Skin Lesions in Feline Leishmaniosis: A Systematic Review
Abstract
1. Introduction
2. Results
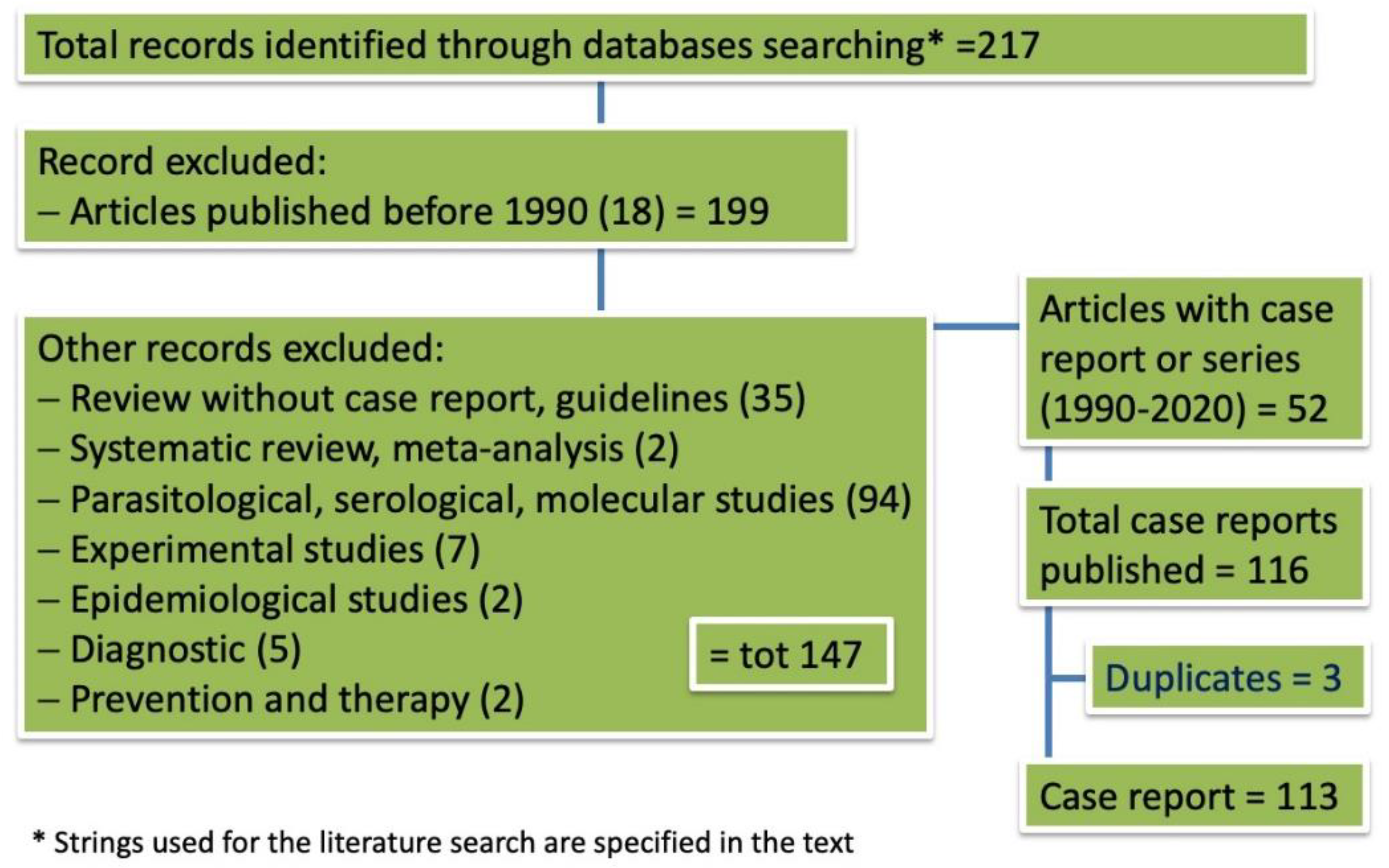
2.1. Outcome of Case Selection
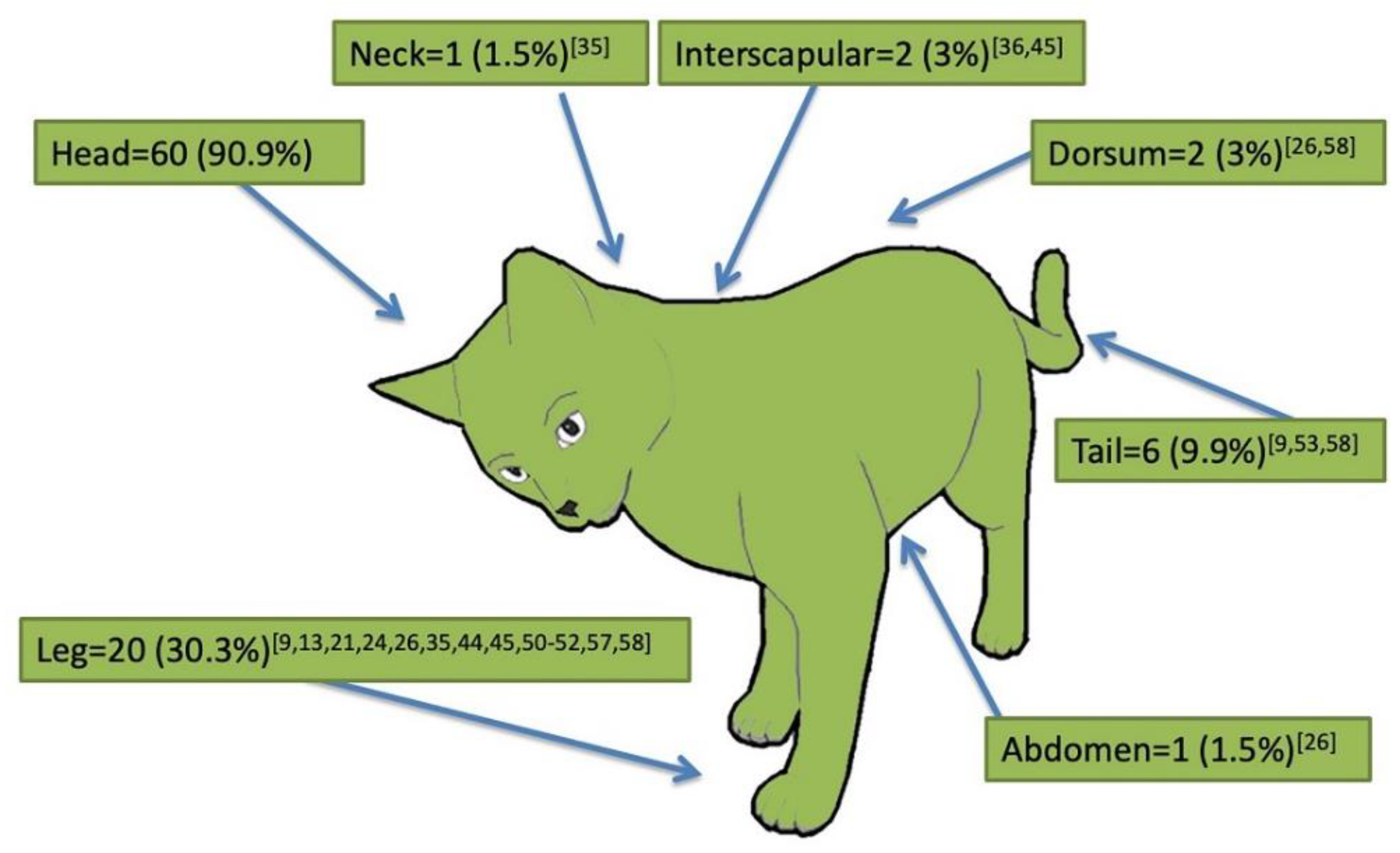
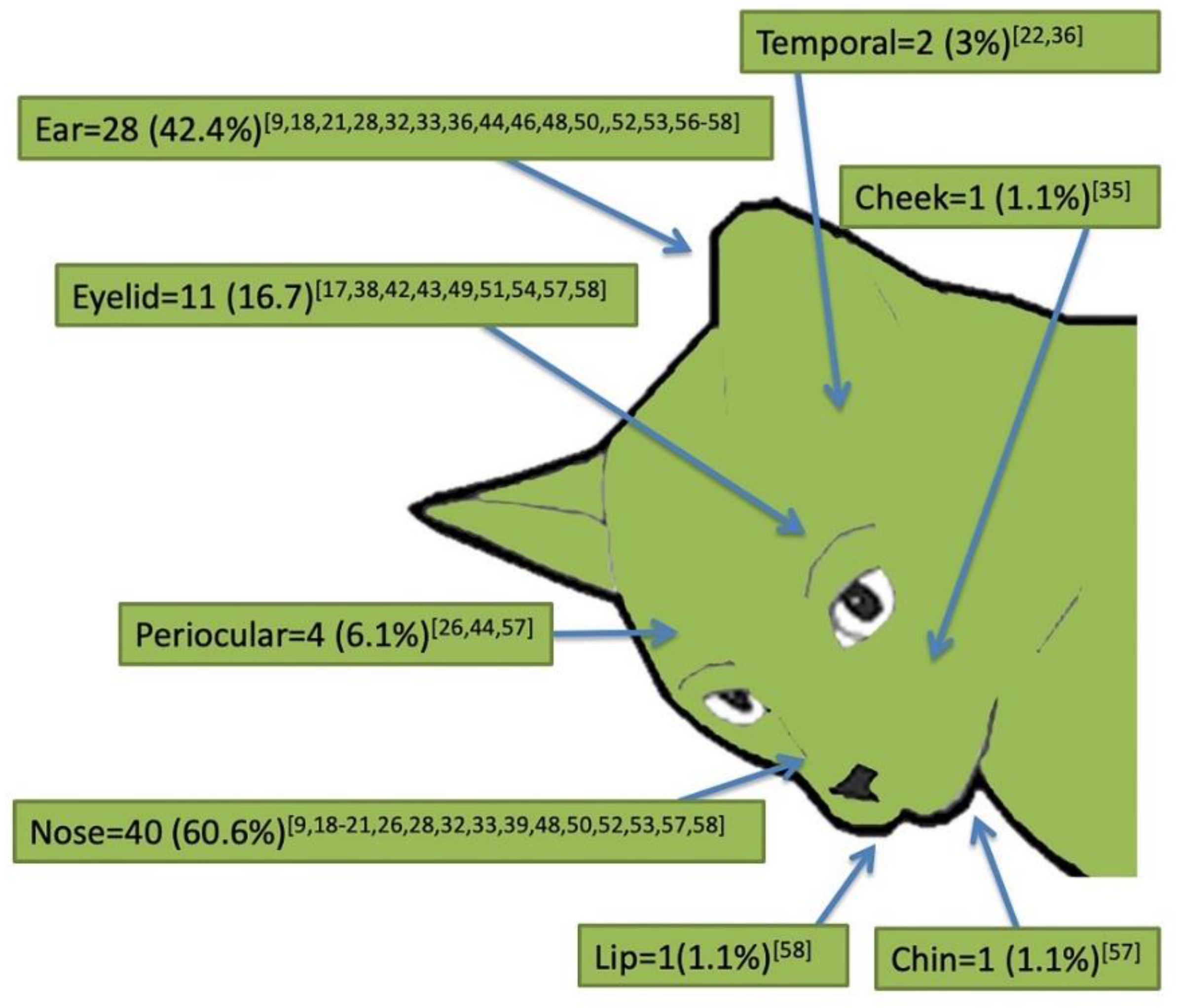
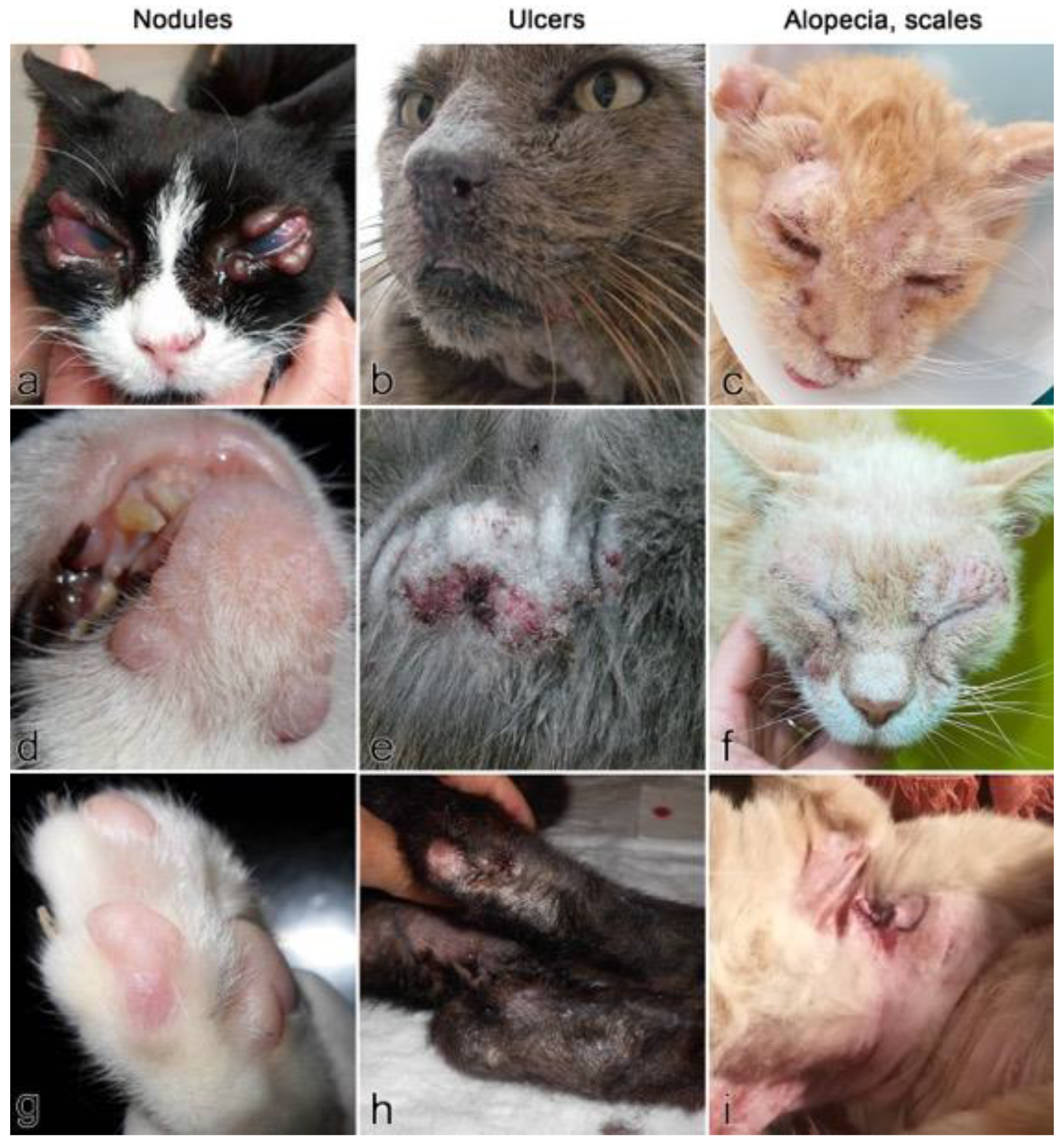
2.2. Signalment and Clinical Presentation of Cutaneous Feline Leishmaniosis (66 Case Reports)
2.3. Unpublished Italian Cases
3. Discussion
4. Materials and Methods
4.1. Searching Strategy
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gramiccia, M. Recent advances in leishmaniosis in pet animals: Epidemiology, diagnostics and anti-vectorial prophylaxis. Vet. Parasitol. 2011, 181, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Koutinas, A.; Mirò, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bordeau, P.; Oliva, G.; Baneth, G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 2009, 165, 1–18. [Google Scholar] [CrossRef]
- Roque, A.L.R.; Jansen, A.M. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int. J. Parasitol. Parasites Wildl. 2014, 3, 251–262. [Google Scholar] [CrossRef]
- Alemayehu, B.; Alemayehu, M. Leishmaniasis: A Review on Parasite, Vector and Reservoir Host. Health Sci. J. 2017, 11, 519. [Google Scholar] [CrossRef]
- Kirkpatrick, C.E.; Farrell, J.P.; Goldschmidt, M.H. Leishmania chagasi and L. donovani: Experimental Infections in Domestic Cats. Exp. Parasitol. 1984, 58, 125–131. [Google Scholar] [CrossRef]
- Sergent, E.; Sergent, E.; Lombard, J.; Quilichini, M. La leishmaniose à Alger. Infection simultanée d’un enfant, d’un chien et d’un chat dans la même habitation. Bull. Soc. Pathol. Exot. 1912, 5, 93–98. [Google Scholar]
- Ondovilla, A.G. Un chat infecté par Leishmania en Espagne. Trabajos 1933, 2, 26–27. [Google Scholar]
- Dunan, N.; Mary, C.; Garbe, L.; Breton, Y. A propos d’un cas de leishmaniosechez un chat de la région marseillaise. Bull. Soc. Fr. Parasitol. 1989, 7, 17–20. [Google Scholar]
- Bonfante-Garrido, R.I.; Urdaneta, I.; Urdaneta, R.; Alvarado, J. Natural infection of cats with Leishmania in Barquisimeto, Venezuela. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 53. [Google Scholar] [CrossRef][Green Version]
- Barnes, J.C.; Stanley, O.; Craig, T.M. Diffuse cutaneous leishmaniasis in a cat. J. Am. Vet. Med. Assoc. 1993, 202, 416–418. [Google Scholar]
- Morsy, T.A.; Abou el Seoud, S.M. Natural infection in two pet cats in a house of a zoonotic cutaneous leishmaniasis patient in Imbaba area, Giza Governorate, Egypt. J. Egypt Soc. Parasitol. 1994, 24, 199–204. [Google Scholar]
- Laruelle-Magalon, C.; Toga, I. A case of feline Leishmaniasis. Prat. Med. Chir. An. Comp. 1996, 31, 255–261. [Google Scholar]
- Passos, V.M.A.; Lasmar, E.B.; Gontijo, C.M.F.; Fernandes, O.; Degrave, W. Natural infection of a domestic cat (Felis domesticus) with Leishmania (Viannia) in the metropolitan region of Belo Horizonte, state of Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz 1996, 91. [Google Scholar] [CrossRef]
