Exposure of Domestic Cats to Three Zoonotic Bartonella Species in the United States
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Serology
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Breitschwerdt, E.B. Bartonellosis: One Health Perspectives for an Emerging Infectious Disease. ILAR J. 2014, 55. [Google Scholar] [CrossRef]
- Regier, Y.; Órourke, F.; Kempf, V.A.J. Bartonella Spp.—A Chance to Establish One Health Concepts in Veterinary and Human Medicine. Parasites Vectors 2016. [Google Scholar] [CrossRef]
- Boulouis, H.-J.; Chao-chin, C.; Henn, J.B.; Kasten, R.W.; Chomel, B.B. Factors Associated with the Rapid Emergence of Zoonotic Bartonella Infections. Vet. Res. 2005, 36, 383–410. [Google Scholar] [CrossRef] [PubMed]
- Chomel, B.B.; Kasten, R.W.; Henn, J.B.; Molia, S. Bartonella Infection in Domestic Cats and Wild Felids. Ann. N. Y. Acad. Sci. 2006, 1078. [Google Scholar] [CrossRef]
- Jameson, P.; Greene, C.; Regnery, R.; Dryden, M.; Marks, A.; Brown, J.; Cooper, J.; Glaus, B.; Greene, R. Prevalence of Bartonella henselae Antibodies in Pet Cats throughout Regions of North America. J. Infect. Dis. 1995, 172. [Google Scholar] [CrossRef] [PubMed]
- Breitschwerdt, E.B.; Broadhurst, J.J.; Cherry, N.A. Bartonella henselae as a Cause of Acute-Onset Febrile Illness in Cats. J. Feline Med. Surg. Open Rep. 2015, 1. [Google Scholar] [CrossRef]
- Stützer, B.; Hartmann, K. Chronic Bartonellosis in Cats. J. Feline Med. Surg. 2012, 14. [Google Scholar] [CrossRef]
- Varanat, M.; Broadhurst, J.; Linder, K.E.; Maggi, R.G.; Breitschwerdt, E.B. Identification of Bartonella henselae in 2 Cats With Pyogranulomatous Myocarditis and Diaphragmatic Myositis. Vet. Pathol. 2012, 49. [Google Scholar] [CrossRef]
- Morelli, S.; Crisi, P.; Di Cesare, A.; De Santis, F.; Barlaam, A.; Santoprete, G.; Parrinello, C.; Palermo, S.; Mancini, P.; Traversa, D. Exposure Of Client-Owned Cats To Zoonotic Vector-Borne Pathogens: Clinic-Pathological Alterations And Infection Risk Analysis. Comp. Immunol. Microbiol. Infect. Dis. 2019, 66, 101344. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B. Feline Bartonellosis and Cat Scratch Disease. Vet. Immunol. Immunopathol. 2008. [Google Scholar] [CrossRef]
