 42 (17.3) | Irregular | 42/42 (100) | 6/42 (14.3) | - | - | - | 2/42 (4.8) | 28/42 (66.7) | 15/42 (35.7) | 30/42 (71.4) |
| Unknown product 17 (7) | Irregular | 17/17 (100) | - | - | - | 1/17 (5.9) | - | 4/17 (23.5) | - | 5/17 (29.4) |
| Fisher’s Exact Test | |||
|---|---|---|---|
| p Value | OR * | 95% CI * | |
| At least one VBP | |||
| Living in Northern Italy | 0.002 | 2.486 | 1.356–4.445 |
| Outdoor lifestyle | 0.022 | 1.870 | 1.079–3.194 |
| At least one TBP | |||
| Inadequate tick prevention | <0.001 | 1.939 | 1.171–3.267 |
| Previous tick infestation | 0.014 | 2.793 | 1.661–4.662 |
| Rickettsia conorii | |||
| Living in Northern Italy | <0.001 | 3. 265 | 1.783–5.746 |
| Outdoor lifestyle | <0.001 | 2.720 | 1.531–4.767 |
| Previous tick infestation | <0.001 | 3.173 | 1.826–5.334 |
| Anaplasma spp. | |||
| Previous tick infestation | 0.006 | 4.500 | 1.472–12.55 |
| Previous flea infestation | 0.049 | 2. 548 | 1.081–5.979 |
| Babesia canis | |||
| Current flea infestation | 0.005 | 4.171 | 1.612–10.04 |
| Previous flea infestation | <0.001 | 4.990 | 2.144–11.85 |
| Multivariate Logistic Regression | |||
| At least one VBP | |||
| Random/irregular treatment | 0.003 | 3.673 | 1.077–12.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, M.; Morelli, S.; Simonato, G.; Di Cesare, A.; Veronesi, F.; Frangipane di Regalbono, A.; Grassi, L.; Russi, I.; Tiscar, P.G.; Morganti, G.; et al. Exposure to Major Vector-Borne Diseases in Dogs Subjected to Different Preventative Regimens in Endemic Areas of Italy. Pathogens 2021, 10, 507. https://doi.org/10.3390/pathogens10050507
Colombo M, Morelli S, Simonato G, Di Cesare A, Veronesi F, Frangipane di Regalbono A, Grassi L, Russi I, Tiscar PG, Morganti G, et al. Exposure to Major Vector-Borne Diseases in Dogs Subjected to Different Preventative Regimens in Endemic Areas of Italy. Pathogens. 2021; 10(5):507. https://doi.org/10.3390/pathogens10050507
Chicago/Turabian StyleColombo, Mariasole, Simone Morelli, Giulia Simonato, Angela Di Cesare, Fabrizia Veronesi, Antonio Frangipane di Regalbono, Laura Grassi, Ilaria Russi, Pietro Giorgio Tiscar, Giulia Morganti, and et al. 2021. "Exposure to Major Vector-Borne Diseases in Dogs Subjected to Different Preventative Regimens in Endemic Areas of Italy" Pathogens 10, no. 5: 507. https://doi.org/10.3390/pathogens10050507
APA StyleColombo, M., Morelli, S., Simonato, G., Di Cesare, A., Veronesi, F., Frangipane di Regalbono, A., Grassi, L., Russi, I., Tiscar, P. G., Morganti, G., Hattab, J., Rizzo, V., & Traversa, D. (2021). Exposure to Major Vector-Borne Diseases in Dogs Subjected to Different Preventative Regimens in Endemic Areas of Italy. Pathogens, 10(5), 507. https://doi.org/10.3390/pathogens10050507













