High-Throughput Microfluidic Real-Time PCR for the Detection of Multiple Microorganisms in Ixodid Cattle Ticks in Northeast Algeria
Abstract
1. Introduction
2. Results
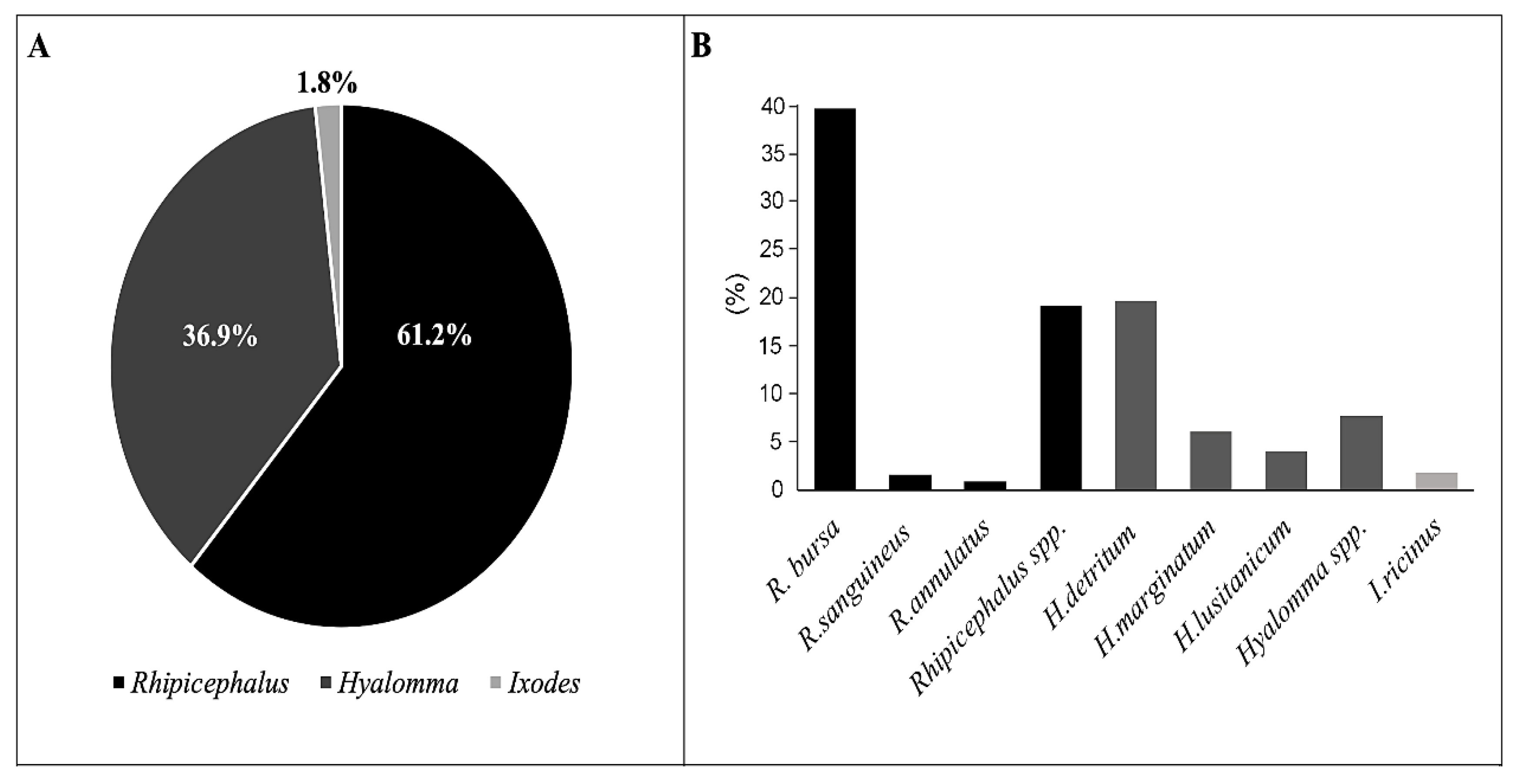
2.1. Taxonomical Identification of Collected Tick Species
2.2. Infection Rates of Microorganisms and Their Co-Infection Rates in Ticks
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Tick Collection and Morphological Identification
4.3. DNA Extraction
4.4. DNA Pre-Amplification
4.5. High-Throughput Microfluidic Real-Time PCR
4.6. Standard/Nested PCR and Sequencing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pesquera, C.; Portillo, A.; Palomar, A.M.; Oteo, J.A. Investigation of tick-borne bacteria (Rickettsia spp., Anaplasma spp., Ehrlichia spp. and Borrelia spp.) in ticks collected from Andean tapirs, cattle and vegetation from a protected area in Ecuador. Parasit. Vectors 2015, 8, 46. [Google Scholar] [CrossRef]
- Rehman, A.; Nijhof, A.M.; Sauter-Louis, C.; Schauer, B.; Staubach, C.; Conraths, F.J. Distribution of ticks infesting ruminants and risk factors associated with high tick prevalence in livestock farms in the semi-arid and arid agro-ecological zones of Pakistan. Parasit. Vectors 2017, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef] [PubMed]
- Silatsa, B.A.; Simo, G.; Githaka, N.; Mwaura, S.; Kamga, R.M.; Oumarou, F.; Keambou, C.; Bishop, R.P.; Djikeng, A.; Kuiate, J.R.; et al. A comprehensive survey of the prevalence and spatial distribution of ticks infesting cattle in different agro-ecological zones of Cameroon. Parasit. Vectors 2019, 12, 489. [Google Scholar] [CrossRef]
- Moutailler, S.; Valiente Moro, C.; Vaumourin, E.; Michelet, L.; Tran, F.H.; Devillers, E.; Cosson, J.F.; Gasqui, P.; Van, V.T.; Mavingui, P.; et al. Co-Infection of Ticks: The Rule Rather Than the Exception. PLoS Negl. Trop. Dis. 2016, 10, e0004539. [Google Scholar] [CrossRef]
- Benchikh Elfegoun, M.C.; Kohil, K.; Gharbi, M.; Afoutni, L.; Benachour, M.L. Cinétique d’infestation par les tiques des bovins de la région subhumide de Constantine en Algérie. Rev. D’éle. Méd. Vét. Pays Trop. 2019, 72, 41–45. [Google Scholar] [CrossRef]
- Bitam, I.; Parola, P.; Matsumoto, K.; Rolain, J.M.; Baziz, B.; Boubidi, S.C.; Harrat, Z.; Belkaid, M.; Raoult, D. First molecular detection of R. conorii, R. aeschlimannii, and R. massiliae in ticks from Algeria. Ann. N. Y. Acad. Sci. 2006, 1078, 368–372. [Google Scholar] [CrossRef]
- Dib, L.; Lafri, I.; Boucheikhchoukh, M.; Dendani, Z.; Bitam, I.; Benakhla, A. Seasonal distribution of Rickettsia spp. in ticks in northeast Algeria. New Microbes New Infect. 2019, 27, 48–52. [Google Scholar] [CrossRef]
- Abdelkadir, K.; Palomar, A.M.; Portillo, A.; Oteo, J.A.; Ait-Oudhia, K.; Khelef, D. Presence of Rickettsia aeschlimannii, ‘Candidatus Rickettsia barbariae’ and Coxiella burnetii in ticks from livestock in Northwestern Algeria. Ticks Tick-Borne Dis. 2019, 10, 924–928. [Google Scholar] [CrossRef]
- Sadeddine, R.; Diarra, A.Z.; Laroche, M.; Mediannikov, O.; Righi, S.; Benakhla, A.; Dahmana, H.; Raoult, D.; Parola, P. Molecular identification of protozoal and bacterial organisms in domestic animals and their infesting ticks from north-eastern Algeria. Ticks Tick-Borne Dis. 2020, 11, 101330. [Google Scholar] [CrossRef]
