Acinetobacter baumannii LOS Regulate the Expression of Inflammatory Cytokine Genes and Proteins in Human Mast Cells
Abstract
1. Introduction
2. Results
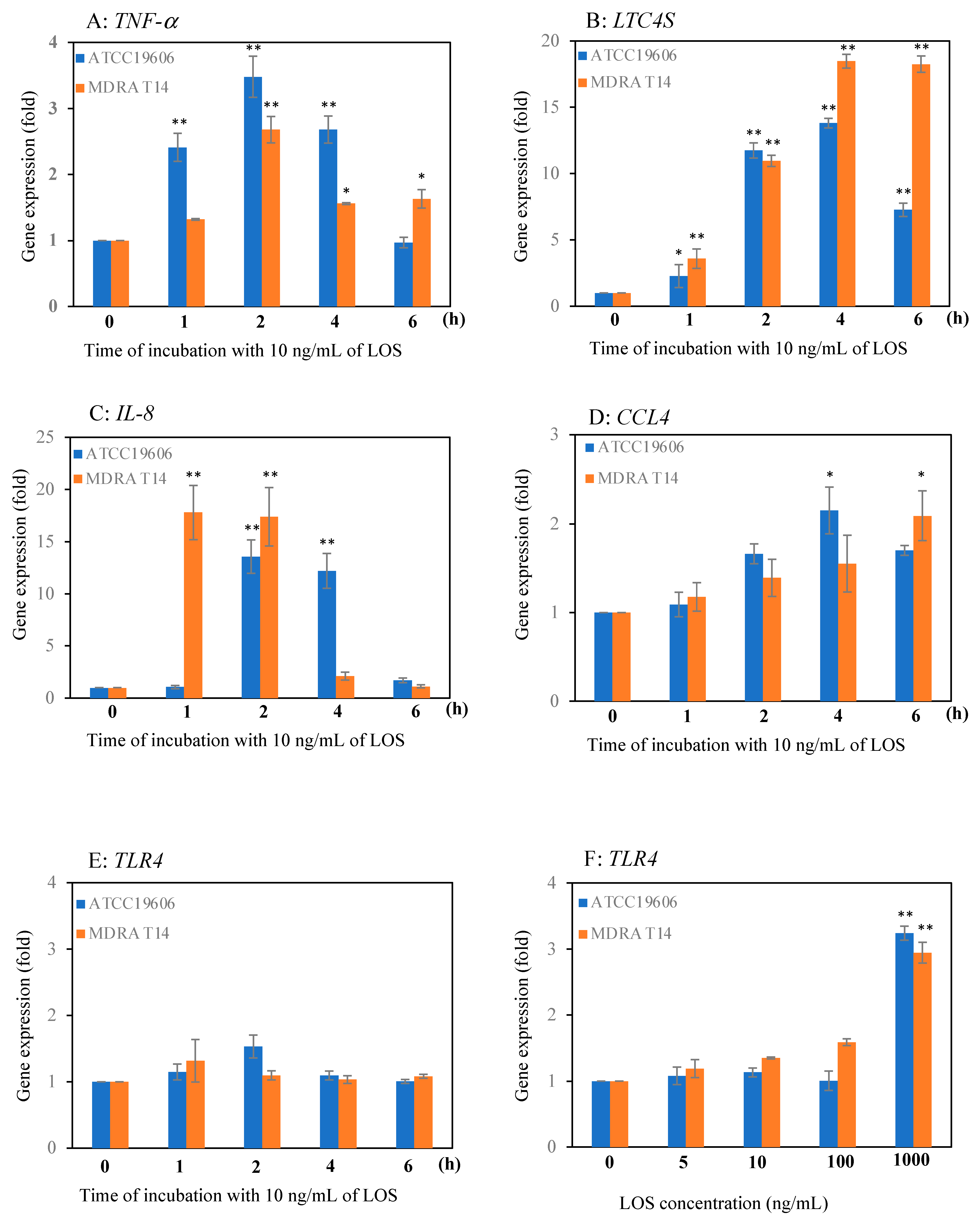
2.1. Effect of LOS on Mast Cell Gene Expression of Pro-Inflammatory Genes
2.2. Effect of LOS on Expression of Chemoattractant Genes in Mast Cells
2.3. Effect of LOS on the Expression of the TLR4 Gene in Mast Cells
2.4. Effect of LOS on Mast Cell Production of Pro-Inflammatory Mediators
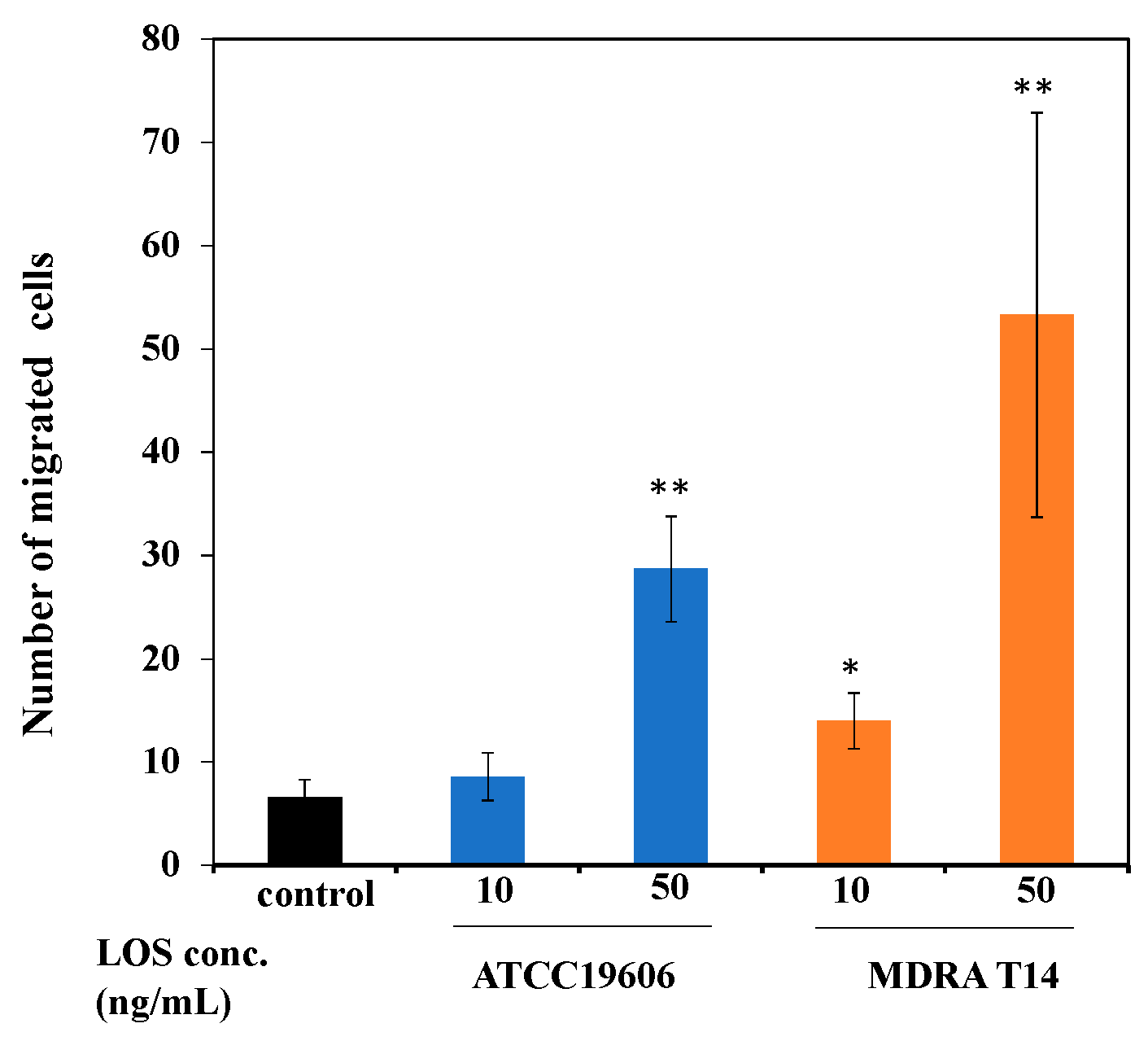
2.5. Effect of Pro-Inflammatory Mediators Released from Mast Cells Stimulated with A. baumannii LOS to Induce Neutrophil Transmigration
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. LAD2 Cell Culture
5.2. LOS Preparation
5.3. Mast Cells Stimulation with LOS
5.4. RNA Preparation
5.5. Complimentary DNA Synthesis
5.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
5.7. Cytokine Assay
5.8. Neutrophil Preparation
5.9. Cell-Migration Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef]
- Fournier, P.E.; Vallenet, P.D.; Barbe, V.; Audic, S.; Ogata, H.; Poirel, L.; Richet, H.; Robert, C.; Mangenot, S.; Abergel, C.; et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006, 2, 62–72. [Google Scholar] [CrossRef]
- Tomaras, A.P.; Dorsey, C.W.; Edelmann, R.E.; Actis, L.A. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: Involvement of a novel chaperone-usher pili assembly system. Microbiology 2003, 149, 3473–3484. [Google Scholar] [CrossRef]
- Choi, C.H.; Lee, E.Y.; Lee, Y.C.; Park, T.I.; Kim, H.J.; Hyun, S.H.; Kim, S.A.; Lee, S.-K.; Lee, J.C. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell. Microbiol. 2005, 7, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Davenport, E.K.; Call, D.R.; Beyenal, H. Differential protection from Tobramycin by extracellular polymeric substances from Acinetobacter baumannii and Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2014, 58, 4755–4761. [Google Scholar] [CrossRef]
