Re-Introduction of Bovine Viral Diarrhea Virus in a Disease-Free Region: Impact on the Affected Cattle Herd and Diagnostic Implications
Abstract
1. Introduction
2. Results
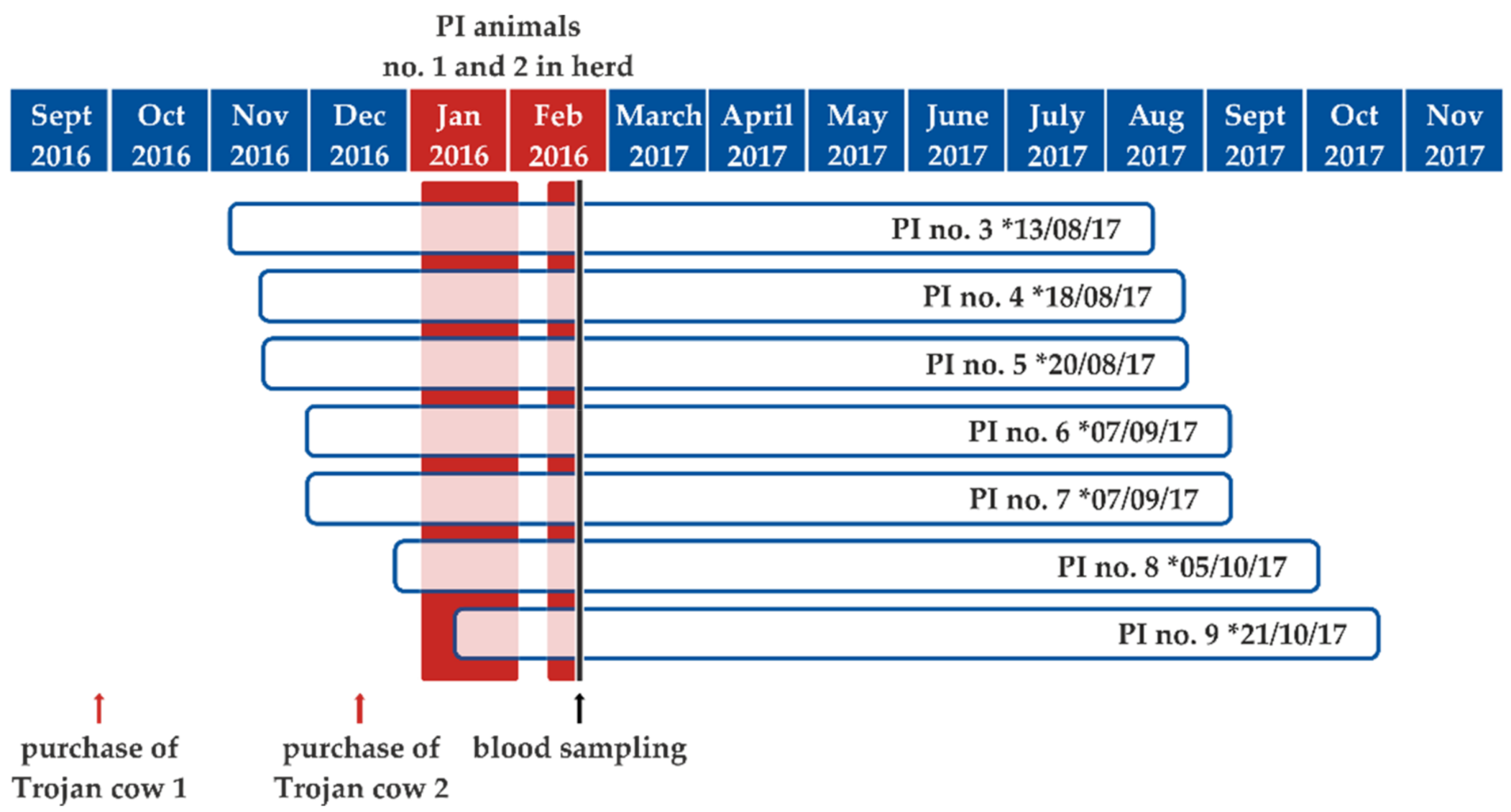
2.1. BVDV Dynamics and Clinical Impact of Virus Introduction into a Naïve Cattle Herd
2.2. Serological Investigation of the In-Contact Dams
3. Discussion
4. Materials and Methods
4.1. Cattle Holding and BVDV Introduction into the Study Herd
4.2. Pathological, Bacteriological, and Virological Investigation
4.3. Serology
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICTV. Genus: Pestivirus. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/positive-sense-rna-viruses/w/flaviviridae/361/genus-pestivirus 2019 (accessed on 3 January 2021).