- Ozon, C.; Marty, P.; Pratlong, F.; Breton, C.; Blein, M.; Lelie`vre, A.; Haas, P. Disseminated feline leishmaniosis due to Leishmania infantum in Southern France. Vet. Parasitol. 1998, 75, 273–277. [Google Scholar] [CrossRef]
- Hervás, J.; De Lara, F.C.M.; Sánchez-Isarria, M.A.; Pellicer, S.; Carrasco, L.; Castillo, J.A.; Gómez-Villamandos, J.C. Two cases of feline visceral and cutaneous leishmaniosis in Spain. J. Fel. Med. Surg. 1999, 1, 101–105. [Google Scholar] [CrossRef]
- Hervás, J.; de Lara, F.C.M.; López, J.; Gómez-Villamandos, J.C.; Guerrero, M.J.; Moreno, A. Granulomatous (pseudotumoral) iridociclitis associated with leishmaniasis in a cat. Vet. Rec. 2001, 149, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Abramo, F.; Barsotti, P.; Leva, S.; Gramiccia, M.; Ludovisi, A.; Mancianti, F. Feline leishmaniosis due to Leishmania infantum in Italy. Vet. Parasitol. 2002, 106, 181–191. [Google Scholar] [CrossRef]
- Pennisi, M.G.; Venza, M.; Reale, S.; Vitale, F.; Lo Giudice, S. Case Report of Leishmaniasis in Four Cats. Vet. Res. Com. 2004, 28, 363–366. [Google Scholar] [CrossRef]
- Savani, E.S.M.M.; de Oliveira Camargo, M.C.G.; de Carvalho, M.R.; Zampieri, R.A.; dos Santos, M.G.; Nicoletti D’Áuria, S.R.; Shaw, J.J.; Floeter-Winter, L.M. The first record in the Americas of an autochthonous case of Leishmania (Leishmania) infantum chagasi in a domestic cat (Felix catus) from Cotia County, São Paulo State, Brazil. Vet. Parasitol. 2004, 120, 229–233. [Google Scholar] [CrossRef]
- Schubach, T.M.P.; Figueiredo, F.B.; Pereira, S.A.; Madeira, M.F.; Santos, I.B.; Andrade, M.V.; Cuzzi, T.; Marzochi, M.C.A. American cutaneous leishmaniasis in two cats from Rio de Janeiro, Brazil: First report of natural infection with Leishmania (Viannia) braziliensis. Trans. Royal Soc. Trop. Med. Hyg. 2004, 98, 165–167. [Google Scholar] [CrossRef]
- De Souza, A.I.; Ilha, I.M.N.; Marin, G.R.B.; Nunes, V.L.B. Feline leishmaniasis due to Leisgmania (Leishmania) amazonensis in Mato Grosso do Sul State, Brazil. Vet. Parasitol. 2005, 128, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Grevot, A.; Hugues, J.P.; Marty, P.; Pratlong, F.; Ozon, C.; Haas, P.; Breton, C.; Bourdoiseau, G. Leishmaniosis due to Leishmania infantum in a FIV and FeLV positive cat with a squamous cell carcinoma diagnosed with histological, serological and isoenzymatic methods. Parasite 2005, 12, 271–275. [Google Scholar] [CrossRef]
- Leiva, M.; Lloret, A.; Peña, T.; Roura, X. Therapy of ocular and visceral leishmaniasis in a ca. Vet. Ophthalmol. 2005, 8, 71–75. [Google Scholar] [CrossRef]
- Rüfenacht, S.; Sager, H.; Müller, N.; Schaerer, V.; Heier, A.; Welle, M.M.; Roosje, P.J. Two cases of feline leishmaniosis in Switzerland. Vet. Rec. 2005, 156, 542–545. [Google Scholar] [CrossRef]
- Maroli, M.; Pennisi, M.G.; Di Muccio, T.; Khoury, C.; Gradoni, L.; Gramiccia, M. Infection of sandflies by a cat naturally infected with Leishmania infantum. Vet. Parasitol. 2007, 145, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, A.; Ossò, M.; Oliva, A.; Anglada, L.; Sarobè, X.; Vives, E. Leishmaniosis felina a propósito de un caso clínico. ¿Nos olvidamos de que existe? Clin. Vet. Peq. Anim. 2008, 28, 233–237. [Google Scholar]
