Serological and Molecular Prevalence of Babesia caballi in Apparently Healthy Horses in Israel
Abstract
1. Introduction
2. Results
2.1. Study Population
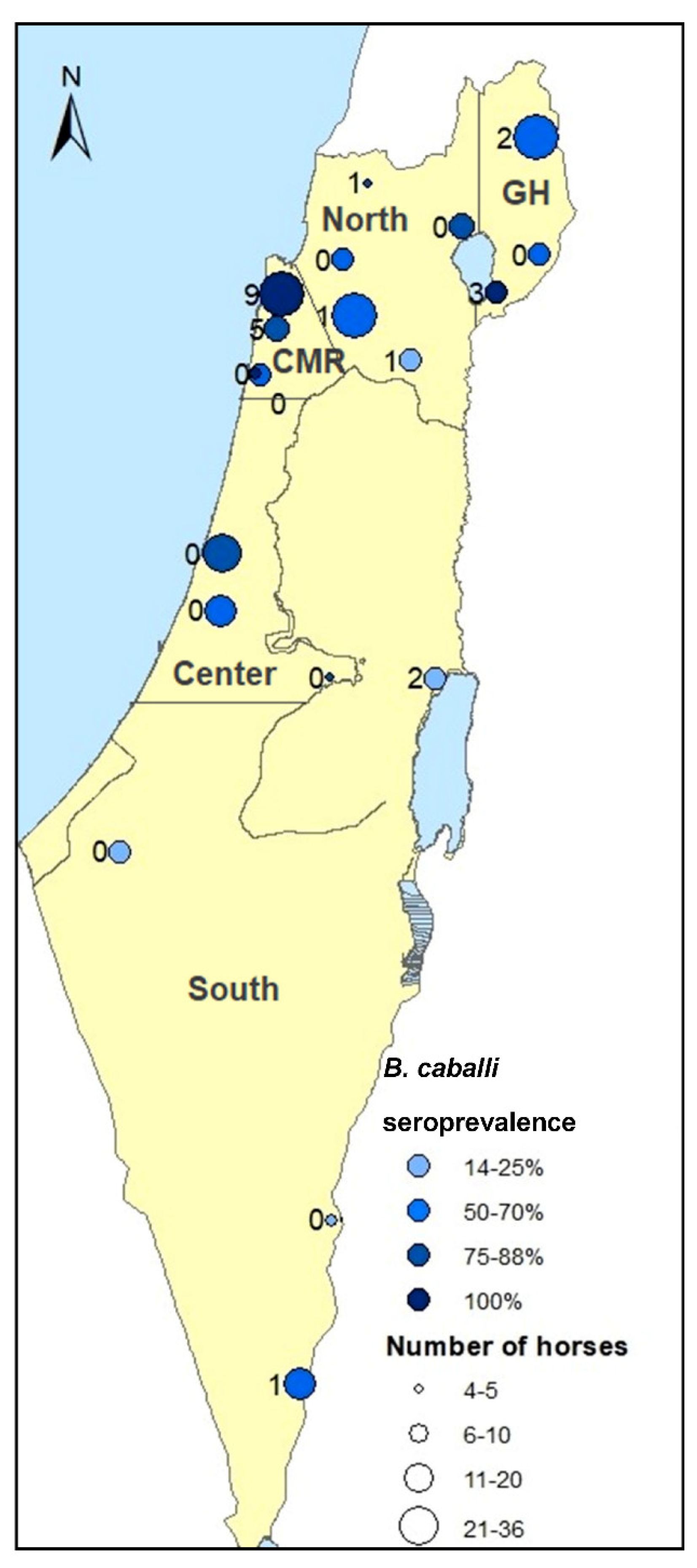
2.2. Detection of B. caballi Infection by IFAT and PCR
2.3. Risk Factors for Serologic Exposure to B. caballi
2.4. Risk Factors for Molecular Identification of B. caballi Infection
2.5. Potential Clinical Consequences of Exposure or Infection
3. Discussion
4. Materials and Methods
4.1. Study Design and Sample Collection
4.2. Serological Screening for B. caballi Exposure
4.3. Identification of B. caballi Infection Using Nested PCR
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothschild, C.M. Equine piroplasmosis. J. Equine Vet. Sci. 2013, 23, 115–120. [Google Scholar] [CrossRef]
- Tirosh-Levy, S.; Gottlieb, Y.; Fry, L.M.; Knowles, D.P.; Steinman, A. Twenty Years of Equine Piroplasmosis Research: Global Distribution, Molecular Diagnosis, and Phylogeny. Pathogens 2020, 9, 926. [Google Scholar] [CrossRef]
- Wise, L.N.; Kappmeyer, L.S.; Mealey, R.H.; Knowles, D.P. Review of equine piroplasmosis. J. Vet. Int. Med. 2013, 27, 1334–1346. [Google Scholar] [CrossRef]
- Friedhoff, K.T.; Tenter, A.M.; Muller, I. Haemoparasites of equines: Impact on international trade of horses. Rev. Sci. Tech. 1990, 9, 1187–1194. [Google Scholar]
- Scoles, G.A.; Ueti, M.W. Vector ecology of equine piroplasmosis. Ann. Rev. Entomol. 2015, 60, 561–580. [Google Scholar] [CrossRef]
- De Waal, D. Equine piroplasmosis: A review. Brit. Vet. J. 1992, 148, 6–14. [Google Scholar] [CrossRef]
- Kappmeyer, L.S.; Perryman, L.E.; Hines, S.A.; Baszler, T.V.; Katz, J.B.; Hennager, S.G.; Knowles, D.P. Detection of equine antibodies to babesia caballi by recombinant B. caballi rhoptry-associated protein 1 in a competitive-inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1999, 37, 2285–2290. [Google Scholar] [CrossRef]
- Bhoora, R.; Quan, M.; Zweygarth, E.; Guthrie, A.J.; Prinsloo, S.A.; Collins, N.E. Sequence heterogeneity in the gene encoding the rhoptry-associated protein-1 (RAP-1) of Babesia caballi isolates from South Africa. Vet. Parasitol. 2010, 169, 279–288. [Google Scholar] [CrossRef]
- Mahmoud, M.S.; El-Ezz, N.T.; Abdel-Shafy, S.; Nassar, S.A.; El Namaky, A.H.; Khalil, W.K.; Knowles, D.; Kappmeyer, L.; Silva, M.G.; Suarez, C.E. Assessment of Theileria equi and Babesia caballi infections in equine populations in Egypt by molecular, serological and hematological approaches. Parasit Vectors 2016, 9, 260. [Google Scholar] [CrossRef]
- Rapoport, A.; Aharonson-Raz, K.; Berlin, D.; Tal, S.; Gottlieb, Y.; Klement, E.; Steinman, A. Molecular characterization of the Babesia caballi rap-1 gene and epidemiological survey in horses in Israel. Infect. Genet. Evol. 2014, 23, 115–120. [Google Scholar] [CrossRef]