- Houe, H. Economic impact of BVDV infection in dairies. Biologicals 2003, 31, 137–143. [Google Scholar] [CrossRef]
- Richter, V.; Lebl, K.; Baumgartner, W.; Obritzhauser, W.; Käsbohrer, A.; Pinior, B. A systematic worldwide review of the direct monetary losses in cattle due to bovine viral diarrhoea virus infection. Vet. J. 2017, 220, 80–87. [Google Scholar] [CrossRef]
- Lindberg, A.; Brownlie, J.; Gunn, G.J.; Houe, H.; Moennig, V.; Saatkamp, H.W.; Sandvik, T.; Valle, P.S. The control of bovine viral diarrhoea virus in Europe: Today and in the future. Rev. Sci. Tech. OIE 2006, 25, 961–979. [Google Scholar] [CrossRef]
- Pinior, B.; Garcia, S.; Minviel, J.J.; Raboisson, D. Epidemiological factors and mitigation measures influencing production losses in cattle due to bovine viral diarrhoea virus infection: A meta-analysis. Transbound. Emerg. Dis. 2019, 66, 2426–2439. [Google Scholar] [CrossRef]
- Santman-Berends, I.; Mars, M.; van Duijn, L.; van Schaik, G. Evaluation of the epidemiological and economic consequences of control scenarios for bovine viral diarrhea virus in dairy herds. J. Dairy Sci. 2015, 98, 7699–7716. [Google Scholar] [CrossRef]
- Lanyon, S.R.; Hill, F.I.; Reichel, M.P.; Brownlie, J. Bovine viral diarrhoea: Pathogenesis and diagnosis. Vet. J. 2014, 199, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.C. The Clinical Manifestations of Bovine Viral Diarrhea Infection. Vet. Clin. N. Am. Food Anim. Pr. 1995, 11, 425–445. [Google Scholar] [CrossRef]
- Brownlie, J. The pathogenesis of bovine virus diarrhoea virus infection. Rev. Sci. Tech. OIE 1990, 9, 43–59. [Google Scholar] [CrossRef]
- Brock, K.V. The persistence of bovine viral diarrhea virus. Biologicals 2003, 31, 133–135. [Google Scholar] [CrossRef]
- Ezanno, P.; Fourichon, C.; Seegers, H. Influence of herd structure and type of virus introduction on the spread of bovine viral diarrhoea virus (BVDV) on the spread of bovine viral diarrhoea virus (BVDV) within a dairy herd. Vet. Res. 2008, 39, 39. [Google Scholar] [CrossRef]
- Bitsch, V.; Hansen, K.E.; Ronsholt, L. Experiences from the Danish programme for eradication of bovine virus diarrhoea (BVD) 1994-1998 with special reference to legislation and causes of infection. Vet. Microbiol. 2000, 77, 137–143. [Google Scholar] [CrossRef]
- Moennig, V.; Becher, P. Control of bovine iral diarrhea. Pathogens 2018, 7, 29. [Google Scholar] [CrossRef]
- Akagami, M.; Seki, S.; Kashima, Y.; Yamashita, K.; Oya, S.; Fujii, Y.; Takayasu, M.; Yaguchi, Y.; Suzuki, A.; Ono, Y.; et al. Risk factors associated with the within-farm transmission of bovine viral diarrhea virus and the incidence of persistently infected cattle on dairy farms from Ibaraki prefecture of Japan. Res. Vet. Sci. 2020, 129, 187–192. [Google Scholar] [CrossRef]
- Wernike, K.; Gethmann, J.; Schirrmeier, H.; Schröder, R.; Conraths, F.J.; Beer, M. Six years (2011–2016) of mandatory nationwide bovine viral diarrhea control in Germany—A success story. Pathogens 2017, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Moennig, V.; Houe, H.; Lindberg, A. BVD control in Europe: Current status and perspectives. Anim. Health Res. Rev. 2005, 6, 63–74. [Google Scholar] [CrossRef]
- Ståhl, K.; Alenius, S. BVDV control and eradication in Europe—An update. Jpn. J. Vet. Res. 2012, 60, S31–S39. [Google Scholar] [PubMed]
- Moennig, V.; Becher, P. Pestivirus control programs: How far have we come and where are we going? Anim. Health Res. Rev. 2015, 16, 83–87. [Google Scholar] [CrossRef]
- Friedrich-Loeffler-Institut. Statistik zur BVD-Bekämpfung in Deutschland 2011–2019. Available online: https://www.fli.de/de/institute/institut-fuer-virusdiagnostik-ivd/referenzlabore/nrl-fuer-bvdmd/ (accessed on 27 November 2020).
