Abstract
Recent global changes have led to an increase in the spread of ticks and tick-borne diseases (TBDs) affecting domestic ruminants and humans, with an annual loss of US $13.9–$18.7 billion. The current study determined the perception and practices of livestock farmers regarding tick infestation. A total of 112 livestock farms were surveyed in Punjab, Pakistan, among which animals from 42 (37.5%) farms were infested with ticks. Only 28.6% (n = 32) of the dairy farmers were consulting veterinarians for ticks control, while 86.7% (n = 97) of the respondents did not consider biosecurity measures in the control of tick transmission. Most of the respondents, 71.4% (n = 80), did not consider manual tick removal from their animals (i.e., by hand, followed by physically crushing) as a risky practice for spreading zoonotic diseases. Improper disposal of bottles of acaricides in the farm drainage was also observed, putting the environment and aquatic life at risk. These wrong practices may contribute to high disease burdens and economic losses, increasing the possibility of transmission of zoonotic TBDs and pollution of the environment. Therefore, an integrated One Health approach is required for the control of TBDs through environmentally friendly approaches.
1. Introduction
Pakistan is an agricultural hub, and domestic animals are the major contributor of Pakistan’s economy. Ectoparasites are major factors for decrease in farm animal production [1]. Among these ectoparasites, ticks are a major concern in the livestock sector of Pakistan. Ticks are hematophagous arachnid ectoparasites that feed on the blood of many animals, including humans [2]. Three key economically important tick families have been classified, namely Ixodidae, Argasidae, and Nuttalliellidae. The Ixodidae is comprised of 949 known species, Argasidae has 200, and Nuttalliellidae has only 1 species. Ticks suck blood from their host, which they locate by responding to cues associated with host odors, breath, body heat, and the vibration of the victim. Ticks are usually found on the udder, ear, groin region, and tails of cattle [3], where they can affect livestock directly by causing irritation and allergic reaction [4,5]. Ticks are also one of the most important biological disease vectors in the environments where they are found, posing a threat to both animal and human health alike.
Ticks cause negative impacts on human and animal health through infestation and transmission of a wide range of pathogens, including viral, bacterial, and protozoal diseases [5]. Ticks and the pathogens they transmit are a growing burden on human and animal health world-wide. To date, several studies from Pakistan reported that more than 80% of bovines were tick-infested with species of Hyalomma and Rhipicephalus [6,7,8,9], which transmit tick-borne pathogens (TBPs) causing babesiosis, theileriosis, and anaplasmosis in ruminants and Crimean Congo haemorrhagic fever (CCHF) in humans [10,11,12,13,14,15]. Humans are infected by tick-borne diseases (TBDs) in many ways, such as via biting of ticks, and/or contact with blood or tissue of the infected animal. As ticks externally attached to their hosts, people involved in livestock handling, including slaughterhouse workers, veterinarians, laborers , laboratory workers, and milkmen, are at high risk of being infected [16,17]. Tick-borne diseases such as Lyme disease, rickettsiosis, CCHF, and tick-borne encephalitis are present in humans in all over the world, including Pakistan [18,19,20,21,22,23,24,25,26,27,28]. Of the approximately 949 identified tick species, circa 10% are vectors of TBPs, including agents of various infectious diseases having profound public health importance [5,13]. These ticks’ saliva may also cause skin lesions and systemic reactions in humans [29,30,31,32]. Several biological and chemical methods have been used to control ticks, but these have proved ineffective and unsatisfactory, mainly due to the development of acaricide resistance in many species [33,34], undesirable non-target toxicity [35,36,37], and the prohibitive costs of chemical tick control treatments [38]. Most of the studies conducted in Pakistan referred to the identification of the adult stages of ticks by a morphological (phenetic) method. However, species identification by morphological means can be challenging, particularly in diagnostic laboratories with limited entomological expertise [39,40]
Climate and environmental changes in Pakistan are likely to increase the tick abundance which will, in turn, increase the risk of human exposure to these arthropods and the incidence of human infections with TBPs [41,42,43,44,45,46]. Tick control in Pakistan is also challenging particularly due to import of exotic breeds of cattle, which is more prone to tick infestation under Pakistan’s climatic conditions [47], and the ability of ticks to quickly spread over large areas through feeding on migrating hosts, coupled with their ability to readily adapt to new habitat conditions [48,49].
Despite the above, few studies have been conducted and published on people’s knowledge, attitude, and practices regarding tick and TBDs, with even fewer from Pakistan, where TBDs are causing devastating economic losses [50,51,52,53,54,55,56]. With this in mind, the current study was designed to evaluate livestock owner’s knowledge, attitude, and practices towards ticks in Punjab, Pakistan, to assess the awareness about ticks and TBDs, zoonotic concerns, and need for a One Health approach towards tick control in this region.
2. Results
2.1. Prevalence of Ticks
We calculated herd prevalence of tick infestation with the criteria of minimum five tick infested animals on the farm. Based on that criteria, 42 (37.5%) farms out of 112 overall were found to be positive for tick infestation. The highest tick prevalence observed was in the Sheikhpura and Vehari districts (50%), followed by Kasur and Muzaffargarh (43.7%). In comparison, the lowest prevalence was seen in Khushab (12.5%), followed by the Gujranwala and Bahawalnagar districts (31.3%) (Figure 1).
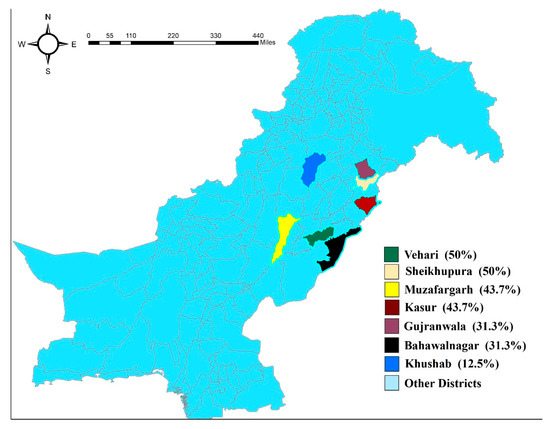
Figure 1.