- Nelson, C.A.; Saha, S.; Mead, P.S. Cat-Scratch Disease in the United States, 2005–2013. Emerg. Infect. Dis. 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- Margileth, A.M.; Baehren, D.F. Chest-Wall Abscess Due to Cat-Scratch Disease (CSD) in an Adult with Antibodies to Bartonella clarridgeiae: Case Report and Review of the Thoracopulmonary Manifestations of CSD. Clin. Infect. Dis. 1998, 27. [Google Scholar] [CrossRef]
- Burzo, M.L.; Antonelli, M.; Pecorini, G.; Favuzzi, A.M.R.; Landolfi, R.; Flex, A. Fever of Unknown Origin and Splenomegaly: A Case Report of Blood Culture Negative Endocarditis. Medicine 2017, 96. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Maggi, R.G.; Lantos, P.M.; Woods, C.W.; Hegarty, B.C.; Bradley, J.M. Bartonella vinsonii subsp. berkhoffii and Bartonella henselae Bacteremia in a Father and Daughter with Neurological Disease. Parasites Vectors 2010, 3. [Google Scholar] [CrossRef]
- Celiker, H.; Kazokoglu, H.; Eraslan, M.; Cerman, E.; Karabas, L. Bartonella henselae Neuroretinitis in Patients without Cat Scratch. Jpn. J. Infect. Dis. 2018, 71. [Google Scholar] [CrossRef]
- Bieraugel, K.; Oehler, D.; Nesmith, M.; Chiovaro, J. Cat Got Your Spleen? Hepatosplenic Bartonella Infection. Am. J. Med. 2015. [Google Scholar] [CrossRef]
- Mirouse, G.; Journe, A.; Casabianca, L.; Moreau, P.E.; Pannier, S.; Glorion, C. Bartonella henselae Osteoarthritis of the Upper Cervical Spine in a 14-Year-Old Boy. Orthop. Traumatol. Surg. Res. 2015, 101. [Google Scholar] [CrossRef]
- Smith, R.A.; Scott, B.; Beverley, D.W.; Lyon, F.; Taylor, R. Encephalopathy with Retinitis Due to Cat-Scratch Disease. Dev. Med. Child Neurol. 2007, 49. [Google Scholar] [CrossRef] [PubMed]
- Shtaya, A.A.; Perek, S.; Kibari, A.; Cohen, S. Bartonella henselae Endocarditis: An Usual Presentation of an Unusual Disease. Eur. J. Case Rep. Intern. Med. 2019, 6. [Google Scholar] [CrossRef]
- Kordick, D.L.; Hilyard, E.J.; Hadfield, T.L.; Wilson, K.H.; Steigerwalt, A.G.; Brenner, D.J.; Breitschwerdt, E.B. Bartonella clarridgeiae, a Newly Recognized Zoonotic Pathogen Causing Inoculation Papules, Fever, and Lymphadenopathy (Cat Scratch Disease). J. Clin. Microbiol. 1997, 35. [Google Scholar] [CrossRef] [PubMed]