- Marcos, R.; Santos, M.; Malhão, F.; Pereira, R.; Femandes, A.C.; Montenegro, L.; Roccabianca, P. Pancytopenia in a cat with visceral leishmaniosis. Vet. Clin. Pathol. 2009, 38, 201–205. [Google Scholar] [CrossRef]
- Souza, A.I.; Nunes, V.L.B.; Borralho, V.M.; Ishikawa, E.A.Y. Domestic feline cutaneous leishmaniasis in the municipality of Ribas do Rio Pardo, Mato Grosso do Sul State, Brazil: Acase report. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 359–365. [Google Scholar] [CrossRef][Green Version]
- Coelho, W.M.D.; de Lima, V.M.F.; Talamini do Amarante, A.F.; Langoni, H.; Richini Pereira, V.B.; Abdelnour, A.; Bresciani, K.D.S. Occurrence of Leishmania (Leishmania) chagasi in a domestic cat (Felis catus) in Andradina, São Paulo, Brazil: Case report. Rev. Bras. Parasitol. Vet. Jaboticabal 2010, 19, 256–258. [Google Scholar] [CrossRef]
- Da Silva, S.M.; Rabelo, P.F.; Gontijo, N.F.; Ribeiro, R.R.; Melo, M.N.; Ribeiro, V.M.; Michalick, M.S.M. First report of infection of Lutzomyia longipalpis by Leishmania (Leishmania) infantum from a naturally infected cat of Brazil. Vet. Parasitol. 2010, 174, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Hatam, G.R.; Adnani, S.J.; Asgari, Q.; Fallah, E.; Motazedian, M.H.; Sadjjadi, S.M.; Sarkari, B. First Report of Natural Infection in Cats with Leishmania infantum in Iran. Vector-Borne Zoonotic Dis. 2010, 10. [Google Scholar] [CrossRef]
- Trainor, K.E.; Porter, B.F.; Logan, K.S.; Hoffman, R.J.; Snowden, K.F. Eight cases of feline cutaneous leishmaniasis in Texas. Vet. Parasitol. 2010, 47, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Rougeron, V.; Catzeflis, F.; Hide, M.; De Meeûs, T.; Banuls, A.L. First clinical case of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis in a domestic cat from French Guiana. Vet. Parasitol. 2011, 181, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Sanches, A.; Pereira, A.G.; Carvalho, J.P. Um caso de leishmaniose felina. Vet. Med. 2011, 63, 29–30. [Google Scholar]
- Ennas, F.; Calderone, S.; Caprì, A.; Pennisi, M.G. Un caso di Leishmaniosi felina in Sardegna. Veterinaria 2012, 2, 55–59. [Google Scholar]
- Pocholle, E.; Reyes-Gomez, E.; Giacomo, A.; Delaunay, P.; Hasseine, L.; Marty, P. Un cas de leishmaniose féline disseminé edans le sud de la France. Parasite 2012, 19, 77–80. [Google Scholar] [CrossRef]
- Verneuil, M. Leishmaniose oculaire féline: À proposd’un cas. J. Fr. Ophtalmol. 2013, 36, e67–e72. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Schaarschmidt-Kiener, D.; Krudewig, C. Ocular signs, diagnosis and long-term treatment with allopurinol in a cat with leishmaniasis. Schweiz. Arch. Tierheilkd. 2014, 156, 289–294. [Google Scholar] [CrossRef]
- Maia, C.; Sousa, C.; Ramos, C.; Cristóvão, J.M.; Faísca, P.; Campino, L. First case of feline leishmaniosis caused by Leishmania infantum genotype E in a cat with a concurrent nasal squamous cell carcinoma. JFMS Open Rep. 2015, 1. [Google Scholar] [CrossRef]