- Noda, H.; Munderloh, U.G.; Kurtti, T.J. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ. Microbiol. 1997, 63, 3926–3932. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Noël, V.; McCoy, K.D.; Bonazzi, M.; Sidi-Boumedine, K.; Morel, O.; Vavre, F.; Zenner, L.; Jourdain, E.; Durand, P.; et al. The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen, Coxiella burnetii. PLoS Pathog. 2015, 11, e1004892. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; Delannoy, S.; Devillers, E.; Umhang, G.; Aspan, A.; Juremalm, M.; Chirico, J.; van der Wal, F.J.; Sprong, H.; Boye Pihl, T.P.; et al. High-throughput screening of tick-borne pathogens in Europe. Front. Cell. Infect. Microbiol. 2014, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Grech-Angelini, S.; Stachurski, F.; Vayssier-Taussat, M.; Devillers, E.; Casabianca, F.; Lancelot, R.; Uilenberg, G.; Moutailler, S. Tick-borne pathogens in ticks (Acari: Ixodidae) collected from various domestic and wild hosts in Corsica (France), a Mediterranean island environment. Transbound. Emerg. Dis. 2020, 67, 745–757. [Google Scholar] [CrossRef]
- Benchikh Elfegoun, M.C.; Gharbi, M.; Djebir, S.; Kohil, K. Dynamique d’activité saisonnière des tiques ixodidés parasites des bovins dans deux étages bioclimatiques du nord-est algérien. Rev. D’éle. Méd. Vét. Pays Trop. 2013, 66, 117–122. [Google Scholar] [CrossRef]
- Sarih, M.; Socolovschi, C.; Boudebouch, N.; Hassar, M.; Raoult, D.; Parola, P. Spotted fever group Rickettsiae in ticks, Morocco. Emerg. Infect. Dis. 2008, 14, 1067–1073. [Google Scholar] [CrossRef]
- Said, Y.; Lahmar, S.; Dhibi, M.; Rjeibi, M.R.; Jdidi, M.; Gharbi, M. First survey of ticks, tick-borne pathogens (Theileria, Babesia, Anaplasma and Ehrlichia) and Trypanosoma evansi in protected areas for threatened wild ruminants in Tunisia. Parasitol. Int. 2020, 81, 102275. [Google Scholar] [CrossRef] [PubMed]
- Bitam, I. Vectors of Rickettsiae in Africa. Ticks Tick-Borne Dis. 2012, 3, 382–386. [Google Scholar] [CrossRef]
- Portillo, A.; Santibáñez, S.; García-Álvarez, L.; Palomar, A.M.; Oteo, J.A. Rickettsioses in Europe. Microbes Infect. 2015, 17, 834–838. [Google Scholar] [CrossRef]
- Beati, L.; Meskini, M.; Thiers, B.; Raoult, D. Rickettsia aeschlimannii sp. nov., a new spotted fever group Rickettsia associated with Hyalomma marginatum ticks. Int. J. Syst. Bacteriol. 1997, 47, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.; Diatta, G.; Fenollar, F.; Sokhna, C.; Trape, J.F.; Raoult, D. Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl. Trop. Dis. 2010, 4, e821. [Google Scholar] [CrossRef] [PubMed]
- Ehounoud, C.B.; Yao, K.P.; Dahmani, M.; Achi, Y.L.; Amanzougaghene, N.; Kacou N’Douba, A.; N’Guessan, J.D.; Raoult, D.; Fenollar, F.; Mediannikov, O. Multiple Pathogens Including Potential New Species in Tick Vectors in Côte d’Ivoire. PLoS Negl. Trop. Dis. 2016, 10, e0004367. [Google Scholar] [CrossRef] [PubMed]
- Fernández Soto, P.; Encinas Grandes, A.; Pérez Sánchez, R. Rickettsia aeschlimannii in Spain: Molecular evidence in Hyalomma marginatum and five other tick species that feed on humans. Emerg. Infect. Dis. 2003, 9, 889–890. [Google Scholar] [PubMed]