- Breitschwerdt, E.B.; Maggi, R.G.; Mozayeni, B.R.; Hegarty, B.C.; Bradley, J.M.; Mascarelli, P.E. PCR Amplification of Bartonella koehlerae from Human Blood and Enrichment Blood Cultures. Parasites Vectors 2010, 3. [Google Scholar] [CrossRef]
- Avidor, B.; Graidy, M.; Efrat, G.; Leibowitz, C.; Shapira, G.; Schattner, A.; Zimhony, O.; Giladi, M. Bartonella koehlerae, a New Cat-Associated Agent of Culture-Negative Human Endocarditis. J. Clin. Microbiol. 2004, 42. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Mascarelli, P.E.; Schweickert, L.A.; Maggi, R.G.; Hegarty, B.C.; Bradley, J.M.; Woods, C.W. Hallucinations, Sensory Neuropathy, and Peripheral Visual Deficits in a Young Woman Infected with Bartonella koehlerae. J. Clin. Microbiol. 2011, 49. [Google Scholar] [CrossRef]
- Vera, C.P.; Maggi, R.G.; Woods, C.W.; Mascarelli, P.E.; Breitschwerdt, E.B. Spontaneous Onset of Complex Regional Pain Syndrome Type i in a Woman Infected with Bartonella koehlerae. Med. Microbiol. Immunol. 2014, 203. [Google Scholar] [CrossRef]
- Guptill, L.; Wu, C.C.; HogenEsch, H.; Slater, L.N.; Glickman, N.; Dunham, A.; Syme, H.; Glickman, L. Prevalence, Risk Factors, and Genetic Diversity of Bartonella henselae Infections in Pet Cats in Four Regions of the United States. J. Clin. Microbiol. 2004, 42. [Google Scholar] [CrossRef] [PubMed]
- Case, J.B.; Chomel, B.; Nicholson, W.; Foley, J.E. Serological Survey of Vector-Borne Zoonotic Pathogens in Pet Cats and Cats from Animal Shelters and Feral Colonies. J. Feline Med. Surg. 2006, 8. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, D.A.; Chomel, B.B.; Kasten, R.W.; Stuckey, M.J.; Scarlet, J.; Liu, H.; Boulouis, H.J.; Haddad, N.; Pedersen, N.C. Bartonella Infection among Cats Adopted from a San Francisco Shelter, Revisited. Appl. Environ. Microbiol. 2015, 81. [Google Scholar] [CrossRef]
- Iredell, J.; Blanckenberg, D.; Arvand, M.; Grauling, S.; Feil, E.J.; Birtles, R.J. Characterization of the Natural Population of Bartonella henselae by Multilocus Sequence Typing. J. Clin. Microbiol. 2003, 41. [Google Scholar] [CrossRef]
