Beneficial Immunomodulatory Effects of Fluticasone Propionate in Chlamydia pneumoniae-Infected Mice
Abstract
1. Introduction
2. Results
2.1. FP Suppressed C. pneumoniae Replication in A549 Cells
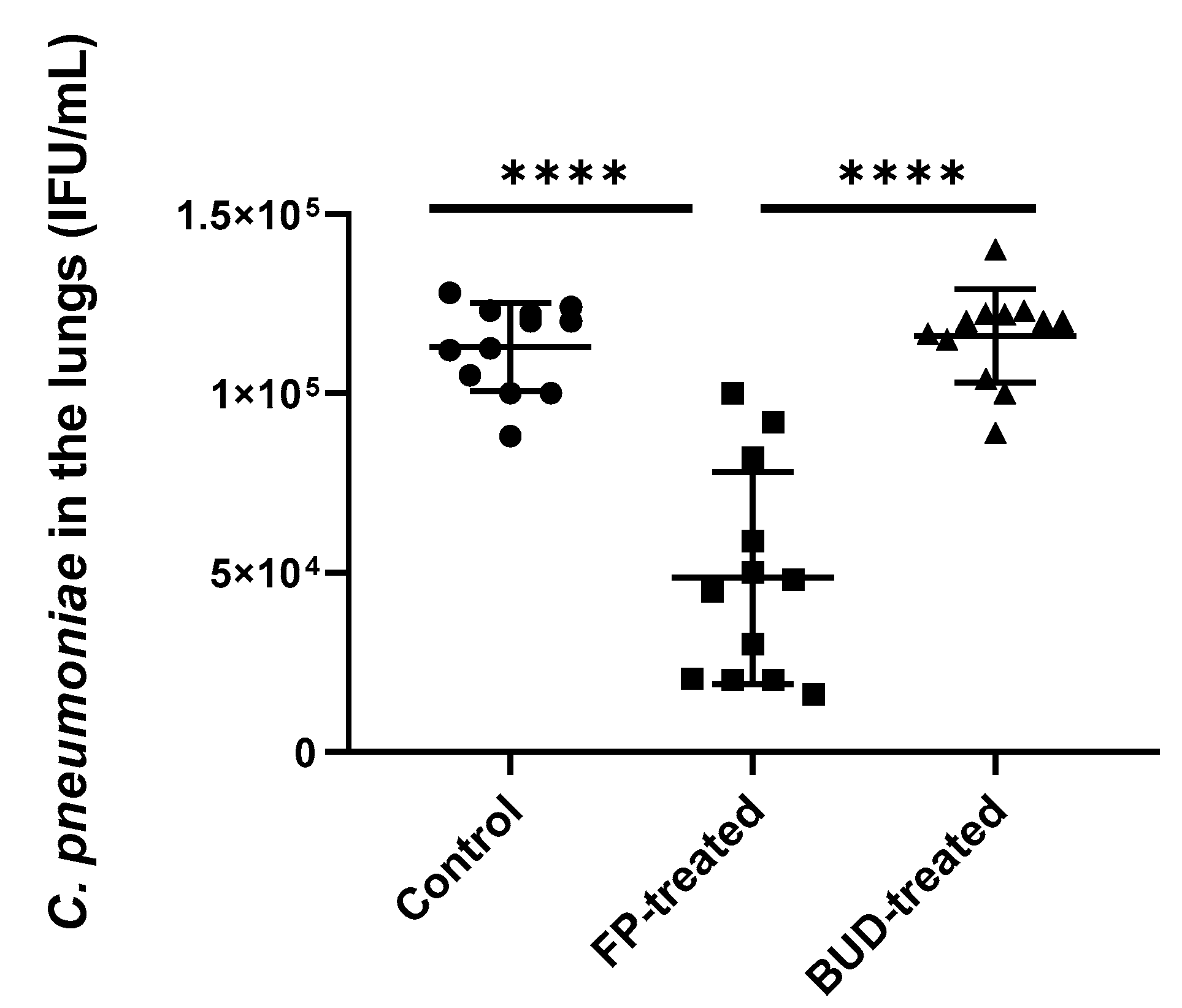
2.2. FP Inhibited C. pneumoniae Growth in the Lungs of Mice
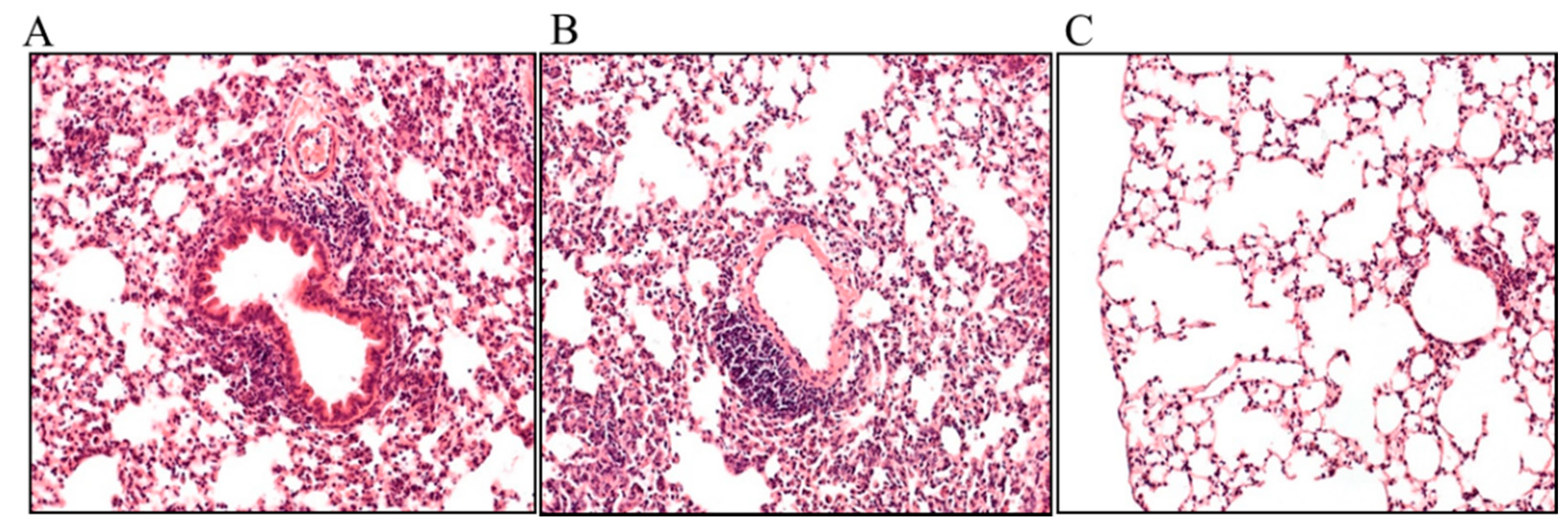
2.3. Effects of FP and BUD on Chlamydia-Infected Lung Tissue Histopathology
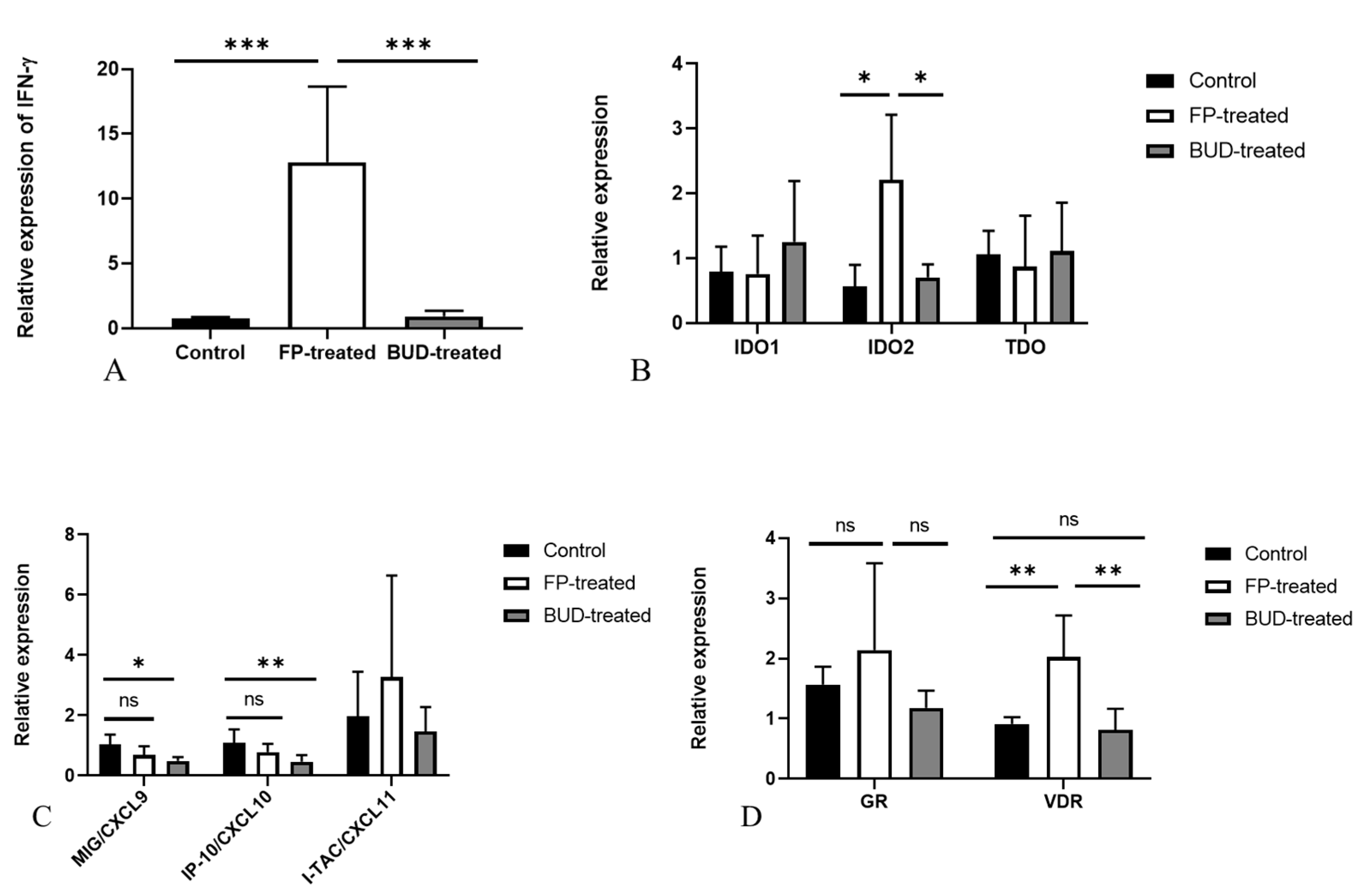
2.4. Effects of FP and BUD Treatment on Gene Expressions Related to IFN-γ and Corticosteroid Responses in C. pneumoniae Infected Mice
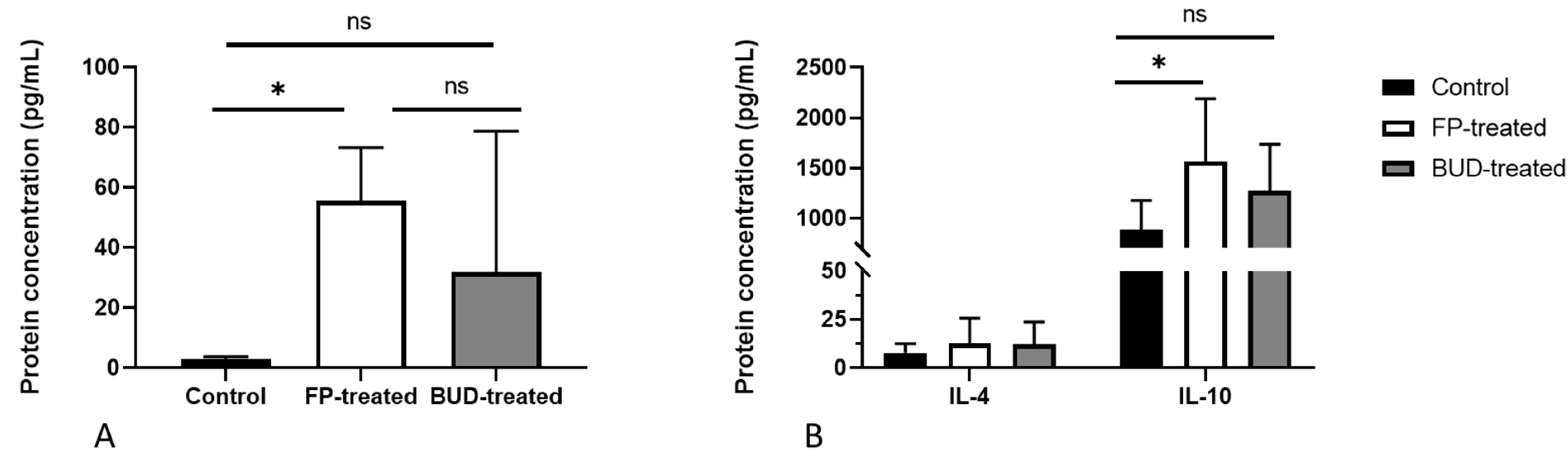
2.5. Anti-Chlamydial IFN-γ and MIG/CXCL9 Protein Production Are Enhanced by FP Treatment
2.6. Effects of BUD and FP Treatment on the Secretion of Th17 and Th2 Cytokines in C. pneumoniae-Infected Lung Tissues
3. Discussion
4. Materials and Methods
4.1. In Vitro Study Design
4.2. Inoculum Preparation and Immunostaining
4.3. Corticosteroid Treatment in Mice
4.4. Animals and Experimental Design
4.5. Culturing of C. pneumoniae from the Lungs
4.6. mRNA Extraction and cDNA Synthesis
4.7. qPCR Validation
4.8. Lung Histology
4.9. Cytokine and Chemokine Measurements from the Lungs
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morjaria, J.B.; Rigby, A.; Morice, A.H. Inhaled Corticosteroid Use and the Risk of Pneumonia and COPD Exacerbations in the UPLIFT Study. Lung 2017, 195, 281–288. [Google Scholar] [CrossRef]
- Sin, D.D.; Tashkin, D.; Zhang, X.; Radner, F.; Sjöbring, U.; Thorén, A.; Calverley, P.M.A.; Rennard, S.I. Budesonide and the Risk of Pneumonia: A Meta-Analysis of Individual Patient Data. Lancet 2009, 374, 712–719. [Google Scholar] [CrossRef]
- McKeever, T.; Harrison, T.W.; Hubbard, R.; Shaw, D. Inhaled Corticosteroids and the Risk of Pneumonia in People with Asthma: A Case-Control Study. Chest 2013, 144, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Janson, C.; Stratelis, G.; Miller-Larsson, A.; Harrison, T.W.; Larsson, K. Scientific Rationale for the Possible Inhaled Corticosteroid Intraclass Difference in the Risk of Pneumonia in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 3055–3064. [Google Scholar] [CrossRef]