- Migliazzo, A.; Vitale, F.; Calderone, S.; Puleio, R.; Binanti, D.; Abramo, F. Feline leishmaniosis: A case with a high parasitic burden. Vet. Dermatol. 2015, 26, 69–70. [Google Scholar] [CrossRef]
- Noè, P.; Domingos, S.L.; Oshiro, E.T.; Lima, R.B.; Pirmez, C.; Pedroso, T.C.; Babo-Terra, V.J. Detection of Leishmania chagasi in cats (Felis catus) from visceral leishmaniasis endemic area in Brazil. Ciência An. 2015, 25, 3–14. [Google Scholar]
- Pimenta, P.; Alves-Pimenta, S.; Barros, J.; Barbosa, P.; Rodrigues, A.; Pereira, M.J.; Maltez, L.; Gama, A.; Cristóvão, J.M.; Campino, L.; et al. Feline leishmaniosis in Portugal: 3 cases (year 2014). Vet. Parasitol. Reg. Stud. Rep. 2015, 1, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.M.; Ramírez, N.N.; Alegre, A.E.; Bastiani, C.E.; De Biasio, M.B. Detección de Leishmania (Viannia) braziliensis en gato doméstico de Corrientes, Argentina, por técnicas de biología molecular. Rev. Vet. 2015, 26, 147–150. [Google Scholar] [CrossRef]
- Basso, M.A.; Marques, C.; Santos, M.; Duarte, A.; Pissarra, H.; Carreira, L.M.; Gomes, L.; Valério-Bolas, A.; Tavares, L.; Santos-Gomes, G.; et al. Successful treatment of feline leishmaniosis using a combination of allopurinol and N-methyl-glucamine antimoniate. JFMS Open Rep. 2016, 2. [Google Scholar] [CrossRef]
- Attipa, C.; Papasouliotis, K.; Solano-Gallego, L.; Baneth, G.; Nachum-Biala, Y.; Savani, E.; Knowles, T.G.; Mengi, S.; Morris, D.; Helps, C.; et al. Follow-up monitoring in a cat with leishmaniosis and coinfection with Hepatozoon felis and “candidatus mycoplasma haemominutum”. JFMS Open Rep. 2017, 1, 6. [Google Scholar] [CrossRef]
- Minard, H.M.; Daniel, A.K.; Pool, R.R.; Snowden, K.F.; Levine, G.J. Pathology in Practice. JAVMA 2017, 251, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Altuzarra, R.; Movilla, R.; Roura, X.; Espada, Y.; Majo, N.; Novellas, R. Computed tomographic features of destructive granulomatous rhinitis with intracranial extension secondary to leishmaniasis in a cat. Vet. Radiol. Ultrasound 2018. [Google Scholar] [CrossRef] [PubMed]
- Arenales, A.; Eckstein, C.; Azevedo, J.; Reginaldo, G.M.S.; Lima, V.M.F.; Rozza, D.B.; Santos, R.L. Granulomatous rhinitis in a case of feline leishmaniasis. Braz. J. Vet. Pathol. 2018, 11, 7–11. [Google Scholar] [CrossRef]
- Leal, R.O.; Pereira, H.; Cartaxeiro, C.; Delgado, E.; Peleteiro, M.D.C.; da Fonseca, I.P. Granulomatous rhinitis secondary to feline leishmaniosis: Report of an unusual presentation and therapeutic complications. JFMS Open Rep. [CrossRef]
- Rivas, A.K.; Alcover, M.; Martinez-Orellana, P.; Montserrat-Sangrà, S.; Nachum-Biala, Y.; Bardagi, M.; Fisa, R.; Riera, C.; Baneth, G.; Solano-Gallego, L. Clinical and diagnostic aspects of feline cutaneous leishmaniosis in Venezuela. Parasit Vectors 2018, 11, 141. [Google Scholar] [CrossRef]
- Brianti, E.; Celi, N.; Napoli, E.; Abbate, J.M.; Arfuso, A.; Gaglio, G.; Iatta, R.; Giannetto, S.; Gramiccia, M.; Otranto, D. Treatment and long term follow up of a cat with leishmaniosis. Parasit Vectors 2019, 12, 121. [Google Scholar] [CrossRef]
