Evidence for the Use of Mucus Swabs to Detect Renibacterium salmoninarum in Brook Trout
Abstract
1. Introduction
2. Results
2.1. Assay Performance
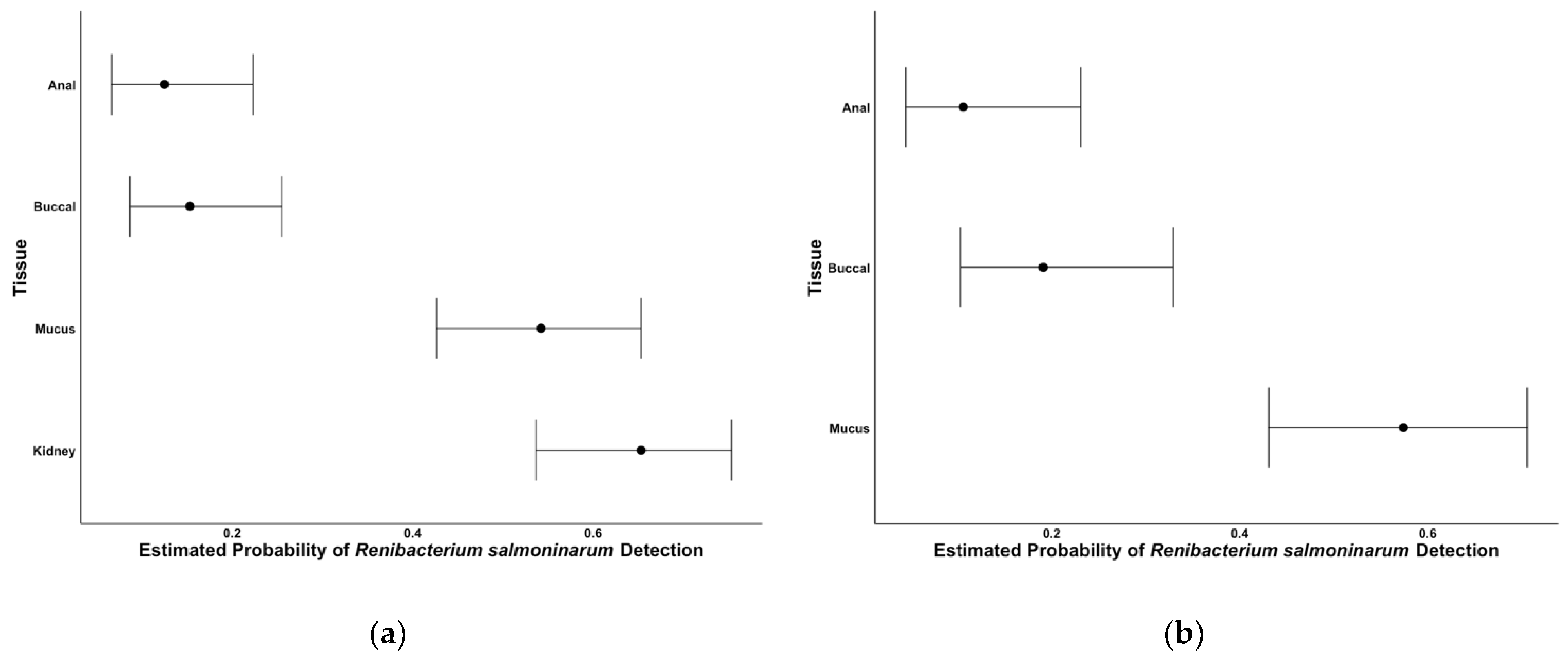
2.2. Tissue Comparisons
2.3. Comparisons When Kidney Tissue Is Positive
3. Discussion
4. Materials and Methods
4.1. Fish and Tissue Collection
4.2. Laboratory Analyses
4.3. Data Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AFS-FHS (American Fisheries Society-Fish Health Section). FHS Blue Book: Suggested Procedures for the Detection and Identification of Certain Finfish and Shellfish Pathogens, 2020th ed.; American Fisheries Society: Bethesda, MD, USA, 2014; Available online: https://units.fisheries.org/fhs/fish-health-section-blue-book-2020/ (accessed on 3 February 2021).