- Germanakis, A.; Chochlakis, D.; Angelakis, E.; Tselentis, Y.; Psaroulaki, A. Rickettsia aeschlimannii infection in a man, Greece. Emerg. Infect. Dis. 2013, 19, 1176–1177. [Google Scholar] [CrossRef]
- Wei, Q.Q.; Guo, L.P.; Wang, A.D.; Mu, L.M.; Zhang, K.; Chen, C.F.; Zhang, W.J.; Wang, Y.Z. The first detection of Rickettsia aeschlimannii and Rickettsia massiliae in Rhipicephalus turanicus ticks, in northwest China. Parasit. Vectors 2015, 8, 2–5. [Google Scholar] [CrossRef]
- Socolovschi, C.; Mediannikov, O.; Raoult, D.; Parola, P. The relationship between spotted fever group Rickettsiae and Ixodid ticks. Vet. Res. 2009, 40, 34. [Google Scholar] [CrossRef] [PubMed]
- Ghafar, A.; Cabezas-Cruz, A.; Galon, C.; Obregon, D.; Gasser, R.B.; Moutailler, S.; Jabbar, A. Bovine ticks harbour a diverse array of microorganisms in Pakistan. Parasit. Vectors 2020, 13, 1. [Google Scholar] [CrossRef]
- Dib, L.; Bitam, I.; Bensouilah, M.; Parola, P.; Raoult, D. First description of Rickettsia monacensis in Ixodes ricinus in Algeria. Clin. Microbiol. Infect. 2009, 15, 261–262. [Google Scholar] [CrossRef]
- Kernif, T.; Messaoudene, D.; Ouahioune, S.; Parola, P.; Raoult, D.; Bitam, I. Spotted fever group rickettsiae identified in Dermacentor marginatus and Ixodes ricinus ticks in Algeria. Ticks Tick-Borne Dis. 2012, 3, 380–381. [Google Scholar] [CrossRef]
- Boucheikhchoukh, M.; Laroche, M.; Aouadi, A.; Dib, L.; Benakhla, A.; Raoult, D.; Parola, P. MALDI-TOF MS identification of ticks of domestic and wild animals in Algeria and molecular detection of associated microorganisms. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Akl, T.; Bourgoin, G.; Souq, M.L.; Appolinaire, J.; Poirel, M.T.; Gibert, P.; Abi Rizk, G.; Garel, M.; Zenner, L. Detection of tick-borne pathogens in questing Ixodes ricinus in the French Pyrenees and first identification of Rickettsia monacensis in France. Parasite 2019, 26, 20. [Google Scholar] [CrossRef] [PubMed]
- McFadden, A.M.J.; Rawdon, T.G.; Meyer, J.; Makin, J.; Morley, C.M.; Clough, R.R.; Tham, K.; Müllner, P.; Geysen, D. An outbreak of haemolytic anaemia associated with infection of Theileria orientalis in naïve cattle. N. Z. Vet. J. 2011, 59, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.O.; Tolf, C.; Tamba, P.; Stefanache, M.; Radbea, G.; Rubel, F.; Waldenström, J.; Dobler, G.; Chițimia-Dobler, L. Babesia, Theileria, and Hepatozoon species in ticks infesting animal hosts in Romania. Parasitol. Res. 2017, 116, 2291–2297. [Google Scholar] [CrossRef]
- Chisu, V.; Alberti, A.; Zobba, R.; Foxi, C.; Masala, G. Molecular characterization and phylogenetic analysis of Babesia and Theileria spp. in ticks from domestic and wild hosts in Sardinia. Acta Trop. 2019, 196, 60–65. [Google Scholar] [CrossRef]
- Bock, R.; Jackson, L.; De Vos, A.; Jorgensen, W. Babesiosis of cattle. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, S.; Esmaeilnejad, B.; Tavassoli, M. A molecular study on Babesia spp. in cattle and ticks in West-Azerbaijan province, Iran. Vet. Res. Forum Int. Q. J. 2017, 8, 299–306. [Google Scholar]