- Belchamber, K.B.; Thomas, C.M.; Dunne, A.E.; Barnes, P.J.; Donnelly, L.E. Comparison of Fluticasone Propionate and Budesonide on COPD Macrophage and Neutrophil Function. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2883–2897. [Google Scholar] [CrossRef]
- Yamaya, M.; Nishimura, H.; Nadine, L.; Kubo, H.; Nagatomi, R. Formoterol and Budesonide Inhibit Rhinovirus Infection and Cytokine Production in Primary Cultures of Human Tracheal Epithelial Cells. Respir. Investig. 2014, 52, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, Y.A.; Busse, W.W.; Brockman-Schneider, R.A.; Evans, M.D.; Jarjour, N.N.; McCrae, C.; Miller-Larsson, A.; Gern, J.E. Budesonide and Formoterol Effects on Rhinovirus Replication and Epithelial Cell Cytokine Responses. Respir. Res. 2013, 14, 98. [Google Scholar] [CrossRef]
- Van den Berge, M.; Jonker, M.R.; Miller-Larsson, A.; Postma, D.S.; Heijink, I.H. Effects of Fluticasone Propionate and Budesonide on the Expression of Immune Defense Genes in Bronchial Epithelial Cells. Pulm. Pharmacol. Ther. 2018, 50, 47–56. [Google Scholar] [CrossRef]
- Yamaya, M.; Nishimura, H.; Deng, X.; Sugawara, M.; Watanabe, O.; Nomura, K.; Shimotai, Y.; Momma, H.; Ichinose, M.; Kawase, T. Inhibitory Effects of Glycopyrronium, Formoterol, and Budesonide on Coronavirus HCoV-229E Replication and Cytokine Production by Primary Cultures of Human Nasal and Tracheal Epithelial Cells. Respir. Investig. 2020, 58, 155–168. [Google Scholar] [CrossRef]
- Jaffuel, D.; Demoly, P.; Gougat, C.; Balaguer, P.; Mautino, G.; Godard, P.; Bousquet, J.; Mathieu, M. Transcriptional Potencies of Inhaled Glucocorticoids. Am. J. Respir. Crit. Care Med. 2000, 162, 57–63. [Google Scholar] [CrossRef]