- Headley, S.A.; Pimentel, L.A.; de Amorim, I.F.G.; Amude, A.M.; Viana, N.E.; Muraro, L.S.; Tafuri, W.L.; dos Santos, M.D. Immunohistochemical characterization of cutaneous leishmaniasis in cats from Central-west Brazil. Vet. Parasitol. Reg. Stud. Rep. 2019, 17, 100290. [Google Scholar] [CrossRef] [PubMed]
- Paniz Mondolfi, A.E.; Colmenares Garmendiac, A.; Mendoza Pérez, Y.M.; Hernández-Pereira, C.E.; Medina, C.; Vargas, F.; Sandoval, D.; Agüero, J.; Román, D.; Forlano-Riera, M.; et al. Autochthonous cutaneous leishmaniasis in urban domestic animals (Felis catus/Canis lupus familiaris) from central-western Venezuela. Acta Trop. 2019, 191, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Valente, J.; Parreira, R.; Cristovao, J.M.; Azinheira, S.; Campino, L.; Maia, C. An Unusual Case of Feline Leishmaniosis with Involvement of the Mammary Glands. Top. Companion An. Med. 2019, 37, 100356. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, L.K.A.R.; de Andrade Gomes, C.F.C.; de Oliveira Nascimento, J.; Bernardi, J.C.M.; Santana Lima, V.F.; de Oliveira, J.B.; do Nascimento Ramos, C.A.; Nascimento Ramos, R.A.; Alves, L.C. Leishmania infantum Infection in a Domestic Cat: A Real Treat or an Occasional Finding? Acta Parasitol. 2020. [Google Scholar] [CrossRef]
- Carneiro, L.A.; dos Santos, T.V.; do Rêgo Lima, L.V.; Ramos, P.K.S.; Campos, M.B.; Silveira, F.T. First report on feline leishmaniasis caused by Leishmania (Leishmania) amazonensis in Amazonian Brazil. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100360. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gallego, A.; Feo Bernabe, L.F.; Dalmau, A.; Esteban-Saltiveri, D.; Font, A.; Leiva, M.; Ortuñez-Navarro, A.; Peña, M.T.; Tabar, M.D.; Real-Sampietro, L.; et al. Feline leishmaniosis: Diagnosis, treatment and outcome in 16 cats. JFMS 2020, 22, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.B.S.; Portela, R.A.; Arruda, L.F.B.; Ferreira, J.S.; Souto, E.P.F.; Araújo, A.L.; Madeira, M.D.F.; Dantas, A.F.M.; de Melo, M.A. Natural Infection by Leishmania infantum in domestic cats (Felis catus) in a municipality of moderate transmission in the Brazilian semi-arid region. Braz. J. Vet. Parasitol. 2020, 29, e016620. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Gomes, J.; Cristóvão, J.; Nunes, M.; Martins, A.; Rebêlo, E.; Campino, L. Feline Leishmania infection in a canine leishmaniasis endemic region, Portugal. Vet. Parasitol. 2010, 174, 336–340. [Google Scholar] [CrossRef]
- Abbate, J.M.; Maia, C.; Pereira, A.; Arfuso, F.; Gaglio, G.; Rizzo, M.; Caracappa, G.; Marino, G.; Pollmeier, M.; Giannetto, S.; et al. Identification of trypanosomatids and blood feeding preferences of phlebotomine sand fly species common in Sicily, Southern Italy. PLoS ONE 2020, 15, e0229536. [Google Scholar] [CrossRef]
- Otranto, D.; Napoli, E.; Latrofa, M.S.; Annoscia, G.; Tarallo, V.D.; Greco, G.; Lorusso, E.; Gulotta, L.; Falsone, L.; Solari Basano, F.S.; et al. Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian Islands: Pathogen and vector circulation in a confined environment. Vet. Parasitol. 2017, 236, 144–151. [Google Scholar] [CrossRef]