- Zeaiter, Z.; Fournier, P.-E.; Raoult, D. Genomic Variation of Bartonella henselae Strains Detected in Lymph Nodes of Patients with Cat Scratch Disease. J. Clin. Microbiol. 2002, 40, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Lashnits, E.; Correa, M.; Hegarty, B.C.; Birkenheuer, A.; Breitschwerdt, E.B. Bartonella Seroepidemiology in Dogs from North America, 2008–2014. J. Vet. Intern. Med. 2018, 32. [Google Scholar] [CrossRef] [PubMed]
- Oteo, J.; Maggi, R.; Portillo, A.; Bradley, J.; García-Álvarez, L.; San-Martín, M.; Roura, X.; Breitschwerdt, E. Prevalence of Bartonella spp. by Culture, PCR and Serology, In Veterinary Personnel From Spain. Parasites Vectors 2017, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.; Morick, D.; Gross, I.; Winkler, R.; Abdeen, Z.; Harrus, S. Bartonellae in Domestic and Stray Cats From Israel: Comparison Of Bacterial Cultures And High-Resolution Melt Real-Time PCR As Diagnostic Methods. Vector Borne Zoonotic Dis. 2013, 13, 857–864. [Google Scholar] [CrossRef]
- Rust, M.K. The Biology and Ecology of Cat Fleas and Advancements in Their Pest Management: A Review. Insects 2017, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.; Rust, M.K.; Reierson, D.A. Influence of Temperature and Humidity on Survival and Development of the Cat Flea, Ctenocephalides felis (Siphonaptera: Pulicidae). J. Med. Entomol. 1981, 18, 78–83. [Google Scholar] [CrossRef]
- Lashnits, E.W.; Dawson, D.E.; Breitschwerdt, E.; Lanzas, C. Ecological and Socioeconomic Factors Associated with Bartonella henselae Exposure in Dogs Tested for Vector-Borne Diseases in North Carolina. Vector Borne Zoonotic Dis. 2019, 19. [Google Scholar] [CrossRef]
- Lashnits, E.; Neupane, P.; Maggi, R.G.; Linder, K.E.; Bradley, J.M.; Balakrishnan, N.; Southern, B.L.; McKeon, G.P.; Chandrashekar, R.; Breitschwerdt, E.B. Detection of Bartonella Spp. in Dogs after Infection with Rickettsia Rickettsii. J. Vet. Intern. Med. 2020, 34. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Chomel, B.B.; Kasten, R.W.; Hew, C.M.; Weber, D.K.; Lee, W.I.; Droz, S.; Koehler, J.E. Experimental Infection of Domestic Cats with Bartonella koehlerae and Comparison of Protein and DNA Profiles with Those of Other Bartonella Species Infecting Felines. J. Clin. Microbiol. 2002, 40. [Google Scholar] [CrossRef]
- Gurfield, A.N.; Boulouis, H.J.; Chomel, B.B.; Heller, R.; Kasten, R.W.; Yamamoto, K.; Piemont, Y. Coinfection with Bartonella clarridgeiae and Bartonella henselae and with Different Bartonella henselae Strains in Domestic Cats. J. Clin. Microbiol. 1997, 35. [Google Scholar] [CrossRef]