- Ossiander, F.J.; Wedemeyer, G. Computer program for sample sizes required to determine disease incidence in fish populations. J. Fish. Res. Board Can. 1973, 30, 1383–1384. [Google Scholar] [CrossRef]
- Richards, M.; Carolyn, A.; Faisal, M. Detection accuracy of Renibacterium salmoninarum in chinook salmon, Oncorhynchus tshawytscha (Walbaum) from non-lethally collected samples: Effects of exposure route and disease severity. Prev. Vet. Med. 2017, 145, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.; Overturf, K.; Hogge, C.; Johnson, K. Detection of Renibacterium salmoninarum in chinook salmon, Oncorhynchus tshawytscha (Walbaum), using quantitative PCR. J. Fish Dis. 2005, 28, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, E.R.; Bellmund, C.A.; Groocock, G.H.; Ting, W.P.; Hambury, K.L.; Getchel, R.G.; Bowser, P. Fin and gill biopsies are effective nonlethal samples for detection of viral hemorrhagic septicemia virus phenotype Ivb. J. Vet. Diagn. 2013, 25, 203–209. [Google Scholar] [CrossRef]
- Bowers, R.M.; Lapatra, S.E.; Dhar, A. Detection and quantitation of infectious pancreatic necrosis virus by real-time reverse transcriptase-polymerase chain reaction using lethal and non-lethal tissue sampling. J. Virol. Methods 2008, 147, 226–234. [Google Scholar] [CrossRef]
- Cipriano, F.; Rocco, C.; Flint, D.E. Use of non-lethal procedures to detect and monitor Aeromonas Salmonicida in potentially endangered or threatened populations of migrating and post-spawning salmon. Dis. Aquat. Org. 1996, 27, 233–236. [Google Scholar] [CrossRef]
- Evenden, A.J.; Grayson, T.H.; Gilpin, M.L.; Munn, C. Renibacterium salmoninarum and bacterial kidney disease—The unfinished jigsaw. Annu. Rev. Fish Dis. 1993, 3, 87–104. [Google Scholar] [CrossRef]
- Mitchum, D.L.; Sherman, L.E.; Baxter, G. Bacterial kidney disease in feral populations of brook trout (Salvelinus fontinalis), brown trout (Salmo trutta), and rainbow trout (Salmo gairdneri). J. Fish Res. Board Can. 1979, 36, 1370–1376. [Google Scholar] [CrossRef]
- Starliper, C.E.; Smith, D.; Shatzer, T. Virulence of Renibacterium salmoninarum to salmonids. J Aquat. Anim. Health 1997, 9, 1–7. [Google Scholar] [CrossRef]
- Jones, D.T.; Moffitt, C.M.; Peters, K.K. Temperature-mediated differences in bacterial kidney disease expression and survival in Renibacterium salmoninarum challenged bull trout and other salmonids. N. Am. J. Fish. Manag. 2007, 27, 695–706. [Google Scholar] [CrossRef]
- Elliott, D.G.; Wiens, G.D.; Hammell, K.L.; Rhodes, L.D. Vaccination against bacterial kidney disease: Chapter 22. In Fish Vaccination; Wiley-Blackwell: Oxford, UK, 2014; pp. 255–272. [Google Scholar]
- Pascho, R.J.; Elliott, D.G.; Chase, D.M. Comparison of traditional and molecular methods for detection of Renibacterium salmoninarum. In Molecular Diagnosis of Salmonid Diseases; Kluwer Academic Press: Dordrecht, The Netherland, 2002; pp. 157–209. [Google Scholar]
- McKibben, C.L.; Pascho, R.L. Shedding of Renibacterium salmoninarum by infected chinook salmon Oncorhynchus tshawytscha. Dis. Aquat. Org. 1999, 38, 75–79. [Google Scholar] [CrossRef]
