From Upper Respiratory Symptoms to Hemophagocytic Lymphohistiocytosis: Case Report of a Human Adenovirus Infection in Haploidentical Hematopoietic Stem Cell Transplant Recipient
Abstract
:1. Introduction
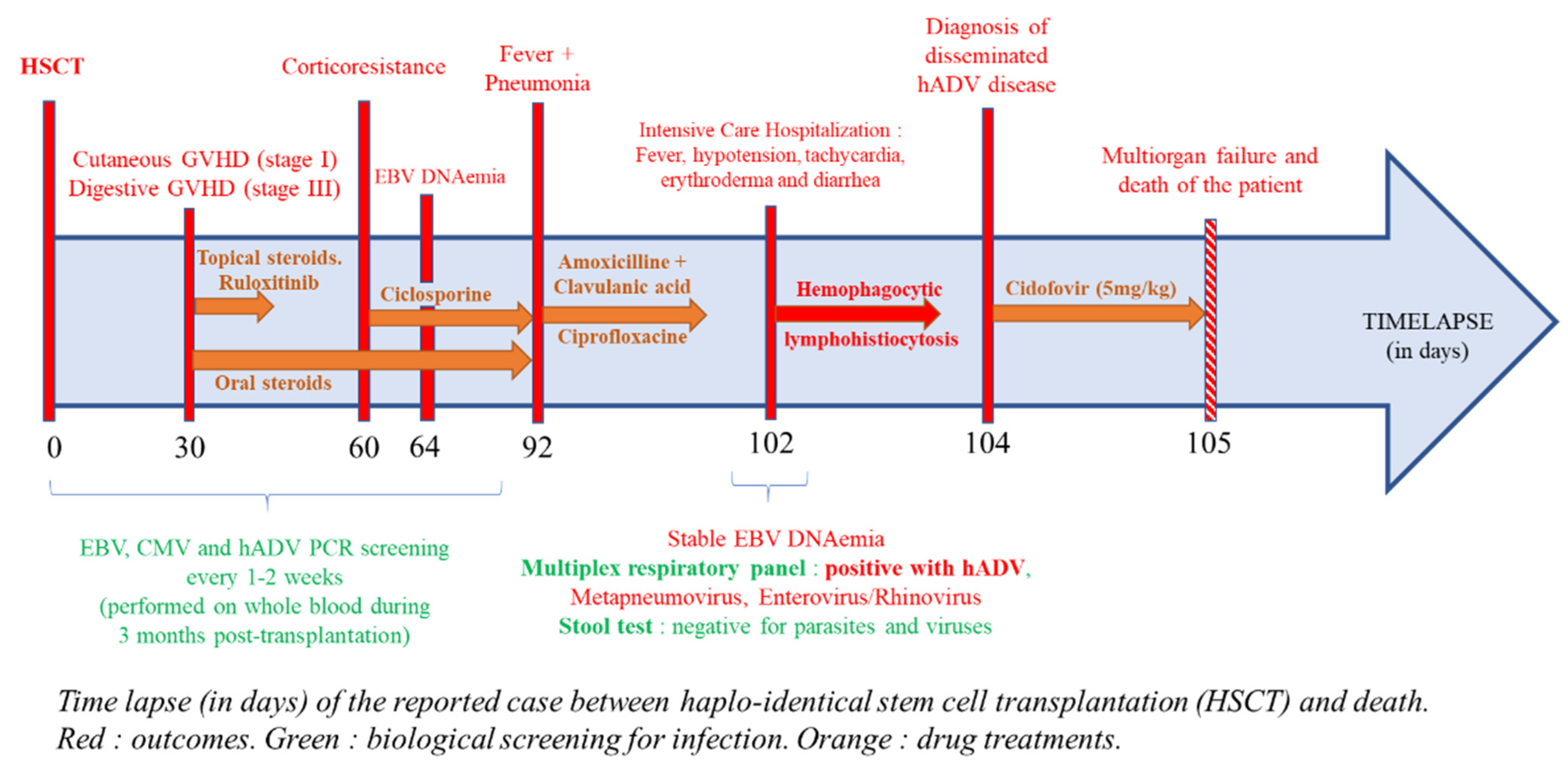
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sedláček, P.; Petterson, T.; Robin, M.; Sivaprakasam, P.; Vainorius, E.; Brundage, T.; Chandak, A.; Mozaffari, E.; Nichols, G.; Voigt, S. Incidence of Adenovirus Infection in Hematopoietic Stem Cell Transplantation Recipients: Findings from the AdVance Study. Biol. Blood Marrow Transplant. 2019, 25, 810–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lion, T. Adenovirus Infections in Immunocompetent and Immunocompromised Patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, K.; Yoshihara, S.; Tamaki, H.; Fujimoto, T.; Ikegame, K.; Kaida, K.; Nakata, J.; Inoue, T.; Kato, R.; Fujioka, T.; et al. Incidence and Treatment Strategy for Disseminated Adenovirus Disease after Haploidentical Stem Cell Transplantation. Ann. Hematol. 2012, 91, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Matthes-Martin, S.; Feuchtinger, T.; Shaw, P.J.; Engelhard, D.; Hirsch, H.H.; Cordonnier, C.; Ljungman, P. Fourth European Conference on Infections in Leukemia European Guidelines for Diagnosis and Treatment of Adenovirus Infection in Leukemia and Stem Cell Transplantation: Summary of ECIL-4 (2011). Transpl. Infect. Dis. 2012, 14, 555–563. [Google Scholar] [CrossRef] [PubMed]
- ECIL-8 European Conference on Infections in Leukaemia: Update on Community-Acquired Respiratory Viruses in Hematology Patients. Available online: http://www.ecil-leukaemia.com/resources.htm (accessed on 24 January 2020).