- Iatta, R.; Furlanello, T.; Colella, V.; Tarallo, V.D.; Latrofa, M.S.; Brianti, E.; Trerotoli, P.; Decaro, N.; Lorusso, E.; Schunack, B.; et al. A nationwide survey of Leishmania infantum infection in cats and associated risk factors in Italy. PLoS Negl. Trop. Dis. 2019, 15, e0007594. [Google Scholar] [CrossRef]
- Pennisi, M.G. Leishmaniosis of companion animals in Europe: An update. Vet. Parasitol. 2015, 208, 35–47. [Google Scholar] [CrossRef]
- Mancianti, F. Feline leishmaniasis: What’s the epidemiological role of the cat? Rev. Parassitol. 2004, 46, 203–206. [Google Scholar]
- Pennisi, M.G.; Hartmann, K.; Lloret, A.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hosie, M.J.; et al. Leishmaniosis in cats: ABCD guidelines on prevention and management. JFMS 2013, 15, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.S.A.; Duarte, S.C.; Sousa, S.R. What do we know about feline leishmaniosis? JFMS 2015, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Cardoso, L.; Baneth, G.; Bourdeau, P.; Koutinas, A.; Miró, G.; Oliva, G.; Solano-Gallego, L. LeishVet update and recommendations on feline leishmaniosis. Parasit Vectors 2015, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G.; Persichetti, M.F. Feline leishmaniosis: Is the cat a small dog? Vet. Parasitol. 2018, 251, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Asfaram, S.; Fakhar, M.; Teshnizi, S.H. Is the cat an important reservoir host for visceral leishmaniasis? A systematic review with meta-analysis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190012. [Google Scholar] [CrossRef]
- Kenner, J.R.; Aronson, N.R.; Bratthauer, G.L.; Turnicky, R.P.; Jackson, J.E.; Tang, D.B. Immunohistochemistry to identify Leishmania parasites in fixed tissues. J. Cutan. Pathol. 1999, 26, 130–136. [Google Scholar] [CrossRef]
- Esteve, L.O.; Saz, S.V.; Hosein, S.; Solano-Gallego, L. Histopathological findings and detection of toll-like receptor 2 in cutaneous lesions of canine leishmaniosis. Vet. Pathol. 2015, 209, 157–163. [Google Scholar] [CrossRef]
- Di Mattia, D.; Fondevilla, D.; Abramo, F.; Fondati, A. A retrospective histopathological, immunohistochemical and molecular study of the presence of Leishmania spp. in the skin of cats with head and neck ulcerative dermatitis. Vet. Dermatol. 2018, 29, 212-e76. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, W.L.; de Lima Santos, R.; Esteves Arantes, R.M.; Gonçalves, R.; de Melo, M.N.; Michalick, M.S.M.; Tafuri, W.L. An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J. Immunol. Methods 2004, 292, 17–23. [Google Scholar] [CrossRef]
- Benassi, J.C.; Benvenga, G.U.; Ferreira, H.L.; Pereira, V.F.; Keid, L.B.; Soares, R.; de Sousa Oliveira, T.M.F. Detection of Leishmania infantum DNA in conjunctival swabs of cats by quantitative real-time PCR. Exp. Parasitol. 2017, 177, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.L.; Ihrke, P.J.; Walder, E.J.; Affolter, V.K. Skin Diseases of the Dog and Cat. Clinical and Histopathological Diagnosis; Blackwell Science: Oxford, UK, 2005. [Google Scholar]