- Mesa, M.G.; Schreck, C. Interaction of infection with Renibacterium salmoninarum and physical stress in juvenile chinook salmon: Physiological responses, disease progression, and mortality. Trans. Am. Fish Soc. 2000, 129, 158–173. [Google Scholar] [CrossRef]
- Rhodes, L.D.; Durkin, C.; Nance, S.L.; Rice, C. Prevalence and analysis of Renibacterium salmoninarum infection among juvenile chinook salmon Oncorhynchus tshawytscha in North Puget Sound. Dis. Aquat. Org. 2006, 71, 179–190. [Google Scholar] [CrossRef]
- Fetherman, E.R.; Neuschwanger, B.; Davis, T.; Wells, C.L.; Kraft, A. Efficacy of erymicin 200 injections for reducing Renibacterium salmoninarum and controlling vertical transmission in an inland rainbow trout brood stock. Pathogens 2020, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.G.; McKibben, C.L.; Conway, P.; Carla, M.; Chase, D.M.; Applegate, L.J. Testing of candidate non-lethal sampling methods for detection of Renibacterium salmoninarum in juvenile chinook salmon Oncorhynchus tshawytscha. Dis. Aquat. Org. 2015, 114, 21–43. [Google Scholar] [CrossRef]
- Suzuki, K.; Misaka, N.; Mizuno, S.; Sasaki, Y. Subclinical infection of Renibacterium salmoninarum in fry and juvenile chum salmon Oncorhynchus keta in Hokkaido, Japan. Fish Path. 2017, 52, 89–95. [Google Scholar] [CrossRef][Green Version]
- Benhamed, S.; Guardiola, F.A.; Mars, M.; Esteban, M.A. Pathogen bacteria adhesion to skin mucus of fishes. Vet. Microbiol. 2014, 171, 1–12. [Google Scholar] [CrossRef]
- Sanders, J.; Fryer, J.L. Renibacterium salmoninarum the causative agent of bacterial kidney disease in salmonid fishes. Int. J. Syst. Evol. Microbiol. 1980, 30, 496–502. [Google Scholar]
- Bullock, G.; Herman, R.L. Bacterial Kidney Disease of Salmonid Fishes Caused by Renibacterium Salmoninarum; No. 78; U.S. Fish and Wildlife Service: Washington, DC, USA, 1988. [Google Scholar]
- O’Bryne-Ring, N.; Dowling, K.; Cotter, D.; Whelan, K.; MacEvilly, U. Changes in mucus cell numbers in the epidermis of the Atlantic salmon at the onset of smoltification. J. Fish Biol. 2003, 63, 1625–1630. [Google Scholar] [CrossRef]
- Pickering, A. Seasonal changes in the epidermis of the brown trout Salmo trutta (L). J. Fish Biol. 1977, 10, 561–566. [Google Scholar] [CrossRef]
- Pottinger, T.G.; Pickering, A.; Blackstock, N. Ectoparasite-induced changes in epidermal mucification of the brown trout, Salmo trutta (L). J. Fish Biol. 1984, 25, 123–128. [Google Scholar] [CrossRef]
- Balfry, S.K.; Albright, L.J.; Evelyn, T. Horizontal transfer of Renibacterium salmoninarum among farmed salmonids via the fecal-oral route. Dis. Aquat. Org. 1996, 25, 63–69. [Google Scholar] [CrossRef]
- Hamel, O.S. Immunosuppression in progeny of chinook salmon infected with Renibacterium salmoninarum: Re-analysis of a brood stock segregation experiment. Dis. Aquat. Org. 2005, 65, 29–41. [Google Scholar] [CrossRef]
- Faisal, M.; Eissa, A. Diagnostic testing patterns of Renibacterium salmoninarum in spawning salmonid stocks in Michigan. J. Wildl. Dis. 2009, 45, 447–456. [Google Scholar] [CrossRef]