Map to show the location of study regions in Punjab, Pakistan. District-wise herd-based prevalence of tick infestation is also shown.
2.2. Production Characteristics and Perceptions about Tick Infestation
Fifty-three (47.3%) farmers kept their animals on the farm, while 59 (52.7%) kept them in their homes. Of the 112 farms surveyed, 29 (25.9%) were located near marshy areas. Twenty-eight (25%) farmers used stall feeding method, while 24 (21.4%) were practicing grazing, however 60 (53.6%) farmers adopted both the feeding strategies. Regarding ticks and TBDs, 64 (57.1%) farmers were aware of the risk factors associated with tick infestation in animals, while 53 livestock owners (47.3%) had knowledge of TBDs and recognized sandy floors as a risk factor for tick presence. A large percentage of the respondents (40.2%; i.e., 45 farmers) considered summer as the most prevalent season for tick infestation, but other seasons were also noted. Regarding tick control measures, 39 farms (34.8%) used herbal-based or traditional treatments, 24 (21.4%) ignored the problem, and only 32 (28.6%) consulted a veterinarian. Seventeen respondents (15.2%) reported that they sold infested animals as a means of controlling tick numbers on their own farms. The average cost of the acaricide per animal per year was reported among 104 respondents as PKR (Pakistani rupees) 4535 (28.87 USD, on 13 March 2021) with the range of 1000–9500 PKR, while 8 respondents never used acaracides. While average frequency of using acaricides on farm per year among 104 respondents was four with the range of 1–11 (Table 1).

Table 1.
Survey questions and frequencies of responses provided by the livestock farmers.
2.3. Practices of Acaricidal Use
In our study, 51 (45.5%) livestock owners were using acaricides regularly, and 49 (43.8%) had no proper disposal procedures in place for used acaricidal bottles and unused products, instead putting these into general waste streams, including farm drainage systems. Thirty-four (30.4%) livestock owners did not use any acaricides in the year before our visit, but they used them before that. With respect to methods of application, 26 (23.2%) farmers used acaricide systemically, while 34 (30.4%) used topical applications for tick control. Forty-three respondents (38.3%) reported the reinfestation of ticks 30 to 60 days after the use of acaricides. Only 15 (13.4%) respondents considered biosecurity measures as a preventive measure against tick infestation (Table 1).
2.4. Zoonotic Perspective
Almost three-quarters of the farmers (71.4%; i.e., 80 respondents) included in this survey practiced manual removal of ticks (bare-handed); out of which 40 (35%) reported tick-bites, where only 32 (28.6%) consulted a physician while 24 (21.4%) opted self-medication. Interestingly, 46 farmers (41.1%) ignored the tick bite, with 27 (24.1%) experiencing restlessness and/or fever-like symptoms, which affected their daily work schedule (Table 1).
2.5. Risk Factors Associated with Tick Prevalence
A Chi-square test was performed to check the association between potential risk factors and tick presence. Significant associations were found between ticks and livestock feeding methods (χ2(1) = 112.49, p = 0.002), ticks and ignoring their presence (χ2(1) = 17.00, p = 0.001), and proper plan of acaricidal (using acaricides at least with three month intervals) use and absence of ticks on the animals (χ2(1) = 12.09, p = 0.001). There was also a statistically significant association between farms using acaricides more than one year ago with the presence of ticks (χ2(1) = 15.80, p = 0.007), and workers with tick bites (χ2(1) = 4.14, p = 0.042). Similarly, a statistically significant association was found between tick bites and ticks being removed barehanded (χ2(1) = 8.18, p = 0.042). Farmers having above a 10th-grade education had sufficient knowledge about tick-borne diseases as a zoonotic risk (χ2(1) = 41.10, p < 0.001) and were aware that sandy floor was a risk factor for tick presence (χ2(1) = 15.01, p < 0.001) (Table 2).

Table 2.
Chi-square analysis of associated risk factors.
For the quantitative variable analysis, a bivariate correlation test was used to check the correlation between the cost of acaricides per animal per year, which was found very weak but negatively correlated (r(1) = −0.363) with the number of tick-infested animals and statistically significant (p < 0.001) (Figure 2). The frequency of using acaricides on farm was found strong but negatively correlated (r(1) = −0.786) with number of tick infested animals on farm and statistically significant (p < 0.001) (Figure 3). A binary logistic regression indicated that marshy areas near the farms (OR = 5.29, p = 0.001) and dogs infested with ticks (OR = 2.738, p = 0.022) were significant predictors for tick presence on the livestock farms. In addition, keeping animals together in herds (OR = 26.085, p <0.001) and on sandy floors (OR = 10.57, p = 0.001) were potential risk factors for tick presence on the farm. The Hosmer–Lemeshow test indicated value of alpha greater than 0.05, which was non-significant to assure the fitness of our logistic regression model (Table 3).
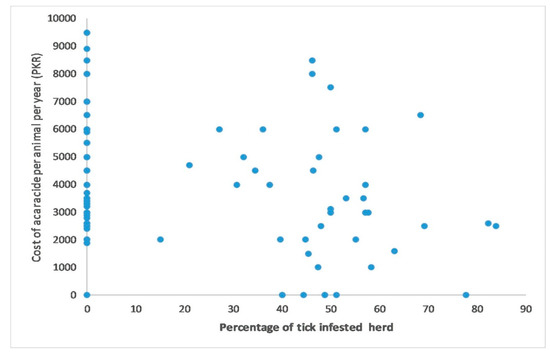
Figure 2.