- Battilani, M.; De Arcangeli, S.; Balboni, A.; Dondi, F. Genetic diversity and molecular epidemiology of Anaplasma. Infect. Genet. Evol. 2017, 49, 195–211. [Google Scholar] [CrossRef]
- Dahmani, M.; Davoust, B.; Tahir, D.; Raoult, D.; Fenollar, F.; Mediannikov, O. Molecular investigation and phylogeny of Anaplasmataceae species infecting domestic animals and ticks in Corsica, France. Parasit. Vectors 2017, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Ferrolho, J.; Antunes, S.; Santos, A.S.; Velez, R.; Padre, L.; Cabezas-Cruz, A.; Santos-Silva, M.M.; Domingos, A. Detection and phylogenetic characterization of Theileria spp. and Anaplasma marginale in Rhipicephalus bursa in Portugal. Ticks Tick-Borne Dis. 2016, 7, 443–448. [Google Scholar] [CrossRef]
- Hornok, S.; Micsutka, A.; Fernández de Mera, I.G.; Meli, M.L.; Gönczi, E.; Tánczos, B.; Mangold, A.J.; Farkas, R.; Lutz, H.; Hofmann-Lehmann, R.; et al. Fatal bovine anaplasmosis in a herd with new genotypes of Anaplasma marginale, Anaplasma ovis and concurrent haemoplasmosis. Res. Vet. Sci. 2012, 92, 30–35. [Google Scholar] [CrossRef]
- Boularias, G.; Azzag, N.; Gandoin, C.; Bouillin, C.; Chomel, B.; Haddad, N.; Boulouis, H.J. Bartonella bovis and Bartonella chomelii infection in dairy cattle and their ectoparasites in Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2020, 70, 101450. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.P.; Amanfu, W.; Losho, T.C. Bovine borreliosis in Botswana. Onderstepoort J. Vet. Res. 2000, 67, 221–223. [Google Scholar]
- Mc Coya, B.N.; Maïgab, O.; Schwana, T.G. Detection of Borrelia theileri in Rhipicephalus geigyi from Mali. Ticks Tick-Borne Dis. 2014, 5, 401–403. [Google Scholar]
- Ahantarig, A.; Trinachartvanit, W.; Baimai, V.; Grubhoffer, L. Hard ticks and their bacterial endosymbionts (or would be pathogens). Folia Microbiol. 2013, 58, 419–428. [Google Scholar] [CrossRef]
- Raoult, D.; Roux, V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 1997, 10, 694–719. [Google Scholar] [CrossRef]
- Gondard, M.; Delannoy, S.; Pinarello, V.; Aprelon, R.; Devillers, E.; Galon, C.; Pradel, J.; Vayssier-Taussat, M.; Albina, E.; Moutailler, S. Upscaling surveillance of tick-borne pathogens in the French Caribbean islands. Pathogens 2020, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Mixson, T.R.; Campbell, S.R.; Gill, J.S.; Ginsberg, H.S.; Reichard, M.V.; Schulze, T.L.; Dasch, G.A. Prevalence of Ehrlichia, Borrelia, and Rickettsial Agents in Amblyomma americanum (Acari: Ixodidae) Collected from Nine States. J. Med. Entomol. 2006, 43, 1261–1268. [Google Scholar] [CrossRef]
- Walker, A.R.; Bouattour, A.; Camicas, J.; Estrada-Peña, A.; Horak, I.; Latif, A.; Pegram, R.; Preston, P. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003; ISBN 095451730X. [Google Scholar]
- Loh, S.M.; Gofton, A.W.; Lo, N.; Gillett, A.; Ryan, U.M.; Irwin, P.J.; Oskam, C.L. Novel Borrelia species detected in echidna ticks, Bothriocroton concolor, in Australia. Parasit. Vectors 2016, 9, 339. [Google Scholar] [CrossRef]
- Rar, V.A.; Fomenko, N.V.; Dobrotvorsky, A.K.; Livanova, N.H.; Rudakova, S.A.; Fedorov, E.G.; Astanin, V.B.; Morozova, O.V. Tick borne pathogen detection, Western Siberia, Russia. Emerg. Infect. Dis. 2005, 11, 1708–1715. [Google Scholar] [CrossRef]