- Elliott, D.G.; Applegate, L.J.; Murray, A.L.; Purcell, M.; McKibben, C. Bench-top validation testing of selected immunological and molecular Renibacterium salmoninarum diagnostic assays by comparison with quantitative bacteriological culture. J. Fish Dis. 2013, 36, 779–809. [Google Scholar] [CrossRef] [PubMed]
- Pascho, R.J.; Elliott, D.G.; Streufert, J. Brood stock segregation of spring chinook salmon Oncorhynchus tshawytscha by use of the enzyme-linked immunosorbent assay (ELISA) and the fluorescent antibody technique (FAT) affects the prevalence and levels of Renibacterium salmoninarum infection in progeny. Dis. Aquat. Org. 1991, 12, 25–40. [Google Scholar]
- Sandell, T.A.; Jacobson, K. Comparison and evaluation of Renibacterium salmoninarum quantitative PCR diagnostic assays using field samples of chinook and coho salmon. Dis Aquat. Org. 2011, 93, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Chase, D.M.; Elliott, D.G.; Pascho, R. Detection and quantification of Renibacterium salmoninarum DNA in salmonid tissues by real-time quantitative polymerase chain reaction analysis. J. Vet. Diagn. 2006, 18, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Bolker, B.; Brooks, M.; Clark, C.; Poulsen, J.; Stevens, M.H.H.; White, J.S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Bates, D.; Machler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
| Model | Contrasts | Odds Ratio | SE | z-Value | p-Value |
|---|---|---|---|---|---|
| Assay Performance | DFAT/PCR | 7.27 | 3.35 | 4.30 | <0.01 |
| DFAT/qPCR | 0.78 | 0.32 | −0.60 | 0.82 | |
| qPCR/PCR | 9.26 | 4.43 | 4.65 | <0.01 | |
| Tissue Comparisons | Kidney/Anal | 13.16 | 5.71 | 5.94 | <0.01 |
| Kidney/Buccal | 10.43 | 4.28 | 5.71 | <0.01 | |
| Kidney/Mucus | 1.59 | 0.54 | 1.36 | 0.53 | |
| Mucus/Anal | 8.27 | 3.54 | 4.94 | <0.01 | |
| Mucus/Buccal | 6.55 | 2.65 | 4.65 | <0.01 | |
| Comparisons when Kidney Tissue is Positive | Mucus/Anal | 11.34 | 6.32 | 4.36 | <0.01 |
| Mucus/Buccal | 5.70 | 2.70 | 3.67 | <0.01 | |
| Buccal/Anal | 1.99 | 1.20 | 1.14 | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication. (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riepe, T.B.; Vincent, V.; Milano, V.; Fetherman, E.R.; Winkelman, D.L. Evidence for the Use of Mucus Swabs to Detect Renibacterium salmoninarum in Brook Trout. Pathogens 2021, 10, 460. https://doi.org/10.3390/pathogens10040460
Riepe TB, Vincent V, Milano V, Fetherman ER, Winkelman DL. Evidence for the Use of Mucus Swabs to Detect Renibacterium salmoninarum in Brook Trout. Pathogens. 2021; 10(4):460. https://doi.org/10.3390/pathogens10040460
Chicago/Turabian StyleRiepe, Tawni B., Victoria Vincent, Vicki Milano, Eric R. Fetherman, and Dana L. Winkelman. 2021. "Evidence for the Use of Mucus Swabs to Detect Renibacterium salmoninarum in Brook Trout" Pathogens 10, no. 4: 460. https://doi.org/10.3390/pathogens10040460
APA StyleRiepe, T. B., Vincent, V., Milano, V., Fetherman, E. R., & Winkelman, D. L. (2021). Evidence for the Use of Mucus Swabs to Detect Renibacterium salmoninarum in Brook Trout. Pathogens, 10(4), 460. https://doi.org/10.3390/pathogens10040460