Correlation between total cost (PKR) of acaracide per animal per year on farm and percentage of tick infested animals at the time of survey on farm.
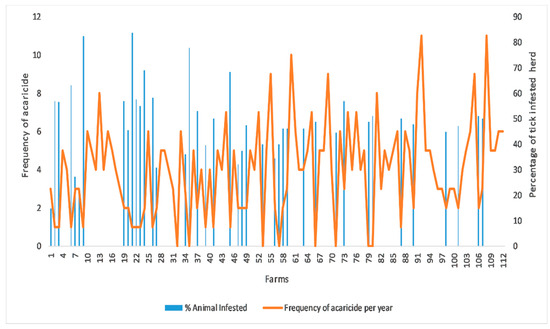
Figure 3.
Correlation between frequency of using acaricides on farms and percentage of tick infested animals at the time of survey on farm.

Table 3.
Binary logistic regression analysis of associated risk factors retained in the final model.
3. Discussion
Ticks and TBDs are among the major veterinary and public health problems world-wide, including Pakistan. In many developing countries, heavy tick infestation and TBDs cause morbidity and mortality in animals and are associated with decreased production of milk, meat, and other livestock products. Among blood-feeding arthropods, ticks transmit more diseases than any other species around the globe, affecting humans, livestock, and companion animals alike [57].
Work elsewhere has reported the high presence of Haemaphysalis cornupunctata and Ha. kashmirensis in Pakistan, alongside Hyalomma tick species, known vectors of Theileria annulata [57]. Economic losses due to ticks in this region rapidly escalate when tick prevalence increases, not only through losses to livestock productivity, but also as a result of expenses incurred for acaricide use [58]. Even with the use of acaricide, some farms suffered high tick infestation, which might be due to lack of awareness about the proper use of acaricides (such as diluting the acaricide to save money) and resistance of ticks to the products used [59]. Economic losses caused by ticks are not properly understood in our study region, due to the diversity of ixodid ticks in Pakistan and the lack of national studies focusing on estimation of economic losses attributed to tick infestation [60,61]. A few studies have tried to genetically characterize ticks in this region, but were imitated due to genetic markers (Cox1 and ITS-2) only being able to separate small numbers of tick specimens; further study would be important for those ticks being vectors of potential tick-borne pathogens needing further/different treatments [62]. Moreover, we found that farmers were not adopting biosecurity measures against tick infestation, which might be linked to continuous infestation with ticks, also contributing to acaricide failures in the long-term (by assisting resistance development or promoting reinfestations) and ultimately leading to large, temporally accrued economic losses, even in small herds.
In the current study, most of the survey respondents were fully reliant on livestock farming for their livelihoods. Most of the farms were heavily infested with ticks, which would have resulted in overall production declines. As there was a strong negative correlation between the frequency of using acaricides with percentage of tick-infested animals, we can consider the frequent and effective use of acaricides as a major reason for difference in the tick prevalence on different farms. The majority of the respondents were unaware of tick infestation as a source of disease transmission in their animals, and only a few knew to contact a qualified veterinarian on observing ticks on their animals. In our study, tick-infested farm dogs played a significant role in enhancing tick presence in livestock, which agrees with previous research elsewhere [63,64]. Similarly, use of herbal or traditional therapeutic approaches as control measures, the lack of proper knowledge on ticks, TBDs, and risk factors, the absence of consultation with a qualified veterinarian or a proper plan of acaricides use, potential promotion of ticks via climate change, sandy floors, and nearby marshy areas due to maximum retainability of moisture and possibility of cracks were possible contributing factors for infestation aligned with previous studies [65]. Farmers in this study reported the summer season as the most high-risk period for tick infestation, which is also consistent with another study conducted in two districts of Punjab; here it was found that the highest tick prevalence of 68.29% and 73.4% (in the Layyah and Muzaffargarh districts, respectively) occurred in July [66]. Our study results related to tick infestation in livestock herds were, however, slightly different (10% lower) from previous studies conducted in Pakistan [66]. We speculate that this might be due to sampling at different times. It is also noted that our results for tick prevalence do align well with another study conducted in Pakistan [67].
The risk of TBPs to humans varies with the tick density, human activities, and occurrence and frequencies of behaviors that cause exposure of people to host-seeking ticks. An improved understanding of human activities and behaviors, specifically in home-based livestock settings, may reduce exposure to tick bites and, ultimately, the risk of TBDs transmission to people [68]. According to a study conducted in Pakistan in 2009, R. microplus, R. annulatus, Hae. punctata, Hya. marginatum, and Hya. anatolicum were collected from humans (farmers and the general public) [69], highlighting the importance of better managing human-tick interactions in this region. The current study has reported several tick bites in home-based livestock settings, noting that most were simply ignored, which may represent an important public health concern for zoonotic transmission of TBDs. Lack of awareness about the zoonotic potential of TBPs likely facilitates not taking proper action to consult the physician for a possible diagnosis. Our results, and those of others, also show that home-based livestock settings, where people maintain a few animals for their livelihood (representing a major livestock sector in Pakistan), are at a greater risk of getting zoonotic diseases [70,71].
Environmental factors and climate change are expected to play a significant role in future patterns of ticks and TBDs, although their relationship has not been yet demonstrated in Pakistan. Ticks are more commonly found in the areas having prolonged extreme temperature ranges, heavy rainfall, and low humidity. Such increase in temperatures and changes in rainfall patterns are highly associated with climate change [71,72,73], which may in turn increase tick infestation where it favors hatching of eggs and tick development. Furthermore, rising temperature may lead to improved survival conditions for ticks and might accelerate their reproduction and lifecycle [74]. In our study, livestock farms located in the high-temperature zones showed notably high tick prevalence, which is aligned with results of a previous study conducted in several districts of Punjab and Canada [75,76,77].