- Sander, A.; Posselt, M.; Oberle, K.; Bredt, W. Seroprevalence of Antibodies to Bartonella henselae in Patients with Cat Scratch Disease and in Healthy Controls: Evaluation and Comparison of Two Commercial Serological Tests. Clin. Diagn. Lab. Immunol. 1998, 5, 486–490. [Google Scholar] [CrossRef]
- Hegarty, B.C.; Bradley, J.M.; Lappin, M.R.; Balakrishnan, N.; Mascarelli, P.E.; Breitschwerdt, E.B. Analysis of Seroreactivity against Cell Culture-Derived Bartonella Spp. Antigens in Dogs. J. Vet. Intern. Med. 2014, 28. [Google Scholar] [CrossRef] [PubMed]
- Neupane, P.; Hegarty, B.C.; Marr, H.S.; Maggi, R.G.; Birkenheuer, A.J.; Breitschwerdt, E.B. Evaluation of Cell Culture-Grown Bartonella Antigens in Immunofluorescent Antibody Assays for the Serological Diagnosis of Bartonellosis in Dogs. J. Vet. Intern. Med. 2018, 32. [Google Scholar] [CrossRef]
- Lashnits, E.; Neupane, P.; Bradley, J.M.; Richardson, T.; Thomas, R.; Linder, K.E.; Breen, M.; Maggi, R.G.; Breitschwerdt, E.B. Molecular Prevalence of Bartonella, Babesia, and Hemotropic Mycoplasma Species in Dogs with Hemangiosarcoma from across the United States. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Lappin, M.R.; Davis, W.L.; Hawley, J.R.; Brewer, M.; Morris, A.; Stanneck, D. A Flea and Tick Collar Containing 10% Imidacloprid and 4.5% Flumethrin Prevents Flea Transmission of Bartonella henselae in Cats. Parasites Vectors 2013, 6. [Google Scholar] [CrossRef]
- Bradbury, C.A.; Lappin, M.R. Evaluation of Topical Application of 10% Imidacloprid-1% Moxidectin to Prevent Bartonella henselae Transmission from Cat Fleas. J. Am. Vet. Med. Assoc. 2010, 236. [Google Scholar] [CrossRef] [PubMed]
- Bouhsira, E.; Franc, M.; Lienard, E.; Bouillin, C.; Gandoin, C.; Geurden, T.; Becskei, C.; Jacquiet, P.; Thomas, A.; Boulouis, H.J. The Efficacy of a Selamectin (Stronghold®) Spot on Treatment in the Prevention of Bartonella henselae Transmission by Ctenocephalides felis in Cats, Using a New High-Challenge Model. Parasitol. Res. 2015, 114. [Google Scholar] [CrossRef] [PubMed]