- Ek, A.; Larsson, K.; Siljerud, S.; Palmberg, L. Fluticasone and Budesonide Inhibit Cytokine Release in Human Lung Epithelial Cells and Alveolar Macrophages. Allergy 1999, 54, 691–699. [Google Scholar] [CrossRef]
- Edsbäcker, S.; Wollmer, P.; Selroos, O.; Borgström, L.; Olsson, B.; Ingelf, J. Do Airway Clearance Mechanisms Influence the Local and Systemic Effects of Inhaled Corticosteroids? Pulm. Pharmacol. Ther. 2008, 21, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Miller-Larsson, A.; Mattsson, H.; Hjertberg, E.; Dahlbäck, M.; Tunek, A.; Brattsand, R. Reversible Fatty Acid Conjugation of Budesonide. Novel Mechanism for Prolonged Retention of Topically Applied Steroid in Airway Tissue. Drug Metab. Dispos. 1998, 26, 623–630. [Google Scholar] [PubMed]
- Wang, P.; Wang, X.; Yang, X.; Liu, Z.; Wu, M.; Li, G. Budesonide Suppresses Pulmonary Antibacterial Host Defense by Down-Regulating Cathelicidin-Related Antimicrobial Peptide in Allergic Inflammation Mice and in Lung Epithelial Cells. BMC Immunol. 2013, 14, 7. [Google Scholar] [CrossRef]
- Kuo, C.C.; Jackson, L.A.; Campbell, L.A.; Grayston, J.T. Chlamydia Pneumoniae (TWAR). Clin. Microbiol. Rev. 1995, 8, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.F.; Kraft, M. Chronic Infection and Severe Asthma. Immunol. Allergy Clin. N. Am. 2016, 36, 483–502. [Google Scholar] [CrossRef] [PubMed]
- Paróczai, D.; Mosolygó, T.; Kókai, D.; Endrész, V.; Virok, D.P.; Somfay, A.; Burián, K. Chlamydia Pneumoniae Influence on Cytokine Production in Steroid-Resistant and Steroid-Sensitive Asthmatics. Pathogens 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Eszik, I.; Lantos, I.; Önder, K.; Somogyvári, F.; Burián, K.; Endrész, V.; Virok, D.P. High Dynamic Range Detection of Chlamydia Trachomatis Growth by Direct Quantitative PCR of the Infected Cells. J. Microbiol. Methods 2016, 120, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Virok, D.P.; Raffai, T.; Kókai, D.; Paróczai, D.; Bogdanov, A.; Veres, G.; Vécsei, L.; Poliska, S.; Tiszlavicz, L.; Somogyvári, F.; et al. Indoleamine 2,3-Dioxygenase Activity in Chlamydia Muridarum and Chlamydia Pneumoniae Infected Mouse Lung Tissues. Front. Cell Infect. Microbiol. 2019, 9, 192. [Google Scholar] [CrossRef]