Various acaricides (e.g., products containing Organophosphates such as Trichlorophon) have been used in Pakistan to control ectoparasites. The continuous use of these synthetic pesticides is likely to be restricted, however as their potentially detrimental impact on humans and the environment becomes better understood, as reported in Pakistan, India, and Australia [71,78,79,80], pest control approaches are increasingly assessed within an integrated ‘One Health’ framework. Many of these compounds have a wide range of toxicity levels, are chemically similar to other toxic compounds [81], and can enter milk and meat production chains in the form of residues, through various direct and indirect routes, causing concerns for human health [81]. In our study, livestock owners that were using acaricides to control ticks on their farms were potentially playing a significant role in environmental pollution, with direct exposure of some notably pesticide-sensitive habitats (e.g., aquatic ecosystems) likely to result from observed practices. Respondents had no proper disposal protocols for pesticide bottles, with any left-over acaricides being emptied into drains directed connected to streams and canals located near to the farm. Our results are in agreement with a previously reported study, where more dead organisms were observed in lakes, streams, rivers, and canals located near to farms where insecticides and pesticides were used and handled in an improper way. Hence, we strongly suggest that proper training is needed for the use of acaricides and, importantly, their disposal.
The current study has conclusively shown that most livestock owners in our study region(s) of Pakistan had little knowledge regarding ticks and TBDs and had not considered an integrated strategy against tick infestation in place. Many risk factors remained neglected on most of the tick-infested farms surveyed due to insufficient awareness, which led to high tick prevalence. Many livestock owners were not hesitant to crush the ticks by hand and commonly reported tick bites, which should be considered as a significant public health concern. Improper disposal of acaricides, including into farm drainage systems, was recorded, a practice that will almost certainly be placing the environment and aquatic life of these regions at risk. The gap in coordination and communication between livestock owners and the Livestock and Dairy Development Department (Extension wing) should be addressed to reduce the burden of TBDs affecting farmers’ economic status, as well as to protect Pakistan’s natural environment. This could potentially be achieved by accelerating the departmental campaigns to raise awareness of farmers about environmentally friendly approaches to preventing and treating tick infestations and promoting healthy and productive livestock. Collaborative research would further help in this area, bringing together epidemiologists, ecologists, and microbiologists to further develop One Health approaches to tick/TBDs management for Pakistan’s livestock farmers.
4. Materials and Methods
4.1. Study Area
This study was conducted in seven districts of Punjab, Pakistan, and selected districts are based on the dense population of livestock, markets of animals, and animal products like milk, meat, skin, and hides, in these areas. These districts cover 41,520 km2, encapsulating almost half of the ruminant population in Punjab. More specifically, our study areas included: Kasur (31.2° N and 74.5° E), part of the Northern irrigated plans where the climate is semi-arid to arid (east to the south-west), with maximum (summer) and minimum (winter) temperatures of 39.5 °C and 6.2 °C, respectively, and average annual rainfall of 300–500 mm in the east and 200–300 mm in the south-west [21]; Gujranwala (32.18° N, 74.19° E), part of the agro-ecological zone known as ‘Barani’ (rain-fed), its Southwest part is semi-arid and hot, the maximum temperature in summer is 38 °C and 4–7 °C in winter, mean monthly rainfall in summer is 85 mm and 30–45 mm in winter; Sheikhupura (31.7° N, 73.9° E), which is part of the Northern irrigated plans with a maximum (summer), and minimum (winter) [21] temperature is 39 °C and 5 °C, respectively, and an average rainfall of 635 mm; Khushab (32.3° N, 72.5° E), with maximum (summer) and minimum (winter) [21] temperatures of 42 °C and 12 °C, respectively, and average monthly rainfall of 45 mm (summer) and 10–25 mm (winter); Vehari (30.04° N, 72.34° E), with a maximum (summer) and minimum (winter) [21] temperature of 45 to 28 °C and 21 and 5 °C, respectively, and mean annual rainfall of 300–500 mm in the east and 200–300 mm in the south-west; Bahawalnagar (30.0° N, 72.24° E); and Muzaffargarh (30.07° N, 71.18° E), with maximum (summer) and minimum (winter) [21] temperatures of 54 °C and 1–5 °C, respectively, and an average rainfall of circa 127 mm.
4.2. Data Collection
Livestock owners with ruminant herds ranging from 10 to 50 animals were included in the study. Farms surveyed were selected based on operational convenience and willingness to participate. A total of 112 livestock owners were visited, and face-to-face interviews were conducted to collect the information required, based on a structured questionnaire that was organized and prepared in English (see Table 1). However, interviews were delivered in local languages (Urdu, Punjabi, Saraiki) after translation, to maximize the accuracy of responses and minimize any confusion concerning the terminology used.
In terms of livestock species covered by the survey, cattle breeds (Sahiwal, Friesian, Cross Friesian, Cholistani, Dajjal, Dhanni, and crossbred cattle), buffalo breeds (Nili Ravi, Kundi, and crossbred buffalo), sheep breeds (Kajli, Thali, Sipli, Lohi, and crossbred sheep), and goat breeds (Beetal, Makhi Cheeni, Rajan Puri, Teddi, Nachi, and crossbred goats) were all found in our studied districts.