- Bachofen, C.; Stalder, H.; Vogt, H.-R.; Wegmüller, M.; Schweizer, M.; Zanoni, R.; Peterhans, E. Bovine viral diarrhea (BVD): From biology to control. Berl. Munch. Tierarztl. Wochenschr. 2014, 126, 452–461. [Google Scholar]
- Booth, R.E.; Brownlie, J. Comparison of bulk milk antibody and youngstock serology screens for determining herd status for Bovine Viral Diarrhoea Virus. BMC Vet. Res. 2016, 12, 1–16. [Google Scholar] [CrossRef]
- Houe, H. Bovine virus diarrhoea virus: Detection of Danish dairy herds with persistently infected animals by means of a screening test of ten young stock. Prev. Vet. Med. 1994, 19, 241–248. [Google Scholar] [CrossRef]
- Pritchard, G. Milk antibody testing in cattle. Practice 2001, 23, 542–549. [Google Scholar] [CrossRef]
- Houe, H. Serological analysis of a small herd sample to predict presence or absence of animals persistently infected with bovine viral diarrhoea virus (BVDV) in dairy herds. Res. Vet. Sci. 1992, 53, 320–323. [Google Scholar]
- Valle, P.S.; Martin, S.W.; Skjerve, E. A Bayesian approach to estimating the performance of a bovine virus diarrhoea virus (BVDV) antibody ELISA bulk-tank milk test. Prev. Vet. Med. 2001, 50, 71–87. [Google Scholar] [CrossRef]
- Ridpath, J.F.; Fulton, R.W.; Kirkland, P.D.; Neill, J.D. Prevalence and antigenic differences observed between bovine viral diarrhea virus subgenotypes isolated from cattle in Australia and feedlots in the Southwestern United States. J. Vet. Diagn. Investig. 2010, 22, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Hanon, J.-B.; de Baere, M.; de la Ferté, C.; Roelandt, S.; van der Stede, Y.; Cay, B. Evaluation of 16 commercial antibody ELISAs for the detection of bovine viral diarrhea virus–specific antibodies in serum and milk using well-characterized sample panels. J. Vet. Diagn. Investig. 2017, 29, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Wernike, K.; Beer, M. Diagnostics in the context of an eradication program: Results of the German bovine viral diarrhea proficiency trial. Vet. Microbiol. 2019, 239, 108452. [Google Scholar] [CrossRef] [PubMed]
- Reardon, F.; Graham, D.A.; Clegg, T.A.; Tratalos, J.A.; O’Sullivan, P.; More, S.J. Quantifying the role of Trojan dams in the between-herd spread of bovine viral diarrhoea virus (BVDv) in Ireland. Prev. Vet. Med. 2018, 152, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Hanon, J.B.; de Baere, M.; de la Ferte, C.; Roelandt, S.; Guillot, G.; van der Stede, Y.; Cay, B. Serological monitoring on milk and serum samples in a BVD eradication program: A field study in Belgium showing antibody ELISA performances and epidemi-ological aspects. Prev. Vet. Med. 2018, 160, 136–144. [Google Scholar] [CrossRef]
- Makoschey, B.; Sonnemans, D.; Bielsa, J.M.; Franken, P.; Mars, M.; Santos, L.; Alvarez, M. Evaluation of the induction of NS3 specific BVDV antibodies using a commercial inactivated BVDV vaccine in immunization and challenge trials. Vaccine 2007, 25, 6140–6145. [Google Scholar] [CrossRef] [PubMed]
- Raue, R.; Harmeyer, S.S.; Nanjiani, I.A. Antibody responses to inactivated vaccines and natural infection in cattle using bovine viral diarrhoea virus ELISA kits: Assessment of potential to differentiate infected and vaccinated animals. Vet. J. 2011, 187, 330–334. [Google Scholar] [CrossRef]
- Hansen, T.R.; Smirnova, N.P.; Van Campen, H.; Shoemaker, M.L.; Ptitsyn, A.A.; Bielefeldt-Ohmann, H. Maternal and fetal response to fetal persistent infection with bovine viral diarrhea virus. Am. J. Reprod. Immunol. 2010, 64, 295–306. [Google Scholar] [CrossRef]