- Marcellini, A.; Swieboda, D.; Guedán, A.; Farrow, S.N.; Casolari, P.; Contoli, M.; Johnston, S.L.; Papi, A.; Solari, R. Glucocorticoids Impair Type I IFN Signalling and Enhance Rhinovirus Replication. Eur. J. Pharmacol. 2020, 893, 173839. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the Immune System. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Nelson, D.E.; Virok, D.P.; Wood, H.; Roshick, C.; Johnson, R.M.; Whitmire, W.M.; Crane, D.D.; Steele-Mortimer, O.; Kari, L.; McClarty, G.; et al. Chlamydial IFN-Gamma Immune Evasion Is Linked to Host Infection Tropism. Proc. Natl. Acad. Sci. USA 2005, 102, 10658–10663. [Google Scholar] [CrossRef] [PubMed]
- Mosolygó, T.; Korcsik, J.; Balogh, E.P.; Faludi, I.; Virók, D.P.; Endrész, V.; Burián, K. Chlamydophila Pneumoniae Re-Infection Triggers the Production of IL-17A and IL-17E, Important Regulators of Airway Inflammation. Inflamm. Res. 2013, 62, 451–460. [Google Scholar] [CrossRef]
- Yeh, J.-J.; Lin, C.-L.; Kao, C.-H. Associations among Chronic Obstructive Pulmonary Disease with Asthma, Pneumonia, and Corticosteroid Use in the General Population. PLoS ONE 2020, 15, e0229484. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Rhee, C.K.; Shim, J.S.; Park, S.Y.; Yoo, K.H.; Kim, B.Y.; Bae, H.W.; Sim, Y.S.; Chang, J.H.; Cho, Y.J.; et al. Inhaled Corticosteroids in Asthma and the Risk of Pneumonia. Allergy Asthma Immunol. Res. 2019, 11, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Heijink, I.H.; Jonker, M.R.; de Vries, M.; van Oosterhout, A.J.M.; Telenga, E.; Ten Hacken, N.H.T.; Postma, D.S.; van den Berge, M. Budesonide and Fluticasone Propionate Differentially Affect the Airway Epithelial Barrier. Respir. Res. 2016, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-R.; Song, J.-H.; Ahn, J.-H.; Lee, G.-S.; Ahn, H.; Yoon, S.-I.; Kang, S.G.; Kim, P.-H.; Jeon, S.-M.; Choi, E.-J.; et al. Antiviral and Anti-Inflammatory Activity of Budesonide against Human Rhinovirus Infection Mediated via Autophagy Activation. Antivir. Res. 2018, 151, 87–96. [Google Scholar] [CrossRef]