- Aharonson-Raz, K.; Rapoport, A.; Hawari, I.M.; Lensky, I.M.; Berlin, D.; Zivotofsky, D.; Klement, E.; Steinman, A. Novel description of force of infection and risk factors associated with Theileria equi in horses in Israel and in The Palestinian Authority. Ticks Tick-Borne Dis. 2014, 5, 366–372. [Google Scholar] [CrossRef]
- Ketter-Ratzon, D.; Tirosh-Levy, S.; Nachum-Biala, Y.; Saar, T.; Qura’n, L.; Zivotofsky, D.; Abdeen, Z.; Baneth, G.; Steinman, A. Characterization of Theileria equi genotypes in horses in Israel, the Palestinian Authority and Jordan. Ticks Tick-Borne Dis. 2017, 8, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Shkap, V.; Cohen, I.; Leibovitz, B.; Savitsky; Pipano, E.; Avni, G.; Shofer, S.; Giger, U.; Kappmeyer, L.; Knowles, D. Seroprevalence of Babesia equi among horses in Israel using competitive inhibition ELISA and IFA assays. Vet. Parasitol. 1998, 76, 251–259. [Google Scholar] [CrossRef]
- Steinman, A.; Zimmerman, T.; Klement, E.; Lensky, I.M.; Berlin, D.; Gottlieb, Y.; Baneth, G. Demographic and environmental risk factors for infection by Theileria equi in 590 horses in Israel. Vet. Parasitol. 2012, 187, 558–562. [Google Scholar] [CrossRef]
- Tirosh-Levy, S.; Gottlieb, Y.; Arieli, O.; Mazuz, M.L.; King, R.; Horowitz, I.; Steinman, A. Genetic characteristics of Theileria equi in zebras, wild and domestic donkeys in Israel and the Palestinian Authority. Ticks Tick Borne Dis. 2020, 11, 101286. [Google Scholar] [CrossRef]
- Tirosh-Levy, S.; Steinman, A.; Levy, H.; Katz, Y.; Shtilman, M.; Gottlieb, Y. Parasite load and genotype are associated with clinical outcome of piroplasm-infected equines in Israel. Parasit Vectors 2020, 13, 267. [Google Scholar] [CrossRef]
- Tirosh-Levy, S.; Gottlieb, Y.; Mazuz, M.L.; Savitsky, I.; Steinman, A. Infection dynamics of Theileria equi in carrier horses is associated with management and tick exposure. Ticks Tick Borne Dis. 2020, 11, 101508. [Google Scholar] [CrossRef]
- Abutarbush, S.M.; Alqawasmeh, D.M.; Mukbel, R.M.; Al-Majali, A.M. Equine babesiosis: Seroprevalence, risk factors and comparison of different diagnostic methods in Jordan. Transbound. Emerg. Dis. 2012, 59, 72–78. [Google Scholar] [CrossRef]
- Qablan, M.A.; Obornik, M.; Petrzelkova, K.J.; Sloboda, M.; Shudiefat, M.F.; Horin, P.; Lukes, J.; Modry, D. Infections by Babesia caballi and Theileria equi in Jordanian equids: Epidemiology and genetic diversity. Parasitology 2013, 140, 1096–1103. [Google Scholar] [CrossRef]
- Onyiche, T.E.; Taioe, M.O.; Molefe, N.I.; Biu, A.A.; Luka, J.; Omeh, I.J.; Yokoyama, N.; Thekisoe, O. Equine piroplasmosis: An insight into global exposure of equids from 1990 to 2019 by systematic review and meta-analysis. Parasitology 2020, 147, 1411–1424. [Google Scholar] [CrossRef]