- Wood, C.R.; Ohneck, E.J.; Edelmann, R.E.; Actis, L.A. A light-regulated type I pilus contributes to Acinetobacter baumannii biofilm, motility, and virulence functions. Infect. Immun. 2018, 86, e00442-18. [Google Scholar] [CrossRef] [PubMed]
- Wroblewska, M.M.; Sawicka-Grzelak, A.; Marchel, H.; Luczak, M.; Sivan, A. Biofilm production by clinical strains of Acinetobacter baumannii isolated from patients hospitalized in two tertiary care hospitals. FEMS Immunol. Med. Microbiol. 2008, 53, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Andremont, A.; Wolff, M.; Bouadma, L. Hospital-acquired pneumonia and ventilator-associated pneumonia: Recent advances in epidemiology and management. Curr. Opin. Pulm. Med. 2013, 19, 216–228. [Google Scholar] [CrossRef]
- Brown, J.M.; Wilson, T.M.; Metcalfe, D.D. The mast cell and allergic diseases: Role in pathogenesis and implications for therapy. Clin. Exp. Allergy 2007, 38, 4–28. [Google Scholar] [CrossRef]
- Féger, F.; Varadaradjalou, S.; Gao, Z.; Abraham, S.N.; Arock, M. The role of mast cells in host defense and their subversion by bacterial pathogens. Trends Immunol. 2002, 23, 151–158. [Google Scholar] [CrossRef]
- Malaviya, R.; Ikeda, T.; Ross, E.; Abraham, S.N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 1996, 381, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Leal-Berumen, I.; Conlon, P.; Marshall, J.S. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J. Immunol. 1994, 152, 5468–5476. Available online: http://www.jimmunol.org/content/152/11/5468 (accessed on 1 June 1994). [PubMed]
- Varadaradjalou, S.; Féger, F.; Thieblemont, N.; Hamouda, N.B.; Pleau, J.-M.; Dy, M.; Arock, M. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur. J. Immunol. 2003, 33, 899–906. [Google Scholar] [CrossRef]