- Gramiccia, M.; Gradoni, L. The current status of zoonotic leishmaniases and approaches to disease control. Int. J. Parasitol. 2005, 35, 1169–1180. [Google Scholar] [CrossRef]
- Passos, S.R.; de Azevedo Rodrigues, T.; Madureira, A.P.; Giunchetti, R.C.; Zanini, M.S. Clinical treatment of cutaneous leishmaniasis in dogs with furazolidone and domperidone. Int. J. Antimicrob. Agents 2014, 44, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, D.; Llinas, J.; Homedes, J.; Sust, M.; Ferrer, L. A single-centre, open-label, controlled, randomized clinical trial to assess the preventive efficacy of a domperidone-based treatment programme against clinical canine leishm, aniasis in a high prevalence area. Prev. Vet. Med. 2014, 115, 56–63. [Google Scholar] [CrossRef]
- Brianti, E.; Falsone, L.; Napoli, E.; Gaglio, G.; Giannetto, S.; Pennisi, M.G.; Priolo, V.; Latrofa, M.S.; Tarallo, V.D.; Basano, F.S.; et al. Prevention of feline leishmaniosis with an imidacloprid 10%/flumethrin 4.5% polymer matrix collar. Parasit. Vectors 2017, 10, 334. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 62, e1000100. [Google Scholar] [CrossRef]



| Continent | Country | Type of Study (No.) | No. of Cases | Breed (No.) | Species (No.) | References |
|---|---|---|---|---|---|---|
| Europe | France | Case report (3) | 3 | DSH (3) | L. infantum (3) | [14,22,36] |
| Italy | Case report (4) | 4 | DSH (4) | L. infantum (2); Leishmania sp. (2) | [17,18,35,51] | |
| Portugal | Case report (5) | 5 | DLH (1); DSH (4) | L. infantum (3); Leishmania sp. (2) | [39,42,44,49,54] | |
| Spain | Case report (2); Case series (1) | 9 | DSH (7); Siamese (2) | L. infantum (1); Leishmania sp. (8) | [26,57] | |
| Switzerland | Case report (1) | 1 | DSH (1) | L. infantum (1) | [38] | |
| United Kingdom | Case report (1) | 1 | DLH (1) | L. infantum (1) | [45] | |
| North America | USA | Case report (1); Case series (1) | 6 | DLH (1); DSH (5) | L. mexicana (6) | [32,46] |
| South America | Argentina | Case report (1) | 1 | DLH (1) | L. braziliensis (1) | [43] |
| Brazil | Case series (4); Case report (5) | 13 | DLH (13) | L. amazonensis (3); L. braziliensis (2); L. infantum (3); L. panamensis (1); Leishmania sp. (4) | [13,19,20,21,48,52,56,58] | |
| French Guiana | Case report (1) | 1 | DLH (1) | L. braziliensis (1) | [33] | |
| Venezuela | Case series (3) | 22 | DSH (19); ND (3) | L. braziliensis (2); L. mexicana (12); L. venezuelensis (2); Leishmania sp. (6) | [9,50,53] |
| Lesihmania Species | Cases | Forms | Sign | References | ||||
|---|---|---|---|---|---|---|---|---|
| Cutaneous | Visceral | Nodule (Ulcerated) | Ulcer/Crust | Alopecia | Scale | |||
| L. amazonensis | 3 | 3 | - | 3 (0) | 1 | - | - | [21,28,56] |
| L. brasiliensis | 6 | 6 | 1 | 6 (4) | 1 | - | - | [20,33,43,50] |
| L. infantum | 14 | 14 | 11 | 11 (4) | 4 | 2 | - | [14,17,19,22,24,36,38,39,44,45,51,54,58] |
| L. mexicana | 18 | 18 | 1 | 11 (4) | 7 | - | 1 | [32,46,50,53] |
| L. panamensis | 1 | 1 | - | 1 (0) | - | - | - | [13] |
| L. venezuelensis | 2 | 2 | - | 2 (0) | - | - | - | [9] |
| Leishmania spp. | 22 | 22 | 14 | 14 (3) | 11 | 4 | 4 | [9,18,26,35,42,48,49,50,52,53,57] |