4.3. Statistical Analysis
All data collected in the form of questionnaires were stored in an excel file (Microsoft Excel 2016). Later, the data were entered in the SPSS (Statistical Package for the Social Sciences) version 25.0. The data were checked and cleaned, and descriptive frequencies were calculated to know the knowledge and practices of livestock owners in selected districts of Punjab, Pakistan. Chi-square test was used to analyze the association of the tick infestation with all other possible risk factors asked and observed during data collection. A CSV was created and imported in open source R software version 3.2.3 and all variables for which maximum association was found in Chi-square test were considered for the logistic regression model to check the contribution of these variables towards tick infestation. The Hosmer–Lemeshow test was performed for the goodness of fit for logistic regression model.
Author Contributions
S.H., O.S. and A.H. conceived the study, conducted the questionnaire, data entry, performed the statistical analysis, drafted the manuscript, and revised manuscript. A.R., J.Z., J.H. and O.S. provided intellectual inputs, O.S., J.L. and D.G. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Olivier Sparagano is a Principal Investigator of an internal research fund of the Department of Infectious Diseases and Public Health of the City University of Hong Kong (Project number 9380108).
Institutional Review Board Statement
This survey-based study was conducted according to all relevant Animal Welfare Acts and to the guidelines of the Declaration of Helsinki and approved by the JCC Human Ethics Committee of the City University of Hong Kong (protocol code JCC2021ay004, 7 January 2021). The consent form was translated into the relevant local languages (Urdu, Punjabi, Saraiki). All participants and their attendees were briefed about the purpose of the research, interview, and questions; the voluntary participation; and the data anonymity of the study.
Informed Consent Statement
Informed verbal consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset generated for this study is available from the corresponding author upon request.
Acknowledgments
The authors thank all the livestock farmers for providing the required information and veterinarians for their assistance in data collection.
Conflicts of Interest
The authors declare that they have no competing interests and this study was conducted in the absence of any commercial or financial relationship.
References
- Ramzan, M.; Unsar, N.; Syed, H.; Ghulam, M.; Alamgir, A. Knowledge, attitude and practices of herdsmen about ticks and tick–borne diseases in district Multan. Pak. Entomol. 2018, 40, 13–18. [Google Scholar]
- Furman, D.P.; Loomis, E.C. The Ticks of California (Acari: Ixodida); University of California Press: Orlando, FL, USA, 1984. [Google Scholar]
- Kabir, M.; Mondal, M.; Eliyas, M.; Mannan, M.; Hashem, M.; Debnath, N.; Miazi, O.; Kashem, M.; Islam, M.; Elahi, M. An epidemiological survey on investigation of tick infestation in cattle at Chittagong District, Bangladesh. Afr. J. Microbiol. Res. 2011, 5, 346–352. [Google Scholar]
- Anderson, J.F.; Magnarelli, L.A. Biology of ticks. Infect. Dis. Clin. N. Am. 2008, 22, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitol. Camb. 2004, 129, S3. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.S.; Iqbal, Z.; Khan, M.N.; Muhammad, G. Point prevalence of hard ticks (Ixodids) infesting domestic ruminants of lower Punjab, Pakistan. Int. J. Agric. Biol. 2008, 10, 349–351. [Google Scholar]
- Iqbal, A.; Sajid, M.S.; Khan, M.N.; Khan, M.K. Frequency distribution of hard ticks (Acari: Ixodidae) infesting bubaline population of district Toba Tek Singh, Punjab, Pakistan. Parasitol. Res. 2013, 112, 535–541. [Google Scholar] [CrossRef]
- Rehman, A.; Nijhof, A.M.; Sauter-Louis, C.; Schauer, B.; Staubach, C.; Conraths, F.J. Distribution of ticks infesting ruminants and risk factors associated with high tick prevalence in livestock farms in the semi-arid and arid agro-ecological zones of Pakistan. Parasites Vectors 2017, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Ghafar, A.; Gasser, R.B.; Rashid, I.; Ghafoor, A.; Jabbar, A. Exploring the prevalence and diversity of bovine ticks in five agro-ecological zones of Pakistan using phenetic and genetic tools. Ticks Tick-Borne Dis. 2020, 11, 101472. [Google Scholar] [CrossRef]
- Jabbar, A.; Abbas, T.; Saddiqi, H.A.; Qamar, M.F.; Gasser, R.B. Tick-borne diseases of bovines in Pakistan: Major scope for future research and improved control. Parasites Vectors 2015, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Budachetri, K.; Mukherjee, N.; Williams, J.; Kausar, A.; Hassan, M.J.; Adamson, S.; Dowd, S.E.; Apanskevich, D.; Arijo, A. A study of ticks and tick-borne livestock pathogens in Pakistan. PLoS Negl. Trop. Dis. 2017, 11, e0005681. [Google Scholar] [CrossRef] [PubMed]
- Uilenberg, G. Veterinary Significance of Ticks and Tick-Borne Diseases, Tick Vector Biology; Springer: Berlin/Heidelberg, Germany, 1992; pp. 23–33. [Google Scholar]
- De la Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef]