- Ruegg, S.R.; Heinzmann, D.; Barbour, A.D.; Torgerson, P.R. Estimation of the transmission dynamics of Theileria equi and Babesia caballi in horses. Parasitology 2008, 135, 555–565. [Google Scholar] [CrossRef]
- Ruegg, S.R.; Torgerson, P.; Deplazes, P.; Mathis, A. Age-dependent dynamics of Theileria equi and Babesia caballi infections in southwest Mongolia based on IFAT and/or PCR prevalence data from domestic horses and ticks. Parasitology 2007, 134, 939–947. [Google Scholar] [CrossRef]
- Kerber, C.E.; Labruna, M.B.; Ferreira, F.; De Waal, D.T.; Knowles, D.P.; Gennari, S.M. Prevalence of equine Piroplasmosis and its association with tick infestation in the State of Sao Paulo, Brazil. Rev. Bras. Parasitol. Vet. 2009, 18, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Yang, J.; Wang, X.; Li, Z.; Jianlin, X.; Li, X.; Xiang, Q.; Li, Y.; Liu, Z.; et al. The first molecular detection and genetic diversity of Babesia caballi and Theileria equi in horses of Gansu province, China. Ticks Tick Borne Dis. 2019, 10, 528–532. [Google Scholar] [CrossRef]
- Xuan, X.; Nagai, A.; Battsetseg, B.; Fukumoto, S.; Makala, L.H.; Inoue, N.; Igarashi, I.; Mikami, T.; Fujisaki, K. Diagnosis of equine piroplasmosis in Brazil by serodiagnostic methods with recombinant antigens. J. Vet. Med. Sci. 2001, 63, 1159–1160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nugraha, A.B.; Cahyaningsih, U.; Amrozi, A.; Ridwan, Y.; Agungpriyono, S.; Taher, D.M.; Guswanto, A.; Gantuya, S.; Tayebwa, D.S.; Tuvshintulga, B.; et al. Serological and molecular prevalence of equine piroplasmosis in Western Java, Indonesia. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 1–6. [Google Scholar] [CrossRef]
- Moretti, A.; Mangili, V.; Salvatori, R.; Maresca, C.; Scoccia, E.; Torina, A.; Moretta, I.; Gabrielli, S.; Tampieri, M.P.; Pietrobelli, M. Prevalence and diagnosis of Babesia and Theileria infections in horses in Italy: A preliminary study. Vet. J. 2010, 184, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Ikadai, H.; Nagai, A.; Xuan, X.; Igarashi, I.; Tsugihiko, K.; Tsuji, N.; Oyamada, T.; Suzuki, N.; Fujisaki, K. Seroepidemiologic studies on Babesia caballi and Babesia equi infections in Japan. J. Vet. Med. Sci. 2002, 64, 325–328. [Google Scholar] [CrossRef][Green Version]
- Al-Obaidi, Q.; Arshad, M.; Al-Sultan, I.; Azlinda, A.; Mohd-Azam, K. Comparison between microscopic examination and competitive ELISA for diagnosis of equine piroplasmosis in Kelantan, Malaysia. Malays. J. Vet. Res. 2016, 7, 23–29. [Google Scholar]