| Case No. | Breed, Gender, Age (yrs) | Region | C–V | Localization | Type of Lesion | Citology | Histology | IFAT Titer | PCR | Comorbidity | Therapy | Treatment Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin | L.N. | ||||||||||||
| 1 | DSH, M, 10 | Sicily | C–V | Head (periocular), dorsum, groin, axillae, abdomen | Alopecia, erosion, pruritus | − | + | + | 1:160 | PCR +ve | FIV −ve, FeLV −ve | Allopurinol (10mg/kg BID 6 months) + Cyproheptadine 2 mg | Alive, under therapy for pruritus, remission and retreated with allopurinol |
| 2 | DSH, M, 9 | Sardinia | C–V | Head, ear, neck, leg (metatarsus hock) | Alopecia, exfoliation | − | + | + | − | qPCR +ve | FIV +ve, FeLV −ve | Allopurinol (10mg/kg BID) + Domperidone (0.5 mg SID) | Alive, second cycle therapy after 6 months, recovered 12 months after the first presentation |
| 3 | DSH, F, 12 | Piemonte | C–V | Head (eyelid, chip, nose, nares) | Multiple ulcerated nodules | + | + | + | 1:1280 | PCR +ve | FIV +ve, FeLV −ve | Allopurinol (10mg/kg BID) + Acetate prendnisolone (2 mg SID) | Complete remission in three months, died one year after from chronic renal disease |
| 4 | DSH, F, 4 | Lazio | C | Head (eyelid, lip, chin), leg (digit) | Multiple nodules | + | − | + | 1:320 | PCR +ve | FIV −ve, FeLV +ve | Allopurinol (5 mg BID) | Complete remission in three weeks, no replapse, died after one month from lymphoma |
| 5 | DSH, M, 12 | Sardinia | C–V | Head (nose, nares), ear, leg, dorsum | Alopecia, ulcer, crust, pruritus | + | + | − | 1:1280 | PCR +ve | FIV +ve, FeLV −ve | Allopurinol (10mg BID) + Antimonial salts (50 mg SID) | Complete remission in one month, died soon after from car crash |
| 6 | DSH, M, 11 | Tuscany | C–V | Head (periocular) ear | Alopecia, crust, exfoliation | + | + | + | 1:3200 | PCR +ve | FIV +ve | Allopurinol (10mg BID) + Antimonial salts (15 mg SID 30 days) | Complete remission after 6 months, euthanasia for hypertrophic myocardiopathy and thrombosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramo, F.; Albanese, F.; Gattuso, S.; Randone, A.; Fileccia, I.; Dedola, C.; Ibba, F.; Ottaiano, P.; Brianti, E. Skin Lesions in Feline Leishmaniosis: A Systematic Review. Pathogens 2021, 10, 472. https://doi.org/10.3390/pathogens10040472
Abramo F, Albanese F, Gattuso S, Randone A, Fileccia I, Dedola C, Ibba F, Ottaiano P, Brianti E. Skin Lesions in Feline Leishmaniosis: A Systematic Review. Pathogens. 2021; 10(4):472. https://doi.org/10.3390/pathogens10040472
Chicago/Turabian StyleAbramo, Francesca, Francesco Albanese, Silvia Gattuso, Alessandra Randone, Ivan Fileccia, Carla Dedola, Fabrizio Ibba, Paola Ottaiano, and Emanuele Brianti. 2021. "Skin Lesions in Feline Leishmaniosis: A Systematic Review" Pathogens 10, no. 4: 472. https://doi.org/10.3390/pathogens10040472
APA StyleAbramo, F., Albanese, F., Gattuso, S., Randone, A., Fileccia, I., Dedola, C., Ibba, F., Ottaiano, P., & Brianti, E. (2021). Skin Lesions in Feline Leishmaniosis: A Systematic Review. Pathogens, 10(4), 472. https://doi.org/10.3390/pathogens10040472