- Censoplano, N.; Gorga, S.; Waldeck, K.; Stillwell, T.; Rabah-Hammad, R.; Flori, H. Neonatal Adenovirus Infection Complicated by Hemophagocytic Lymphohistiocytosis Syndrome. Pediatrics 2018, 141, S475–S480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-I.; Yu, H.-H.; Lee, J.-H.; Wang, L.-C.; Lin, Y.-T.; Yang, Y.-H.; Chiang, B.-L. Clinical Analysis of Macrophage Activation Syndrome in Pediatric Patients with Autoimmune Diseases. Clin. Rheumatol. 2012, 31, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; Wodell, R.A.; August, C.S.; Bayever, E. Adenovirus-Related Hemophagocytic Syndrome after Bone Marrow Transplantation. Bone Marrow Transpl. 1990, 6, 349–352. [Google Scholar]
- Mellon, G.; Henry, B.; Aoun, O.; Boutolleau, D.; Laparra, A.; Mayaux, J.; Sanson, M.; Caumes, E. Adenovirus Related Lymphohistiocytic Hemophagocytosis: Case Report and Literature Review. J. Clin. Virol. 2016, 78, 53–56. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, A.M.; Champlin, R.E.; Mirza, N.; Gajewski, J.; Giralt, S.; Rolston, K.V.; Raad, I.; Jacobson, K.; Kontoyiannis, D.; Elting, L.; et al. Adenovirus Infections in Adult Recipients of Blood and Marrow Transplants. Clin. Infect. Dis. 2001, 32, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Echavarria, M. Adenoviruses in Immunocompromised Hosts. Clin. Microbiol. Rev. 2008, 21, 704–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lion, T.; Kosulin, K.; Landlinger, C.; Rauch, M.; Preuner, S.; Jugovic, D.; Pötschger, U.; Lawitschka, A.; Peters, C.; Fritsch, G.; et al. Monitoring of Adenovirus Load in Stool by Real-Time PCR Permits Early Detection of Impending Invasive Infection in Patients after Allogeneic Stem Cell Transplantation. Leukemia 2010, 24, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.L.; Everhart, K.; Daly, J.A.; Hopper, A.; Harrington, A.; Schreckenberger, P.; McKinley, K.; Jones, M.; Holmberg, K.; Kensinger, B. Multicenter Evaluation of BioFire FilmArray Respiratory Panel 2 for Detection of Viruses and Bacteria in Nasopharyngeal Swab Samples. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ison, M.G. Adenovirus Infections in Transplant Recipients. Clin. Infect. Dis. 2006, 43, 331–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demey, B.; Brault, C.; Maizel, J.; Francois, C. From Upper Respiratory Symptoms to Hemophagocytic Lymphohistiocytosis: Case Report of a Human Adenovirus Infection in Haploidentical Hematopoietic Stem Cell Transplant Recipient. Pathogens 2021, 10, 340. https://doi.org/10.3390/pathogens10030340
Demey B, Brault C, Maizel J, Francois C. From Upper Respiratory Symptoms to Hemophagocytic Lymphohistiocytosis: Case Report of a Human Adenovirus Infection in Haploidentical Hematopoietic Stem Cell Transplant Recipient. Pathogens. 2021; 10(3):340. https://doi.org/10.3390/pathogens10030340
Chicago/Turabian StyleDemey, Baptiste, Clément Brault, Julien Maizel, and Catherine Francois. 2021. "From Upper Respiratory Symptoms to Hemophagocytic Lymphohistiocytosis: Case Report of a Human Adenovirus Infection in Haploidentical Hematopoietic Stem Cell Transplant Recipient" Pathogens 10, no. 3: 340. https://doi.org/10.3390/pathogens10030340
APA StyleDemey, B., Brault, C., Maizel, J., & Francois, C. (2021). From Upper Respiratory Symptoms to Hemophagocytic Lymphohistiocytosis: Case Report of a Human Adenovirus Infection in Haploidentical Hematopoietic Stem Cell Transplant Recipient. Pathogens, 10(3), 340. https://doi.org/10.3390/pathogens10030340