- Leigh, R.; Mostafa, M.M.; King, E.M.; Rider, C.F.; Shah, S.; Dumonceaux, C.; Traves, S.L.; McWhae, A.; Kolisnik, T.; Kooi, C.; et al. An Inhaled Dose of Budesonide Induces Genes Involved in Transcription and Signaling in the Human Airways: Enhancement of Anti- and Proinflammatory Effector Genes. Pharmacol. Res. Perspect. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Umland, S.P.; Nahrebne, D.K.; Razac, S.; Beavis, A.; Pennline, K.J.; Egan, R.W.; Billah, M.M. The Inhibitory Effects of Topically Active Glucocorticoids on IL-4, IL-5, and Interferon-Gamma Production by Cultured Primary CD4+ T Cells. J. Allergy Clin. Immunol. 1997, 100, 511–519. [Google Scholar] [CrossRef]
- Smith-Norowitz, T.A.; Loeffler, J.; Huang, Y.; Klein, E.; Norowitz, Y.M.; Hammerschlag, M.R.; Joks, R.; Kohlhoff, S. Chlamydia Pneumoniae Immunoglobulin E Antibody Levels in Patients with Asthma Compared with Non-Asthma. Heliyon 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Smith-Norowitz, T.A.; Chotikanatis, K.; Weaver, D.; Ditkowsky, J.; Norowitz, Y.M.; Hammerschlag, M.R.; Joks, R.; Kohlhoff, S. Chlamydia Pneumoniae-Induced Tumour Necrosis Factor Alpha Responses Are Lower in Children with Asthma Compared with Non-Asthma. BMJ Open Respir. Res. 2018, 5, e000239. [Google Scholar] [CrossRef] [PubMed]
- Black, P.N.; Scicchitano, R.; Jenkins, C.R.; Blasi, F.; Allegra, L.; Wlodarczyk, J.; Cooper, B.C. Serological Evidence of Infection with Chlamydia Pneumoniae Is Related to the Severity of Asthma. Eur. Respir. J. 2000, 15, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Byrne, G.I.; Lehmann, L.K.; Landry, G.J. Induction of Tryptophan Catabolism Is the Mechanism for Gamma-Interferon-Mediated Inhibition of Intracellular Chlamydia Psittaci Replication in T24 Cells. Infect. Immun. 