- Regnery, R.L.; Spruill, C.L.; Plikaytis, B.D. Genotypic identification of Rickettsiae and estimation of intra species sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991, 173, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Veikkolainen, V.; Vesterinen, E.J.; Lilley, T.M.; Pulliainen, A.T. Bats as reservoir hosts of human bacterial pathogen, Bartonella mayotimonensis. Emerg. Infect. Dis. 2014, 20, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Masatani, T.; Hayashi, K.; Andoh, M.; Tateno, M.; Endo, Y.; Asada, M.; Kusakisako, K.; Tanaka, T.; Gokuden, M.; Hozumi, N.; et al. Detection and molecular characterization of Babesia, Theileria, and Hepatozoon species in hard ticks collected from Kagoshima, the southern region in Japan. Ticks Tick-Borne Dis. 2017, 8, 581–587. [Google Scholar] [CrossRef] [PubMed]
| Species | Borrelia spp. | A.marginale | A.centrale | R. aeschlimannii | R.massiliae | R.monacensis | R.helvetica | Bartonella spp. | T.orientalis | B.bigemina | FLE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R. bursa (n = 51) | 4 | 2 | 0 | 11 | 0 | 0 | 0 | 3 | 8 | 0 | 15 |
| (7.8%) | (3.9%) | (21.5%) | (5.8%) | (15.6%) | (29.4%) | ||||||
| (0.4–15.2%) | (0–9.2%) | (10.2–32.5%) | (0–12.2%) | (5.7–25.6%) | (16.8–41.9%) | ||||||
| R. sanguineus (n = 07) | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 2 | 0 | 5 | 1 |
| (14.2%) | (57.1%) | (28.5%) | (71.4%) | (14.2%) | |||||||
| (0–40%) | (20.4–93.8%) | (0–61.9%) | (37.9–100%) | (0–40.2%) | |||||||
| R. annulatus (n = 01) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| (100%) | |||||||||||
| (0–100%) | |||||||||||
| Rhipicephalus spp. (n = 50) | 1 | 6 | 1 | 5 | 0 | 0 | 0 | 3 | 11 | 0 | 21 |
| (2%) | (12%) | (2%) | (10%) | (6%) | (22%) | (42%) | |||||
| (0–5.8%) | (3–21%) | (0–5.8%) | (1.6–18.3%) | (0–12.5%) | (10.5–33.4%) | (28.3–55.6%) | |||||
| H. detritum (n = 41) | 3 | 2 | 0 | 2 | 2 | 0 | 0 | 3 | 6 | 0 | 37 |
| (7.3%) | (4.8%) | (4.8%) | (4.8%) | (7.3%) | (14.3%) | (90.2%) | |||||
| (0–15.2%) | (0–11.3%) | (0–11.3%) | (0–11.3%) | (0–15.2%) | (3.5–25%) | (81.1–99.3%) | |||||
| H. marginatum (n = 15) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 12 |
| (6.6%) | (6.6%) | (80%) | |||||||||
| (0–19.1%) | (0–19.1%) | (59.7–100%) | |||||||||
| H. lusitanicum (n = 04) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 4 |
| (25%) | (100%) | ||||||||||
| (0–67.4%) | (25–100%) | ||||||||||
| Hyalomma spp. (n = 53) | 6 | 1 | 0 | 8 | 5 | 0 | 0 | 4 | 10 | 0 | 47 |
| (11.3%) | (1.8%) | (15.1%) | (9.4%) | (7.5%) | (18.8%) | (88.6%) | |||||
| (2.7–19.8%) | (0–5.3%) | (5.9–25.6%) | (1.5–17.2%) | (0.2–14.1%) | (8.2–29.3%) | (80–97.1%) | |||||
| I. ricinus (n = 13) | 1 | 5 | 0 | 0 | 0 | 5 | 2 | 0 | 6 | 0 | 11 |
| (7.6%) | (38.4%) | (38.4%) | (15.3%) | (46.1%) | (84.6%) | ||||||
| (0–22%) | (12–64.8%) | (12–64.8%) | (0–34.8%) | (19–73.1%) | (64.9–100%) | ||||||
| Total (n = 235) | 16 | 16 | 1 | 27 | 11 | 5 | 2 | 16 | 42 | 6 | 148 |
| 6.8% | 6.8% | 0.4% | 11.5% | 4.6% | 2.1% | 0.8% | 6.8% | 17.8% | 2.5% | 62.9% | |
| (3.5–10%) | (3.5–10%) | (0–1.2%) | (7.4–15.5%) | (1.9–7.2%) | (0.2–3.9%) | (0–1.9%) | (3.5–10%) | (13–22.6%) | (0.5–4.4%) | (56.7–69%) |
| Genus | Species | Numbers | |