- Evans, C.A.; Pinior, B.; Larska, M.; Graham, D.; Schweizer, M.; Guidarini, C.; de Caro, N.; Ridpath, J.; Gates, M.C. Global knowledge gaps in the prevention and control of bovine viral diarrhoea (BVD) virus. Transbound. Emerg. Dis. 2019, 66, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Scharnböck, B.; Roch, F.-F.; Richter, V.; Funke, C.; Firth, C.L.; Obritzhauser, W.; Baumgartner, W.; Käsbohrer, A.; Pinior, B. A meta-analysis of bovine viral diarrhoea virus (BVDV) prevalences in the global cattle population. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.E.S.; Pelaquim, I.F.; Flores, E.F.; Massi, R.P.; Valdiviezo, M.J.J.; Pretto-Giordano, L.G.; Alfieri, A.A.; Saut, J.P.E.; Headley, S.A. Mycoplasma bovis and viral agents associated with the development of bovine respiratory disease in adult dairy cows. Transbound. Emerg. Dis. 2020, 67, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Hay, K.; Morton, J.; Clements, A.; Mahony, T.; Barnes, T. Population-level effects of risk factors for bovine respiratory disease in Australian feedlot cattle. Prev. Vet. Med. 2017, 140, 78–86. [Google Scholar] [CrossRef]
- Hay, K.; Barnes, T.; Morton, J.; Gravel, J.; Commins, M.; Horwood, P.; Ambrose, R.; Clements, A.; Mahony, T. Associations between exposure to viruses and bovine respiratory disease in Australian feedlot cattle. Prev. Vet. Med. 2016, 127, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Laureyns, J.; Pardon, B.; Letellier, C.; Deprez, P. Periparturient infection with bovine viral diarrhea virus type 1 causes hemorrhagic proctocolitis in a cow. Can. Vet. J. Rev. Vet. Can. 2011, 52, 1135–1139. [Google Scholar]
- Wernike, K.; Hoffmann, B.; Kalthoff, D.; König, P.; Beer, M. Development and validation of a triplex real-time PCR assay for the rapid detection and differentiation of wild-type and glycoprotein E-deleted vaccine strains of Bovine herpesvirus type 1. J. Virol. Methods 2011, 174, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Horwood, P.F.; Mahony, T.J. Multiplex real-time RT-PCR detection of three viruses associated with the bovine respiratory disease complex. J. Virol. Methods 2011, 171, 360–363. [Google Scholar] [CrossRef]
- Selim, A.; Gaede, W. Evaluation of reverse transcription-PCR protocols based on the fusion gene for diagnosis of bovine respiratory syncytial virus infections. Biotehnol. Stoc. 2013, 29, 53–64. [Google Scholar] [CrossRef]
- Ehricht, R.; Slickers, P.; Goellner, S.; Hotzel, H.; Sachse, K. Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Mol. Cell. Probes 2006, 20, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Wernike, K.; Schirrmeier, H.; Strebelow, H.-G.; Beer, M. Eradication of bovine viral diarrhea virus in Germany—Diversity of subtypes and detection of live-vaccine viruses. Vet. Microbiol. 2017, 208, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Ford, B.A.; Burnham, C.-A.D. Optimization of routine identification of clinically relevant Gram-negative bacteria by use of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and the Bruker Biotyper. J. Clin. Microbiol. 2013, 51, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Loeffler-Institut. Official Collection of Test Methods for Bovine Viral Diarrhea. (In German). Available online: https://openagrar.bmel-forschung.de/receive/openagrar_mods_00005312.2017/ (accessed on 14 January 2018).