1986, 53, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Burian, K.; Endresz, V.; Deak, J.; Kormanyos, Z.; Pal, A.; Nelson, D.; Virok, D.P. Transcriptome Analysis Indicates an Enhanced Activation of Adaptive and Innate Immunity by Chlamydia-Infected Murine Epithelial Cells Treated with Interferon γ. J. Infect. Dis. 2010, 202, 1405–1414. [Google Scholar] [CrossRef]
- Balogh, E.P.; Faludi, I.; Virók, D.P.; Endrész, V.; Burián, K. Chlamydophila Pneumoniae Induces Production of the Defensin-like MIG/CXCL9, Which Has in Vitro Antichlamydial Activity. Int. J. Med. Microbiol. 2011, 301, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.M.; Carroll, M.L.; Li, H.; Poh, A.M.; Kirkegard, D.; Towers, M.; Upham, J.W. Budesonide and Formoterol Reduce Early Innate Anti-Viral Immune Responses in Vitro. PLoS ONE 2011, 6, e27898. [Google Scholar] [CrossRef] [PubMed]
- Dora, D.; Rivard, C.; Yu, H.; Bunn, P.; Suda, K.; Ren, S.; Lueke Pickard, S.; Laszlo, V.; Harko, T.; Megyesfalvi, Z.; et al. Neuroendocrine Subtypes of Small Cell Lung Cancer Differ in Terms of Immune Microenvironment and Checkpoint Molecule Distribution. Mol. Oncol. 2020, 14, 1947–1965. [Google Scholar] [CrossRef]
- Maneechotesuwan, K.; Supawita, S.; Kasetsinsombat, K.; Wongkajornsilp, A.; Barnes, P.J. Sputum Indoleamine-2, 3-Dioxygenase Activity Is Increased in Asthmatic Airways by Using Inhaled Corticosteroids. J. Allergy Clin. Immunol. 2008, 121, 43–50. [Google Scholar] [CrossRef]
- Feng, E.; Wan, R.; Yang, S.; Yan, Z.; Wang, S.; He, W.; Zhang, Y.; Yin, H.; Chen, Z.; Liu, R. Expression Levels of Induced Sputum IL-8 and IL-10 and Drug Intervention Effects in Patients with Acute Exacerbated COPD Complicated with Chronic Cor Pulmonale at High Altitude. Exp. Ther. Med. 2013, 6, 747–752. [Google Scholar] [CrossRef]
- Li, H.-T.; Lin, Y.-S.; Ye, Q.-M.; Yang, X.-N.; Zou, X.-L.; Yang, H.-L.; Zhang, T.-T. Airway Inflammation and Remodeling of Cigarette Smoking Exposure Ovalbumin-Induced Asthma Is Alleviated by CpG Oligodeoxynucleotides via Affecting Dendritic Cell-Mediated Th17 Polarization. Int. Immunopharmacol. 2020, 82, 106361. [Google Scholar] [CrossRef]