|---|---|---|---|
| Bacteria | Borrelia | B. burgdorferi senso stricto, B. garinii, B. afzelii, B. valaisiana, B. lusitaniae, B. spielmanii, B. bissettii, B. miyamotoi. | 8 |
| Anaplasma | A. marginale, A. platys, A. phagocytophilum, A.ovis, A. centrale, A. bovis. | 6 | |
| Ehrlichia | E. ruminantium, Neoehrlichia mikurensis. | 2 | |
| Rickettsia | R. conorii, R. slovaca, R. massiliae, R. prowazekii, R. aeschlimannii, R. andeanae, R. typhi, R. akari | 8 | |
| Bartonella | B. henselae | 1 | |
| Francisella | F. tularensis, Francisella-like endosymbionts. | 2 | |
| Coxiella | C. burnettii. | 1 | |
| Parasites | Babesia | B. microti, B. ovis, B. bigemina, B. bovis, B. caballi, B. divergens. | 6 |
| Theileria | T. mutans, T. velifera. | 2 | |
| Hepatozoon | Hepatozoon spp. | ||
| Total | 10 | 36 |
| Pathogen | Target Gene | Primers (F, R; 5′-3′) and Probe (P) | Length (bp) |
|---|---|---|---|
| Borrelia burgdorferi s.s. | rpoB | F-GCTTACTCACAAAAGGCGTCTT | 83 |
| R-GCACATCTCTTACTTCAAATCCT | |||
| P-AATGCTCTTGGACCAGGAGGACTTTCA | |||
| Borrelia garinii | rpoB | F-TGGCCGAACTTACCCACAAAA | 88 |
| R-ACATCTCTTACTTCAAATCCTGC | |||
| P-TCTATCTCTTGAAAGTCCCCCTGGTCC | |||
| Borrelia afzelii | fla | F-GGAGCAAATCAAGATGAAGCAAT | 116 |
| R-TGAGCACCCTCTTGAACAGG | |||
| P-TGCAGCCTGAGCAGCTTGAGCTCC | |||
| Borrelia valaisiana | ospA | F-ACTCACAAATGACAGATGCTGAA | 135 |
| R-GCTTGCTTAAAGTAACAGTACCT | |||
| P-TCCGCCTACAAGATTTCCTGGAAGCTT | |||
| Borrelia lusitaniae | rpoB | F-CGAACTTACTCATAAAAGGCGTC | 87 |
| R-TGGACGTCTCTTACTTCAAATCC | |||
| P-TTAATGCTCTCGGGCCTGGGGGACT | |||
| Borrelia spielmanii | fla | F-ATCTATTTTCTGGTGAGGGAGC | 71 |
| R-TCCTTCTTGTTGAGCACCTTC | |||
| P-TTGAACAGGCGCAGTCTGAGCAGCTT | |||
| Borrelia bissettii | rpoB | F-GCAACCAGTCAGCTTTCACAG | 118 |
| R-CAAATCCTGCCCTATCCCTTG | |||
| P-AAAGTCCTCCCGGCCCAAGAGCATTAA | |||
| Borrelia miyamotoi | glpQ | F-CACGACCCAGAAATTGACACA | 94 |
| R-GTGTGAAGTCAGTGGCGTAAT | |||
| P-TCGTCCGTTTTCTCTAGCTCGATTGGG | |||
| Borreliaspp. | 23S rRNA | F-GAGTCTTAAAAGGGCGATTTAGT | 73 |
| R-CTTCAGCCTGGCCATAAATAG | |||
| P-AGATGTGGTAGACCCGAAGCCGAGT | |||
| Anaplasma marginale | msp1 | F-CAGGCTTCAAGCGTACAGTG | 85 |
| R-GATATCTGTGCCTGGCCTTC | |||
| P-ATGAAAGCCTGGAGATGTTAGACCGAG | |||
| Anaplasma platys | groEL | F-TTCTGCCGATCCTTGAAAACG | 75 |
| R-CTTCTCCTTCTACATCCTCAG | |||
| P-TTGCTAGATCCGGCAGGCCTCTGC | |||
| Anaplasma phagocytophilum | msp2 | F-GCTATGGAAGGCAGTGTTGG | 77 |
| R-GTCTTGAAGCGCTCGTAACC | |||
| P-AATCTCAAGCTCAACCCTGGCACCAC | |||
| Anaplasma ovis | msp4 | F-TCATTCGACATGCGTGAGTCA | 92 |
| R-TTTGCTGGCGCACTCACATC | |||
| P-AGCAGAGAGACCTCGTATGTTAGAGGC | |||
| Anaplasma centrale | groEL | F-AGCTGCCCTGCTATACACG | 79 |
| R-GATGTTGATGCCCAATTGCTC | |||
| P-CTTGCATCTCTAGACGAGGTAAAGGGG | |||
| Anaplasma bovis | groEL | F-GGGAGATAGTACACATCCTTG | 73 |
| R-CTGATAGCTACAGTTAAGCCC | |||
| P-AGGTGCTGTTGGATGTACTGCTGGACC | |||
| Anaplasmaspp. | 16S rRNA | F-CTTAGGGTTGTAAAACTCTTTCAG | 160 |
| R-CTTTAACTTACCAAACCGCCTAC | |||
| P-ATGCCCTTTACGCCCAATAATTCCGAACA | |||
| Ehrlichiaspp. | 16S rRNA | F-GCAACGCGAAAAACCTTACCA | 98 |
| R-AGCCATGCAGCACCTGTGT | |||
| P-AAGGTCCAGCCAAACTGACTCTTCCG | |||
| Ehrlichia ruminantium | gltA | F-CCAGAAAACTGATGGTGAGTTAG | 116 |
| R-AGCCTACATCAGCTTGAATGAAG | |||