- Dalton, M.J. Use of Bartonella Antigens for Serologic Diagnosis of Cat-Scratch Disease at a National Referral Center. Arch. Intern. Med. 1995, 155. [Google Scholar] [CrossRef]
- Bai, Y.; Osinubi, M.O.V.; Osikowicz, L.; McKee, C.; Vora, N.M.; Rizzo, M.R.; Recuenco, S.; Davis, L.; Niezgoda, M.; Ehimiyein, A.M.; et al. Human Exposure to Novel Bartonella Species from Contact with Fruit Bats. Emerg. Infect. Dis. 2018, 24. [Google Scholar] [CrossRef] [PubMed]
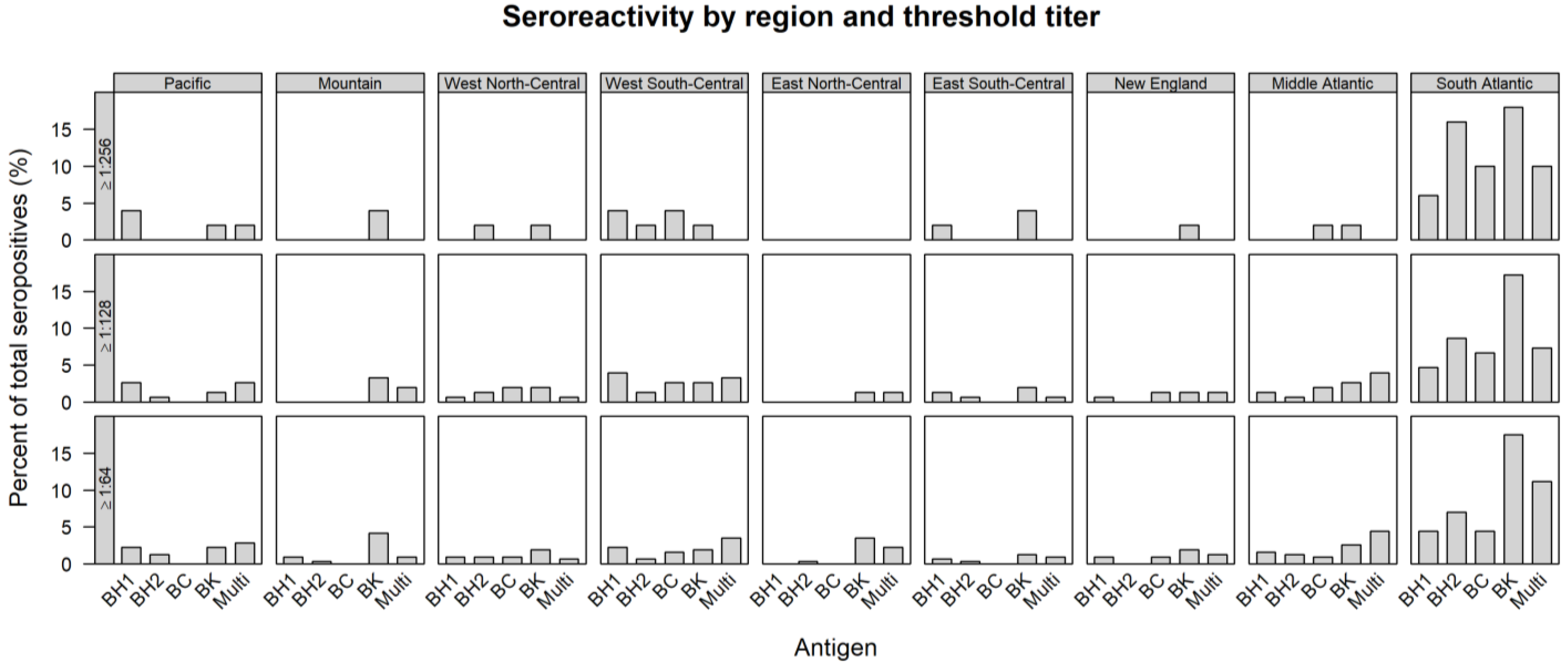
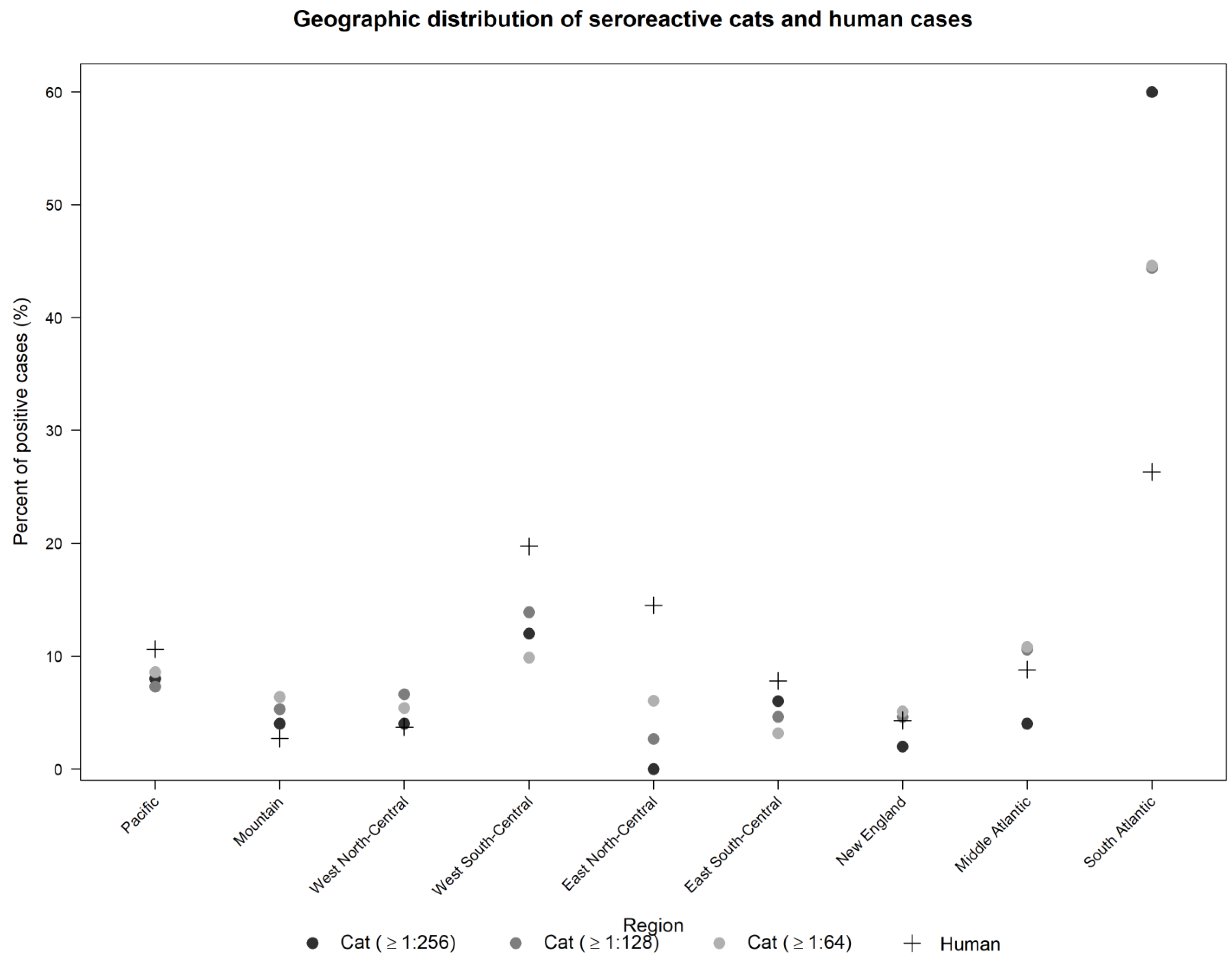
| REGION | TOTAL TESTED | ≥1:64 | ≥1:128 | ≥1:256 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B.h. 1 | B.h. 2 | B.c. | B.k. | Multi | B.h. 1 | B.h. 2 | B.c. | B.k. | Multi | B.h. 1 | B.h. 2 | B.c. | B.k. | Multi | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Pacific | 414 | 7 (1.7) | 4 (1.0) | 0 (0.0) | 7 (1.7) | 9 (2.2) | 4 (1.0) | 1 (0.2) | 0 (0.0) | 2 (0.5) | 4 (1.0) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.2) |
| Mountain | 100 | 3 (3.0) | 1 (1.0) | 0 (0.0) | 13 (13.0) | 3 (3.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (5.0) | 3 (3.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.0) | 0 (0.0) |
| West North-Central | 41 | 3 (7.3) | 3 (7.3) | 3 (7.3) | 6 (14.6) | 2 (4.9) | 1 (2.4) | 2 (4.9) | 3 (7.3) | 3 (7.3) | 1 (2.4) | 0 (0.0) | 1 (2.4) | 0 (0.0) | 1 (2.4) | 0 (0.0) |
| West South-Central | 87 | 7 (8.0) | 2 (2.3) | 5 (5.7) | 6 (6.9) | 11 (12.6) | 6 (6.9) | 2 (2.3) | 4 (4.6) | 4 (4.6) | 5 (5.7) | 2 (2.3) | 1 (1.1) | 2 (2.3) | 1 (1.1) | 0 (0.0) |
| East North-Central | 95 | 0 (0.0) | 1 (1.1) | 0 (0.0) | 11 (11.6) | 7 (7.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.1) | 2 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| East South-Central | 29 | 2 (6.9) | 1 (3.4) | 0 (0.0) | 4 (13.4) | 3 (10.3) | 2 (6.9) | 1 (3.4) | 0 (0.0) | 3 (10.3) | 1 (3.4) | 1 (3.4) | 0 (0.0) | 0 (0.0) | 2 (6.9) | 0 (0.0) |
| New England | 76 | 3 (3.9) | 0 (0.0) | 3 (3.9) | 6 (7.9) | 4 (5.3) | 1 (1.3) | 0 (0.0) | 2 (2.6) | 2 (2.6) | 2 (2.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.3) | 0 (0.0) |
| Middle Atlantic | 117 | 5 (4.3) | 4 (3.4) | 3 (2.6) | 8 (6.8) | 14 (12.0) | 2 (1.7) | 1 (0.9) | 3 (2.6) | 4 (3.4) | 6 (5.1) | 0 (0.0) | 0 (0.0) | 1 (0.9) | 1 (0.9) | 0 (0.0) |