- Honda, K.; Wada, H.; Nakamura, M.; Nakamoto, K.; Inui, T.; Sada, M.; Koide, T.; Takata, S.; Yokoyama, T.; Saraya, T.; et al. IL-17A Synergistically Stimulates TNF-α-Induced IL-8 Production in Human Airway Epithelial Cells: A Potential Role in Amplifying Airway Inflammation. Exp. Lung Res. 2016, 42, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Ren, J.; Tang, X.; Jing, Y.; Xing, D.; Zhao, G.; Yao, Z.; Yang, X.; Bai, H. IL-17A Synergizes with IFN-γ to Upregulate INOS and NO Production and Inhibit Chlamydial Growth. PLoS ONE 2012, 7, e39214. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ananaba, G.A.; Patrickson, J.; Pitts, S.; Yi, Y.; Yan, F.; Eko, F.O.; Lyn, D.; Black, C.M.; Igietseme, J.U.; et al. Chlamydial Infection in Vitamin D Receptor Knockout Mice Is More Intense and Prolonged than in Wild-Type Mice. J. Steroid Biochem. Mol. Biol. 2013, 135, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.A.; Miller, C.W.; El-Abbassi, A.M.; Cutchins, D.C.; Cutchins, C.; Grant, W.B.; Peiris, A.N. Antimicrobial Implications of Vitamin D. Dermatoendocrinology 2011, 3, 220–229. [Google Scholar] [CrossRef]
- Qiu, S.-L.; Kuang, L.-J.; Tang, Q.-Y.; Duan, M.-C.; Bai, J.; He, Z.-Y.; Zhang, J.-Q.; Li, M.-H.; Deng, J.-M.; Liu, G.-N.; et al. Enhanced Activation of Circulating Plasmacytoid Dendritic Cells in Patients with Chronic Obstructive Pulmonary Disease and Experimental Smoking-Induced Emphysema. Clin. Immunol. 2018, 195, 107–118. [Google Scholar] [CrossRef]
- Theegarten, D.; Anhenn, O.; Hotzel, H.; Wagner, M.; Marra, A.; Stamatis, G.; Mogilevski, G.; Sachse, K. A Comparative Ultrastructural and Molecular Biological Study on Chlamydia Psittaci Infection in Alpha-1 Antitrypsin Deficiency and Non-Alpha-1 Antitrypsin Deficiency Emphysema versus Lung Tissue of Patients with Hamartochondroma. BMC Infect. Dis 2004, 4, 38. [Google Scholar] [CrossRef]