- Munkhjargal, T.; Sivakumar, T.; Battsetseg, B.; Nyamjargal, T.; Aboulaila, M.; Purevtseren, B.; Bayarsaikhan, D.; Byambaa, B.; Terkawi, M.A.; Yokoyama, N.; et al. Prevalence and genetic diversity of equine piroplasms in Tov province, Mongolia. Infect. Genet. Evol. 2013, 16, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Asgarali, Z.; Coombs, D.K.; Mohammed, F.; Campbell, M.D.; Caesar, E. A serological study of Babesia caballi and Theileria equi in Thoroughbreds in Trinidad. Vet. Parasitol. 2007, 144, 167–171. [Google Scholar] [CrossRef]
- Acici, M.; Umur, S.; Guvenc, T.; Arslan, H.H.; Kurt, M. Seroprevalence of equine babesiosis in the Black Sea region of Turkey. Parasitol. Int. 2008, 57, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Mujica, F.F.; Perrone, T.; Forlano, M.; Coronado, A.; Melendez, R.D.; Barrios, N.; Alvarez, R.; Granda, F. Serological prevalence of Babesia caballi and Theileria equi in horses of Lara State, Venezuela. Vet. Parasitol. 2011, 178, 180–183. [Google Scholar] [CrossRef]
- Rosales, R.; Rangel-Rivas, A.; Escalona, A.; Jordan, L.S.; Gonzatti, M.I.; Aso, P.M.; Perrone, T.; Silva-Iturriza, A.; Mijares, A. Detection of Theileria equi and Babesia caballi infections in Venezuelan horses using Competitive-Inhibition ELISA and PCR. Vet. Parasitol. 2013, 196, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Tirosh-Levy, S.; Gottlieb, Y.; Apanaskevich, D.A.; Mumcuoglu, K.Y.; Steinman, A. Species distribution and seasonal dynamics of equine tick infestation in two Mediterranean climate niches in Israel. Parasit Vectors 2018, 11, 546. [Google Scholar] [CrossRef]
- Tirosh-Levy, S.; Gottlieb, Y.; Mimoun, L.; Mazuz, M.L.; Steinman, A. Transplacental Transmission of Theileria equi Is Not a Common Cause of Abortions and Infection of Foals in Israel. Animals 2020, 10, 341. [Google Scholar] [CrossRef] [PubMed]
| nPCR-Negative (%) | nPCR-Positive (%) | Total | |
|---|---|---|---|
| IFAT-Negative | 72 (92.3) | 6 (7.7) | 78 |
| IFAT-Positive | 160 (89.4) | 19 (10.6) | 179 |
| Total | 232 | 25 | 257 |
| N | IFAT-Positive (%) | p (χ2) | p (GEE) | OR (95% CI) | |
|---|---|---|---|---|---|
| North | 69 | 40 (58) | <0.001 | 0.138 | 3.45 (0.67–17.77) |
| Carmel | 64 | 59 (92.2) | <0.001 | 10.01 (2.98–33.59) | |
| Center | 47 | 35 (74.5) | 0.062 | 5.45 (0.92–32.30) | |
| South | 35 | 14 (40) | 0.227 | 3.11 (0.49–19.57) | |
| Golan heights | 42 | 31 (73.8) | |||
| Gelding | 128 | 103 (80.5) | <0.001 | 0.037 | 2.01 (1.04–3.89) |
| Stallion | 6 | 4 (66.7) | 0.325 | 2.61 (0.39–17.54) | |
| Mare | 123 | 72 (58.5) | ref | ||
| Mixed Breed | 186 | 142 (76.3) | <0.001 | 0.234 | 1.58 (0.74–3.33) |
| Pure breed | 70 | 36 (51.4) | |||
| Stall | 46 | 36 (78.3) | <0.001 | 0.841 | 0.85 (0.16–4.33) |
| Paddock | 91 | 41 (45.1) | 0.064 | 0.27 (0.07–1.08) | |
| Pasture | 120 | 102 (85) | ref | ||
| Ticks | 98 | 84 (85.7) | <0.001 | 0.127 | 6.98 (0.58–84.34) |
| No ticks | 159 | 95 (59.7) | ref | ||
| Haemaphysalis parva | 5 | 4 (80) | 1 | ||