| Sample Number | Neutralizing Titer | Commercial ELISA Kit | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | ||
| 165285100-36 | 1/113 | positive | positive | positive | positive | positive | positive | positive | positive |
| 165291382-46 | 1/14 | positive | negative | negative | negative | negative | negative | negative | negative |
| 165285930-52 | 1/14 | positive | doubtful | negative | positive | negative | negative | positive | negative |
| 165286867-59 | 1/143 | positive | positive | negative | positive | positive | negative | positive | positive |
| 165284904-62 | 1/18 | positive | negative | negative | negative | negative | negative | negative | negative |
| 165284894-64 | 1/227 | positive | positive | positive | positive | positive | positive | positive | positive |
| 165285390-97 | 1/57 | positive | positive | doubtful | positive | positive | negative | positive | doubtful |
| 165284012-98 | <1/5 | negative | negative | negative | negative | negative | negative | negative | negative |
| 165287229-114 | <1/5 | negative | negative | negative | negative | negative | negative | negative | negative |
| 165286100-115 | <1/5 | negative | negative | negative | negative | negative | negative | negative | negative |
| 165285846-116 | <1/5 | negative | negative | negative | negative | negative | negative | negative | negative |
| 165283770-117 | <1/5 | negative | negative | negative | negative | negative | negative | negative | negative |
| 165286969-120 | <1/5 | negative | negative | negative | negative | negative | negative | negative | positive |
| 165284481-123 | <1/5 | negative | negative | negative | negative | negative | negative | negative | doubtful |
| 165285579-137 | <1/5 | negative | negative | negative | negative | negative | negative | negative | doubtful |
| 165286082-142 | 1/22 | positive | positive | doubtful | positive | positive | negative | positive | doubtful |
| 165285832-147 | 1/11 | positive | positive | negative | negative | positive | negative | negative | negative |
| 165284512-163 | <1/5 | negative | doubtful | negative | negative | negative | negative | negative | negative |
| ∑ positive or doubtful | 9/18 (50.0%) | 9/18 (50.0%) | 8/18 (44.4%) | 4/18 (22.2%) | 6/18 (33.3%) | 6/18 (33.3%) | 2/18 (11.1%) | 6/18 (33.3%) | 8/18 (44.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albrecht, K.; Linder, M.; Heinrich, A.; Höche, J.; Beer, M.; Gaede, W.; Wernike, K. Re-Introduction of Bovine Viral Diarrhea Virus in a Disease-Free Region: Impact on the Affected Cattle Herd and Diagnostic Implications. Pathogens 2021, 10, 360. https://doi.org/10.3390/pathogens10030360
Albrecht K, Linder M, Heinrich A, Höche J, Beer M, Gaede W, Wernike K. Re-Introduction of Bovine Viral Diarrhea Virus in a Disease-Free Region: Impact on the Affected Cattle Herd and Diagnostic Implications. Pathogens. 2021; 10(3):360. https://doi.org/10.3390/pathogens10030360
Chicago/Turabian StyleAlbrecht, Kerstin, Miriam Linder, Anja Heinrich, Jennifer Höche, Martin Beer, Wolfgang Gaede, and Kerstin Wernike. 2021. "Re-Introduction of Bovine Viral Diarrhea Virus in a Disease-Free Region: Impact on the Affected Cattle Herd and Diagnostic Implications" Pathogens 10, no. 3: 360. https://doi.org/10.3390/pathogens10030360
APA StyleAlbrecht, K., Linder, M., Heinrich, A., Höche, J., Beer, M., Gaede, W., & Wernike, K. (2021). Re-Introduction of Bovine Viral Diarrhea Virus in a Disease-Free Region: Impact on the Affected Cattle Herd and Diagnostic Implications. Pathogens, 10(3), 360. https://doi.org/10.3390/pathogens10030360