| P-AGTGTAAACTTGCTGTTGCTAAGGTAGCATG | |||
| Neoehrlichia mikurensis | groEL | F-AGAGACATCATTCGCATTTTGGA | 96 |
| R-TTCCGGTGTACCATAAGGCTT | |||
| P-AGATGCTGTTGGATGTACTGCTGGACC | |||
| Rickettsia conorii | 23S-5S ITS | F-CTCACAAAGTTATCAGGTTAAATAG | 118 |
| R-CGATACTCAGCAAAATAATTCTCG | |||
| P-CTGGATATCGTGGCAGGGCTACAGTAT | |||
| Rickettsia slovaca | 23S-5S ITS | F-GTATCTACTCACAAAGTTATCAGG | 138 |
| R-CTTAACTTTTACTACAATACTCAGC | |||
| P-TAATTTTCGCTGGATATCGTGGCAGGG | |||
| Rickettsia massiliae | 23S-5S ITS | F-GTTATTGCATCACTAATGTTATACTG | 128 |
| R-GTTAATGTTGTTGCACGACTCAA | |||
| P-TAGCCCCGCCACGATATCTAGCAAAAA | |||
| Rickettsia prowazekii | gltA | F-CAAGTATCGGTAAAGATGTAATCG | 151 |
| R-TATCCTCGATACCATAATATGCC | |||
| P-ATATAAGTAGGGTATCTGCGGAAGCCGAT | |||
| Rickettsia aeschlimannii | ITS | F-CTCACAAAGTTATCAGGTTAAATAG | 134 |
| R-CTTAACTTTTACTACGATACTTAGCA | |||
| P-TAATTTTTGCTGGATATCGTGGCGGGG | |||
| Rickettsiaandeanae | OmpB | F-GGCGGACAGGTAACTTTTGG | 165 |
| R-AAGGATCATAGTATCAGGAACTG | |||
| P- ACACATAGTTGACGTTGGTACAGACGGTAC | |||
| Rickettsiatyphi | OmpB | F-CAGGTCATGGTATTACTGCTCA | 133 |
| R-GCAGCAGTAAAGTCTATTGATCC | |||
| P-ACAAGCTGCTACTACAAAAAGTGCTCAAAATG | |||
| Rickettsiaakari | OmpB | F-GTGCTGTTGCAGGTGGTAC | 101 |
| R-TAAAGTAATACCGTGTAATGCAGC | |||
| P-ATTACCAGCACCGTTACCTATATCACCGG | |||
| Rickettsiaspp. | gltA | F-GTCGCAAATGTTCACGGTACTT | 78 |
| R-TCTTCGTGCATTTCTTTCCATTG | |||
| P-TGCAATAGCAAGAACCGTAGGCTGGATG | |||
| Bartonella henselae | pap31 | F-CCGCTGATCGCATTATGCCT | 107 |
| R-AGCGATTTCTGCATCATCTGCT | |||
| P-ATGTTGCTGGTGGTGTTTCCTATGCAC | |||
| Bartonellaspp. | ssrA | F-CGTTATCGGGCTAAATGAGTAG | 118 |
| R-ACCCCGCTTAAACCTGCGA | |||
| P-TTGCAAATGACAACTATGCGGAAGCACGTC | |||
| Francisella tularensis | tul4 | F-ACCCACAAGGAAGTGTAAGATTA | 76 |
| R-GTAATTGGGAAGCTTGTATCATG | |||
| P-AATGGCAGGCTCCAGAAGGTTCTAAGT | |||
| Francisella-like endosymbionts | fopA | F-GGCAAATCTAGCAGGTCAAGC | 91 |
| R-CAACACTTGCTTGAACATTTCTAG | |||
| P-AACAGGTGCTTGGGATGTGGGTGGTG | |||
| Coxiella burnettii | IS1111 | F-TGGAGGAGCGAACCATTGGT | 86 |
| R-CATACGGTTTGACGTGCTGC | |||
| P-ATCGGACGTTTATGGGGATGGGTATCC | |||
| Coxiella burnettii | idc | F-AGGCCCGTCCGTTATTTTACG | 74 |
| R-CGGAAAATCACCATATTCACCTT | |||
| P-TTCAGGCGTTTTGACCGGGCTTGGC | |||
| Babesia microti | CCTeta | F-ACAATGGATTTTCCCCAGCAAAA | 145 |
| R-GCGACATTTCGGCAACTTATATA | |||
| P-TACTCTGGTGCAATGAGCGTATGGGTA | |||
| Babesia ovis | 18SrRNA | F-TCTGTGATGCCCTTAGATGTC | 92 |
| R-GCTGGTTACCCGCGCCTT | |||
| P-TCGGAGCGGGGTCAACTCGATGCAT | |||
| Babesia bigemina | 18SrRNA | F-ATTCCGTTAACGAACGAGACC | 99 |
| R-TTCCCCCACGCTTGAAGCA | |||
| P-CAGGAGTCCCTCTAAGAAGCAAACGAG | |||
| Babesia bovis | CCTeta | F-GCCAAGTAGTGGTAGACTGTA | 100 |
| R-GCTCCGTCATTGGTTATGGTA | |||
| P-TAAAGACAACACTGGGTCCGCGTGG | |||
| Babesia caballi | Rap1 | F-GTTGTTCGGCTGGGGCATC | 94 |
| R-CAGGCGACTGACGCTGTGT | |||
| P-TCTGTCCCGATGTCAAGGGGCAGGT | |||
| Babesia divergens | hsp70 | F-CTCATTGGTGACGCCGCTA | 83 |
| R-CTCCTCCCGATAAGCCTCTT | |||
| P-AGAACCAGGAGGCCCGTAACCCAGA | |||
| Theileria mutans | ITS | F-CCTTATTAGGGGCTACCGTG | 119 |
| R-GTTTCAAATTTGAAGTAACCAAGTG | |||
| P-ATCCGTGAAAAACGTGCCAAACTGGTTAC | |||
| Theileria velifera | 18S rRNA | F-TGTGGCTTATCTGGGTTCGC | 151 |