| South Atlantic | 403 | 14 (3.5) | 22 (5.5) | 14 (3.5) | 55 (13.6) | 35 (8.7) | 7 (1.7) | 13 (3.2) | 10 (2.5) | 26 (6.5) | 11 (2.7) | 3 (0.7) | 8 (2.0) | 5 (1.2) | 9 (2.2) | 5 (1.2) |
| Total | 1362 | 44 (3.2) | 38 (2.8) | 28 (2.1) | 116 (8.5) | 88 (6.5) | 23 (1.7) | 20 (1.5) | 22 (1.6) | 51 (3.7) | 35 (2.6) | 8 (0.6) | 10 (0.7) | 8 (0.6) | 18 (1.3) | 6 (0.4) |
| Antigen | ≥1:64 | ≥1:128 | ≥1:256 | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| B.h. 1 | 44 | 14.0 | 23 | 15.2 | 8 | 16.0 |
| B.h. 2 | 38 | 12.1 | 20 | 13.2 | 10 | 20.0 |
| B.c. | 28 | 8.9 | 22 | 14.6 | 8 | 16.0 |
| B.k. | 116 | 36.9 | 51 | 33.8 | 18 | 36.0 |
| B.h. 1, B.h. 2 | 16 | 5.1 | 14 | 9.3 | 2 | 4.0 |
| B.h. 1, B.c. | 4 | 1.3 | 1 | 0.7 | 0 | 0 |
| B.h. 1, B.k. | 12 | 3.8 | 3 | 2.0 | 0 | 0 |
| B.h. 2, B.k. | 15 | 4.8 | 6 | 4.0 | 2 | 4.0 |
| B.h. 2, B.c. | 5 | 1.6 | 3 | 2.0 | 1 | 2.0 |
| B.c., B.k. | 7 | 2.2 | 2 | 1.3 | 1 | 2.0 |
| B.h. 1, B.h. 2, B.c. | 2 | 0.6 | 0 | 0 | 0 | 0 |
| B.h. 2, B.c., B.k. | 3 | 1.0 | 1 | 0.7 | 0 | 0 |
| B.h. 1, B.c., B.k. | 7 | 2.2 | 2 | 1.3 | 0 | 0 |
| B.h. 1, B.h. 2, B.k. | 15 | 4.8 | 1 | 0.7 | 0 | 0 |
| B.h. 1, B.h. 2, B.c., B.k. | 2 | 0.6 | 2 | 1.3 | 0 | 0 |
| Total | 314 | 151 | 50 | |||
| Antigen Reactivity | ≥1:64 | ≥1:128 | ≥1:256 |
|---|---|---|---|
| B.c. when infected with B.h. 1 and B.h. 2 | 0.07 (0.03–0.18) | ||
| B.k. when infected with B.h. 1 and B.h. 2 | 0.06 (0.03–0.12) | ||
| B.c. when infected with B.h. 1 and B.k | 0.56 (0.36–0.88) | ||
| B.c. when infected with B.h. 2 | 0.11 (0.07–0.16) | 0.09 (0.04–0.25) | |
| B.k. when infected with B.h. 1 | 0.31 (0.24–0.40) | 0.13 (0.11–0.17) | |
| B.k. when infected with B.h. 2 | 0.34 (0.26–0.44) | 0.28 (0.23–0.34) | 0.15 (0.09–0.25) |
| B.h. 2 when infected with B.h. 1 | 0.37 (0.33–0.42) | 0.56 (0.44–0.70) | 0.22 (0.14–0.33) |
| B.c. when infected with B.h. 1 | 0.11 (0.07–0.31) | 0.01 (0.01–0.23) | |
| B.c. when infected with B.k. | 0.07 (0.05–0.09) | 0.04 (0.03–0.05) |
| Life Stage | Approx. Age | Tested | ≥1:64 | ≥1:128 | ≥1:256 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % Life Stage | % Total Reactive | n | % Life Stage | % Total Reactive | n | % Life Stage | % Total Reactive | |||
| Young Cat | 0–2.9 y | 448 | 86 | 19.2 | 28.6 | 49 | 10.9 | 34.3 | 15 | 3.3 | 31.9 |