- Uilenberg, G. General review of tick-borne diseases of sheep and goats world-wide. Parassitologia 1997, 39, 161–165. [Google Scholar] [PubMed]
- Ahmed, J.S.; Luo, J.; Schnittger, L.; Seitzer, U.; Jongejan, F.; Yin, H. Phylogenetic position of small-ruminant infecting piroplasms. Ann. N. Y. Acad. Sci. 2006, 1081, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.D.; Surtees, R.; Walter, C.T.; Ariza, A.; Bergeron, É.; Nichol, S.T.; Hiscox, J.A.; Edwards, T.A.; Barr, J.N. Structure, function, and evolution of the Crimean-Congo hemorrhagic fever virus nucleocapsid protein. J. Virol. 2012, 86, 10914–10923. [Google Scholar] [CrossRef]
- Charrel, R.N.; Attoui, H.; Butenko, A.; Clegg, J.; Deubel, V.; Frolova, T.; Gould, E.; Gritsun, T.; Heinz, F.; Labuda, M. Tick-borne virus diseases of human interest in Europe. Clin. Microbiol. Infect. 2004, 10, 1040–1055. [Google Scholar] [CrossRef]
- Kasi, K.K.; Sas, M.A.; Sauter-Louis, C.; von Arnim, F.; Gethmann, J.M.; Schulz, A.; Wernike, K.; Groschup, M.H.; Conraths, F.J. Epidemiological investigations of Crimean-Congo haemorrhagic fever virus infection in sheep and goats in Balochistan, Pakistan. Ticks Tick-Borne Dis. 2020, 11, 101324. [Google Scholar] [CrossRef] [PubMed]
- Kasi, K.K.; von Arnim, F.; Schulz, A.; Rehman, A.; Chudhary, A.; Oneeb, M.; Sas, M.A.; Jamil, T.; Maksimov, P.; Sauter-Louis, C. Crimean-Congo haemorrhagic fever virus in ticks collected from livestock in Balochistan, Pakistan. Transbound. Emerg. Dis. 2020, 67, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, P.; Labuda, M. Dynamics of infection in tick vectors and at the tick-host interface. Adv. Virus Res. 2003, 60, 233–272. [Google Scholar]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Benes, C.; Danielová, V.; Kriz, B. Sixty years of research of tick-borne encephalitis—A basis of the current knowledge of the epidemiological situation in Central Europe. Epidemiol. Mikrobiol. Imunol. Cas. Spol. Epidemiol. Mikrobiol. Ceske Lek. Spol. JE Purkyne 2011, 60, 135–155. [Google Scholar]
- Süss, J. Tick-borne encephalitis 2010: Epidemiology, risk areas, and virus strains in Europe and Asia—An overview. Ticks Tick-Borne Dis. 2011, 2, 2–15. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Papa, A. Crimean-Congo hemorrhagic fever: Epidemiological trends and controversies in treatment. BMC Med. 2011, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Amicizia, D.; Domnich, A.; Panatto, D.; Lai, P.L.; Cristina, M.L.; Avio, U.; Gasparini, R. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum. Vaccines Immunother. 2013, 9, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Petrulionienė, A.; Radzišauskienė, D.; Ambrozaitis, A.; Čaplinskas, S.; Paulauskas, A.; Venalis, A. Epidemiology of Lyme Disease in a Highly Endemic European Zone. Medicina 2020, 56, 115. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef]
- Moneret-Vautrin, D.A.; Beaudouin, E.; Kanny, G.; Guérin, L.; Roche, J.-F. Anaphylactic shock caused by ticks (Ixodes ricinus). J. Allergy Clin. Immunol. 1998, 101, 144. [Google Scholar] [PubMed]
- Fernández-Soto, P.; Davila, I.; Laffond, E.; Lorente, F.; Encinas-Grandes, A.; Pérez-Sánchez, R. Tick-bite-induced anaphylaxis in Spain. Ann. Trop. Med. Parasitol. 2001, 95, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Castelli, E.; Caputo, V.; Morello, V.; Tomasino, R.M. Local reactions to tick bites. Am. J. Dermatopathol. 2008, 30, 241–248. [Google Scholar] [CrossRef]
- Buczek, W.; Buczek, A.M.; Bartosik, K.; Buczek, A. Comparison of Skin Lesions Caused by Ixodes ricinus Ticks and Lipoptena cervi Deer Keds Infesting Humans in the Natural Environment. Int. J. Environ. Res. Public Health 2020, 17, 3316. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Pound, J.; Davey, R. Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology 2004, 129, S353–S366. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.Z.; Zaman, M.A.; Colwell, D.D.; Gilleard, J.; Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet. Parasitol. 2014, 203, 6–20. [Google Scholar] [CrossRef]
- Ray, D.E.; Ray, D.; Forshaw, P.J. Pyrethroid insecticides: Poisoning syndromes, synergies, and therapy. J. Toxicol. Clin. Toxicol. 2000, 38, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Litovitz, T.L.; Klein-Schwartz, W.; Rodgers, G.C.; Cobaugh, D.J.; Youniss, J.; Omslaer, J.C.; May, M.E.; Woolf, A.D.; Benson, B.E. 2001 Annual report of the American Association of Poison Control Centers toxic exposure surveillance system. Am. J. Emerg. Med. 2002, 20, 391–452. [Google Scholar] [CrossRef]
- Dahlgren, L.; Johnson, R.M.; Siegfried, B.D.; Ellis, M.D. Comparative toxicity of acaricides to honey bee (Hymenoptera: Apidae) workers and queens. J. Econ. Entomol. 2012, 105, 1895–1902. [Google Scholar] [CrossRef]
- De Meneghi, D.; Stachurski, F.; Adakal, H. Experiences in tick control by acaricide in the traditional cattle sector in Zambia and Burkina Faso: Possible environmental and public health implications. Front. Public Health 2016, 4, 239. [Google Scholar] [CrossRef] [PubMed]