| Not present | 252 | 175 (69.4) | |||
| Hyalomma excavatum | 54 | 41 (75.9) | 0.259 | ||
| Not present | 203 | 138 (68) | |||
| Hyalomma marginatum | 79 | 67 (84.8) | <0.001 | 0.191 | 2.63 (0.62–11.20) |
| Not present | 178 | 112 (62.9) | |||
| Rhipicephalus annulatus | 2 | 2 (100) | 1 | ||
| Not present | 255 | 177 (69.4) | |||
| Rhipicephalus turanicus | 38 | 28 (73.7) | 0.558 | ||
| Not present | 219 | 151 (68.9) |
| N | nPCR-Positive (%) | p (χ2) | p (GEE) | OR (95% CI) | |
|---|---|---|---|---|---|
| North | 69 | 3 (4.3) | 0.001 | 0.798 | 0.81 (0.16–4.01) |
| Carmel | 64 | 14 (21.9) | 0.025 | 3.82 (1.19–12.32) | |
| Center | 47 | 0 | na | na | |
| South | 35 | 3 (8.6) | 0.686 | 1.62 (0.16–16.89) | |
| Golan heights | 42 | 5 (11.9) | ref | ||
| Gelding | 128 | 15 (11.7) | 0.284 | ||
| Stallion | 6 | 1 (16.7) | |||
| Mare | 123 | 9 (7.3) | |||
| Mixed Breed | 186 | 24 (12.9) | 0.006 | 0.083 | 9.86 (0.74–130.92) |
| Pure breed | 70 | 1 (1.4) | ref | ||
| Stall | 46 | 0 | 0.001 | na | na |
| Paddock | 91 | 5 (5.5) | 0.916 | 1.11 (0.16–7.79) | |
| Pasture | 120 | 20 (16.7) | ref | ||
| Ticks | 98 | 16 (16.3) | 0.005 | 0.667 | 1.34 (0.31–6.36) |
| No ticks | 159 | 9 (5.7) | ref | ||
| Haemaphysalis parva | 5 | 0 | 1 | ||
| Not present | 252 | 25 (9.9) | |||
| Hyalomma excavatum | 54 | 7 (13) | 0.367 | ||
| Not present | 203 | 18 (8.9) | |||
| Hyalomma marginatum | 79 | 13 (16.5) | 0.015 | 0.528 | 0.624 (0.14–2.69) |
| Not present | 178 | 12 (6.7) | ref | ||
| Rhipicephalus annulatus | 2 | 0 | 1 | ||
| Not present | 255 | 25 (9.8) | |||
| Rhipicephalus turanicus | 38 | 5 (13.2) | 0.388 | ||
| Not present | 219 | 20 (9.1) | |||
| Age | 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirosh-Levy, S.; Mazuz, M.L.; Savitsky, I.; Pinkas, D.; Gottlieb, Y.; Steinman, A. Serological and Molecular Prevalence of Babesia caballi in Apparently Healthy Horses in Israel. Pathogens 2021, 10, 445. https://doi.org/10.3390/pathogens10040445
Tirosh-Levy S, Mazuz ML, Savitsky I, Pinkas D, Gottlieb Y, Steinman A. Serological and Molecular Prevalence of Babesia caballi in Apparently Healthy Horses in Israel. Pathogens. 2021; 10(4):445. https://doi.org/10.3390/pathogens10040445
Chicago/Turabian StyleTirosh-Levy, Sharon, Monica L. Mazuz, Igor Savitsky, Dana Pinkas, Yuval Gottlieb, and Amir Steinman. 2021. "Serological and Molecular Prevalence of Babesia caballi in Apparently Healthy Horses in Israel" Pathogens 10, no. 4: 445. https://doi.org/10.3390/pathogens10040445
APA StyleTirosh-Levy, S., Mazuz, M. L., Savitsky, I., Pinkas, D., Gottlieb, Y., & Steinman, A. (2021). Serological and Molecular Prevalence of Babesia caballi in Apparently Healthy Horses in Israel. Pathogens, 10(4), 445. https://doi.org/10.3390/pathogens10040445