| Adult | 3–10.9 y | 575 | 142 | 24.7 | 47.2 | 59 | 10.3 | 41.3 | 21 | 3.7 | 44.7 |
| Senior | ≥11 y | 273 | 73 | 26.7 | 24.3 | 35 | 12.8 | 24.5 | 11 | 4.0 | 23.4 |
| Pacific | Mountain | West North-Central | West South-Central | East North-Central | East South-Central | New England | Middle Atlantic | South Atlantic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State | n | State | n | State | n | State | n | State | n | State | n | State | n | State | n | State | n |
| CA | 407 | AZ | 2 | IA | 7 | AR | 16 | IL | 28 | AL | 6 | CT | 9 | NJ | 2 | DC | 3 |
| WA | 7 | CO | 88 | KS | 14 | LA | 5 | IN | 7 | KY | 2 | MA | 64 | NY | 97 | DE | 1 |
| NM | 6 | MN | 2 | OK | 5 | MI | 22 | MS | 3 | NH | 3 | PA | 18 | FL | 47 | ||
| WY | 4 | MO | 17 | TX | 61 | OH | 31 | TN | 18 | GA | 7 | ||||||
| NE | 1 | WI | 7 | MD | 21 | ||||||||||||
| NC | 264 | ||||||||||||||||
| SC | 14 | ||||||||||||||||
| VA | 45 | ||||||||||||||||
| WV | 1 | ||||||||||||||||
| Total | 414 | Total | 100 | Total | 41 | Total | 87 | Total | 95 | Total | 29 | Total | 76 | Total | 117 | Total | 403 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osikowicz, L.M.; Horiuchi, K.; Goodrich, I.; Breitschwerdt, E.B.; Chomel, B.; Biggerstaff, B.J.; Kosoy, M. Exposure of Domestic Cats to Three Zoonotic Bartonella Species in the United States. Pathogens 2021, 10, 354. https://doi.org/10.3390/pathogens10030354
Osikowicz LM, Horiuchi K, Goodrich I, Breitschwerdt EB, Chomel B, Biggerstaff BJ, Kosoy M. Exposure of Domestic Cats to Three Zoonotic Bartonella Species in the United States. Pathogens. 2021; 10(3):354. https://doi.org/10.3390/pathogens10030354
Chicago/Turabian StyleOsikowicz, Lynn M., Kalanthe Horiuchi, Irina Goodrich, Edward B. Breitschwerdt, Bruno Chomel, Brad J. Biggerstaff, and Michael Kosoy. 2021. "Exposure of Domestic Cats to Three Zoonotic Bartonella Species in the United States" Pathogens 10, no. 3: 354. https://doi.org/10.3390/pathogens10030354
APA StyleOsikowicz, L. M., Horiuchi, K., Goodrich, I., Breitschwerdt, E. B., Chomel, B., Biggerstaff, B. J., & Kosoy, M. (2021). Exposure of Domestic Cats to Three Zoonotic Bartonella Species in the United States. Pathogens, 10(3), 354. https://doi.org/10.3390/pathogens10030354