- Caporale, D.A.; Rich, S.M.; Spielman, A.; Telford, S.R.; Kocher, T.D. Discriminating between Ixodes ticks by means of mitochondrial DNA sequences. Mol. Phylogenetics Evol. 1995, 4, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.R. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003. [Google Scholar]
- Parola, P.; Socolovschi, C.; Jeanjean, L.; Bitam, I.; Fournier, P.-E.; Sotto, A.; Labauge, P.; Raoult, D. Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS Negl. Trop. Dis. 2008, 2, e338. [Google Scholar] [CrossRef] [PubMed]
- Süss, J.; Klaus, C.; Gerstengarbe, F.W.; Werner, P.C. What makes ticks tick? Climate change, ticks, and tick-borne diseases. J. Travel Med. 2008, 15, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.; Jaenson, D.G.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasites Vectors 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Mysterud, A.; Stigum, V.M.; Seland, I.V.; Herland, A.; Easterday, W.R.; Jore, S.; Østerås, O.; Viljugrein, H. Tick abundance, pathogen prevalence, and disease incidence in two contrasting regions at the northern distribution range of Europe. Parasites Vectors 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Jahfari, S.; Hofhuis, A.; Fonville, M.; van der Giessen, J.; van Pelt, W.; Sprong, H. Molecular detection of tick-borne pathogens in humans with tick bites and erythema migrans, in the Netherlands. PLoS Negl. Trop. Dis. 2016, 10, e0005042. [Google Scholar] [CrossRef] [PubMed]
- Černý, J.; Lynn, G.; Hrnková, J.; Golovchenko, M.; Rudenko, N.; Grubhoffer, L. Management Options for Ixodes ricinus-Associated Pathogens: A Review of Prevention Strategies. Int. J. Environ. Res. Public Health 2020, 17, 1830. [Google Scholar] [CrossRef] [PubMed]
- Buczek, A.; Bartosik, K.; Wisniowski, L.; Tomasiewicz, K. Changes in population abundance of adult Dermacentor reticulatus (Acari: Amblyommidae) in long-term investigations in eastern Poland. Ann. Agric. Environ. Med. 2013, 20, 269–272. [Google Scholar]
- Hasle, G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front. Cell. Infect. Microbiol. 2013, 3, 48. [Google Scholar] [CrossRef]
- Buczek, A.M.; Buczek, W.; Buczek, A.; Bartosik, K. The Potential Role of Migratory Birds in the Rapid Spread of Ticks and Tick-Borne Pathogens in the Changing Climatic and Environmental Conditions in Europe. Int. J. Environ. Res. Public Health 2020, 17, 2117. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, N.; Kakar, A.; Ghani, A.; Iqbal, A.; Achakzai, W.M.; Sadozai, S.; Shafiq, M.; Mengal, M.A. Ixodid ticks (Arachnida: Acari) prevalence associated with risk factors in the bovine host in District Quetta, Balochistan. Pak. J. Zool. 2017, 46, 2113–2121. [Google Scholar] [CrossRef]
- Ahmed, A.; Saqlain, M.; Tanveer, M.; Tahir, A.H.; Ud-Din, F.; Shinwari, M.I.; Khan, G.M.; Anwer, N. Knowledge, attitude and perceptions about Crimean Congo Haemorrhagic Fever (CCHF) among occupationally high-risk healthcare professionals of Pakistan. BMC Infect. Dis. 2021, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Kumar, R.; Ahmed, J.; Ghaffar, A.; Mureed, S. Knowledge, attitude and practice of Crimean-Congo hemorrhagic fever among rural population of Baluchistan, Pakistan. A public health nutritional assessment of elderly in Islamabad: A mixed method study. J. Public Health 2013, 3, 11–13. [Google Scholar]
- Shadick, N.A.; Daltroy, L.H.; Phillips, C.B.; Liang, U.S.; Liang, M.H. Determinants of tick-avoidance behaviors in an endemic area for Lyme disease. Am. J. Prev. Med. 1997, 13, 265–270. [Google Scholar] [CrossRef]
- Riccò, M.; Gualerzi, G.; Ranzieri, S.; Ferraro, P.; Bragazzi, N.L. Knowledge, attitudes, practices (KAP) of Italian occupational physicians towards tick borne encephalitis. Trop. Med. Infect. Dis. 2020, 5, 117. [Google Scholar] [CrossRef]
- Noden, B.H.; Garner, K.D.; Lalman, D.; Talley, J.L. Knowledge, Attitudes, and Practices Regarding Ticks, Tick-Borne Pathogens, and Tick Prevention among Beef Producers in Oklahoma. Southwest. Entomol. 2020, 45, 341–350. [Google Scholar] [CrossRef]
- Buczek, A.; Pilch, J.; Buczek, W. Tick preventive behaviors and practices adopted by medical students from Poland, Germany, and Thailand in relation to socio-demographic conditions and their knowledge of ticks and tick-borne diseases. Insects 2020, 11, 863. [Google Scholar] [CrossRef]
- Hoogstraal, H.; Varma, M. Haemaphysalis cornupunctata sp. n. and H. kashmirensis sp. n. from Kashmir, with Notes on H. sundrai Sharif and H. sewelli Sharif of India and Pakistan (Ixodoidea, Ixodidae). J. Parasitol. 1962, 48, 185–194. [Google Scholar] [CrossRef]
- Batool, M.; Nasir, S.; Rafique, A.; Yousaf, I.; Yousaf, M. Prevalence of tick infestation in farm animals from Punjab, Pakistan. Pak. Vet. J. 2019, 39, 185–187. [Google Scholar]
- Ghosh, S.; Kumar, R.; Nagar, G.; Kumar, S.; Sharma, A.K.; Srivastava, A.; Kumar, S.; Kumar, K.A.; Saravanan, B. Survey of acaricides resistance status of Rhipiciphalus (Boophilus) microplus collected from selected places of Bihar, an eastern state of India. Ticks Tick-Borne Dis. 2015, 6, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.C.; Estrada-Peña, A.; Krücken, J.; Rehman, A.; Nijhof, A.M. Morphological and phylogenetic analyses of Rhipicephalus microplus ticks from Bangladesh, Pakistan and Myanmar. Ticks Tick-Borne Dis. 2018, 9, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Sands, A.F.; Apanaskevich, D.A.; Matthee, S.; Horak, I.G.; Harrison, A.; Karim, S.; Mohammad, M.K.; Mumcuoglu, K.Y.; Rajakaruna, R.S.; Santos-Silva, M.M. Effects of tectonics and large scale climatic changes on the evolutionary history of Hyalomma ticks. Mol. Phylogenet. Evol. 2017, 114, 153–165. [Google Scholar] [CrossRef]