| R-CCATTACTTTGGTACCTAAAACC | |||
| P-TTGCGTTCCCGGTGTTTTACTTTGAGAAAG | |||
| Theileriaspp. | 18S | F-TGAACGAGGAATGCCTAGTATG | 104 |
| R-CACCGGATCACTCGATCGG | |||
| P-TAGGAGCGACGGGCGGTGTGTAC | |||
| Hepatozoonspp. | 18S rRNA | F-ATTGGCTTACCGTGGCAGTG | 175 |
| R-AAAGCATTTTAACTGCCTTGTATTG | |||
| P-ACGGTTAACGGGGGATTAGGGTTCGAT | |||
| Tick species | 16SrRNA | F-AAATACTCTAGGGATAACAGCGT | 99 |
| R-TCTTCATCAAACAAGTATCCTAATC | |||
| P-CAACATCGAGGTCGCAAACCATTTTGTCTA | |||
| Rhipicephalussanguineus | ITS2 | F-TTGAACGCTACGGCAAAGCG | 110 |
| R-CCATCACCTCGGTGCAGTC | |||
| P-ACAAGGGCCGCTCGAAAGGCGAGA | |||
| Ixodes ricinus | ITS2 | F-CGAAACTCGATGGAGACCTG | 77 |
| R-ATCTCCAACGCACCGACGT | |||
| P-TTGTGGAAATCCCGTCGCACGTTGAAC | |||
| Escherichia coli | eae | F-CATTGATCAGGATTTTTCTGGTGATA | 102 |
| R-CTCATGCGGAAATAGCCGTTA | |||
| P-ATAGTCTCGCCAGTATTCGCCACCAATACC |
| Pathogen | Target Gene | Primer Name | Sequence (5′-3′) | Amplicon Size (bp) | T | Reference |
|---|---|---|---|---|---|---|
| Borreliaspp. | FlaB | FlaB280 F | GCAGTTCARTCAGGTAACGG | 645 | 55 | [50] |
| FlaL R | GCAATCATAGCCATTGCAGATTGT | |||||
| FlaB_737F | GCATCAACTGTRGTTGTAACATTAACAGG | |||||
| FlaLL R | ACATATTCAGATGCAGACAGAGGT | 407 | ||||
| Anaplasmaspp. | 16S rRNA | EHR1 F | GAACGAACGCTGGCGGCAAGC | 693 | 60 | [51] |
| EHR2 R | AGTA(T/C)CG(A/G)ACCAGATAGCCGC | |||||
| EHR3 F | TGCATAGGAATCTACCTAGTAG | |||||
| EHR2 R | AGTA(T/C)CG(A/G)ACCAGATAGCCGC | 629 | 55 | |||
| Rickettsiaspp. | gltA | Rsfg877 | GGG GGC CTG CTC ACG GCG G | 381 | 56 | [52] |
| Rsfg1258 | ATT GCA AAA AGT ACA GTG AAC A | |||||
| Bartonellaspp. | ftsZ | 257 F | GCCTTCAAGGAGTTGATTTTGTTGTTGCCA | 580 | 55 | [53] |
| 258 R | ACGACCCATTTCATGCATAACAGAAC | |||||
| Babesia/ Theileria /Hepatozoonspp. | 18S rRNA | BTH 18S 1st F | GTGAAACTGCGAATGGCTCATTAC | 1500 | 58 | [54] |
| BTH 18S 1st R | AAGTGATAAGGTTCACAAAACTTCCC | |||||
| BTH 18S 2nd F | GGCTCATTACAACAGTTATAGTTTATTTG | |||||
| BTH 18S 2nd R | CGGTCCGAATAATTCACCGGAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boularias, G.; Azzag, N.; Galon, C.; Šimo, L.; Boulouis, H.-J.; Moutailler, S. High-Throughput Microfluidic Real-Time PCR for the Detection of Multiple Microorganisms in Ixodid Cattle Ticks in Northeast Algeria. Pathogens 2021, 10, 362. https://doi.org/10.3390/pathogens10030362
Boularias G, Azzag N, Galon C, Šimo L, Boulouis H-J, Moutailler S. High-Throughput Microfluidic Real-Time PCR for the Detection of Multiple Microorganisms in Ixodid Cattle Ticks in Northeast Algeria. Pathogens. 2021; 10(3):362. https://doi.org/10.3390/pathogens10030362
Chicago/Turabian StyleBoularias, Ghania, Naouelle Azzag, Clemence Galon, Ladislav Šimo, Henri-Jean Boulouis, and Sara Moutailler. 2021. "High-Throughput Microfluidic Real-Time PCR for the Detection of Multiple Microorganisms in Ixodid Cattle Ticks in Northeast Algeria" Pathogens 10, no. 3: 362. https://doi.org/10.3390/pathogens10030362
APA StyleBoularias, G., Azzag, N., Galon, C., Šimo, L., Boulouis, H.-J., & Moutailler, S. (2021). High-Throughput Microfluidic Real-Time PCR for the Detection of Multiple Microorganisms in Ixodid Cattle Ticks in Northeast Algeria. Pathogens, 10(3), 362. https://doi.org/10.3390/pathogens10030362