- Virók, D.P.; Eszik, I.; Mosolygó, T.; Önder, K.; Endrész, V.; Burián, K. A Direct Quantitative PCR-Based Measurement of Herpes Simplex Virus Susceptibility to Antiviral Drugs and Neutralizing Antibodies. J. Virol. Methods 2017, 242, 46–52. [Google Scholar] [CrossRef]
- Burián, K.; Hegyesi, H.; Buzás, E.; Endrész, V.; Kis, Z.; Falus, A.; Gönczöl, E. Chlamydophila (Chlamydia) Pneumoniae Induces Histidine Decarboxylase Production in the Mouse Lung. Immunol. Lett. 2003, 89, 229–236. [Google Scholar] [CrossRef]
- Zuśka-Prot, M.; Maślanka, T. Effect of Inhaled and Systemic Glucocorticoid Treatment on CD4+ Regulatory and Effector T Cells in a Mouse Model of Allergic Asthma. Int. Immunopharmacol. 2017, 45, 98–109. [Google Scholar] [CrossRef]
- Becker, A.B.; Abrams, E.M. Asthma Guidelines: The Global Initiative for Asthma in Relation to National Guidelines. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 99–103. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paróczai, D.; Sejben, A.; Kókai, D.; Virok, D.P.; Endrész, V.; Burián, K. Beneficial Immunomodulatory Effects of Fluticasone Propionate in Chlamydia pneumoniae-Infected Mice. Pathogens 2021, 10, 338. https://doi.org/10.3390/pathogens10030338
Paróczai D, Sejben A, Kókai D, Virok DP, Endrész V, Burián K. Beneficial Immunomodulatory Effects of Fluticasone Propionate in Chlamydia pneumoniae-Infected Mice. Pathogens. 2021; 10(3):338. https://doi.org/10.3390/pathogens10030338
Chicago/Turabian StyleParóczai, Dóra, Anita Sejben, Dávid Kókai, Dezső P. Virok, Valéria Endrész, and Katalin Burián. 2021. "Beneficial Immunomodulatory Effects of Fluticasone Propionate in Chlamydia pneumoniae-Infected Mice" Pathogens 10, no. 3: 338. https://doi.org/10.3390/pathogens10030338
APA StyleParóczai, D., Sejben, A., Kókai, D., Virok, D. P., Endrész, V., & Burián, K. (2021). Beneficial Immunomodulatory Effects of Fluticasone Propionate in Chlamydia pneumoniae-Infected Mice. Pathogens, 10(3), 338. https://doi.org/10.3390/pathogens10030338