- Irshad, N.; Qayyum, M.; Hussain, M.; Khan, M.Q. Prevalence of tick infestation and theileriosis in sheep and goats. Pak. Vet. J. 2010, 30, 178–180. [Google Scholar]
- Cromley, E.K.; Cartter, M.L.; Mrozinski, R.D.; Ertel, S.-H. Residential setting as a risk factor for Lyme disease in a hyperendemic region. Am. J. Epidemiol. 1998, 147, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Broderick, T.F.; Malawista, S.E. Erythema chronicum migrans and lyme arthritis: Epidemiologic evidence for a tick vector1. Am. J. Epidemiol. 1978, 108, 312–321. [Google Scholar] [CrossRef]
- Riaz, M.; Ullah, M. Epidemiological survey on diversity and seasonal distribution of hard ticks in sheep and goats in Multan, Pakistan. J. Biodivers. Environ. Sci. 2017, 10, 50–60. [Google Scholar]
- Sajid, M.S.; Iqbal, Z.; Khan, M.N.; Muhammad, G.; Khan, M.K. Prevalence and associated risk factors for bovine tick infestation in two districts of lower Punjab, Pakistan. Prev. Vet. Med. 2009, 92, 386–391. [Google Scholar] [CrossRef]
- Ramzan, M.; Khan, M.S.; Avais, M.; Khan, J.A.; Pervez, K.; Shahzad, W. Prevalence of ecto parasites and comparative efficacy of different drugs against tick infestation in cattle. J. Anim. Pl. Sci. 2008, 18, 17–19. [Google Scholar]
- Nelson, C.A.; Saha, S.; Kugeler, K.J.; Delorey, M.J.; Shankar, M.B.; Hinckley, A.F.; Mead, P.S. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg. Infect. Dis. 2015, 21, 1625. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Khan, M.A.; Zahid, H.; Yaseen, P.M.; Khan, M.Q.; Nawab, J.; Rehman, Z.U.; Ateeq, M.; Khan, S.; Ibrahim, M. Seasonal Dynamics, Record of Ticks Infesting Humans, Wild and Domestic Animals and Molecular Phylogeny of Rhipicephalus microplus in Khyber Pakhtunkhwa Pakistan. Front. Physiol. 2019, 10, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Eisen, L.; Beard, C.B. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med Entomol. 2016, 53, 349–386. [Google Scholar] [CrossRef] [PubMed]
- Baker, J. Resistance to ixodicides by ticks in Africa south of the Equator with some thoughts on tick control in this area. In Proceedings of the Tick Borne Diseases and their Vectors, Proceedings of an International Conference, Innsbruck, Austria, 23–30 August 1978. [Google Scholar]
- Ashraf, S.; Parveen, A.; Asif, M.; Awais, M.M.; Khan, A.; Aktas, M.; Ozubek, S.; Alanazi, A.D.; Alyousif, M.S.; Iqbal, F. A Report on the Tick Burden, Molecular Detection and Phylogenetic Analysis of Anaplasma Marginale in the Blood Samples of Cattle Collected from District Layyah in Punjab (Pakistan). Curr. Microbiol. 2020, 78, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Warren, F.J.; Lemmen, D.S. Canada in a Changing Climate: Sector Perspectives on Impacts and Adaptation; Government of Canada: Ottawa, ON, Canada, 2014.
- Ogden, N.H.; Lindsay, L.R. Effects of climate and climate change on vectors and vector-borne diseases: Ticks are different. Trends Parasitol. 2016, 32, 646–656. [Google Scholar] [CrossRef]
- Atif, F.; Khan, M.; Iqbal, H.; Ali, Z.; Ullah, S. Prevalence of cattle tick infestation in three districts of the Punjab, Pakistan. Pak. J. Sci. 2012, 64, 49. [Google Scholar]
- Gasmi, S.; Bouchard, C.; Ogden, N.H.; Adam-Poupart, A.; Pelcat, Y.; Rees, E.E.; Milord, F.; Leighton, P.A.; Lindsay, R.L.; Koffi, J.K. Evidence for increasing densities and geographic ranges of tick species of public health significance other than Ixodes scapularis in Québec, Canada. PLoS ONE 2018, 13, e0201924. [Google Scholar] [CrossRef]
- Ogden, N.; Bigras-Poulin, M.; O’callaghan, C.; Barker, I.; Lindsay, L.; Maarouf, A.; Smoyer-Tomic, K.; Waltner-Toews, D.; Charron, D. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int. J. Parasitol. 2005, 35, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.S.; Iqbal, Z.; Khan, M.N.; Muhammad, G.; Needham, G.; Khan, M.K. Prevalence, associated determinants, and in vivo chemotherapeutic control of hard ticks (Acari: Ixodidae) infesting domestic goats (Capra hircus) of lower Punjab, Pakistan. Parasitol. Res. 2011, 108, 601–609. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Food and Agriculture Organization of the United Nations (FAO). Data Sheet on Pesticides No. 65: Bis (Tributyltin) Oxide; (VBC/PDS/DS/85.65); World Health Organization: Geneva, Switzerland, 1984. [Google Scholar]
- Roulston, W.; Wharton, R.; Schnitzerling, H.; Sutherst, R.; Sullivan, N. Mixtures of chlorphenamidine with other acaricides for the control of organophosphorus-resistant strains of cattle tick Boophilus microplus. Aust. Vet. J. 1971, 47, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Kunz, S.; Kemp, D. Insecticides and acaricides: Resistance and environmental impact. Rev. Sci. Tech. (Int. Off. Epizoot.) 1994, 13, 1249–1286. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).