- Kudo, Y.; Fukushi, N.; Yoshioka, M.; Kawasoe, Y.; Iriguchi, S.; Imajo, N.; Yasui, Y.; Matsui, N.; Akagi, M. Bacterial components regulate the expression of Toll-like receptor 4 on human mast cells. Inflamm. Res. 2007, 56, 70–75. [Google Scholar] [CrossRef]
- Kikuchi-Ueda, T.; Kamoshida, G.; Ubagai, T.; Nakano, R.; Nakano, A.; Akuta, T.; Hikosaka, K.; Tansho-Nagakawa, S.; Kikuchi, H.; Ono, Y. The TNF-α of mast cells induces pro-inflammatory responses during infection with Acinetobacter baumannii. Immunobiology 2017, 222, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Pantophlet, R.; Brade, L.; Brade, H. Identification of Acinetobacter baumannii strains with monoclonal antibodies against the O antigens of their lipopolysaccharides. Clin. Diagn. Lab. Immunol. 1999, 6, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; Scannon, P.J.; Vincent, J.-L.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Urb, M.; Sheppard, D.C. The roles of mast cells in the defense against pathogens. PLoS Pathog. 2012, 8, e1002619. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, R.; Abraham, S.N. Role of mast cell leukotrienes in neutrophil recruitment and bacterial clearance in infectious peritonitis. J. Leukoc. Biol. 2000, 67, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Wiezbicki, M.; Brzezińska-Blaszczyk, E. Diverse effects of bacterial cell wall components on mast cell degranulation, cysteinyl leukotriene generation and migration. Microbiol. Immunol. 2009, 53, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Henderson, W.R., Jr.; Lewis, D.B.; Albert, R.K.; Zhang, Y.; Lamm, W.J.; Chiang, G.K.; Jones, F.; Eriksen, P.; Tien, Y.T.; Jones, M.; et al. The importance of leukotrienes in airway inflammation in a mouse model of asthma. J. Exp. Med. 1996, 184, 1483–1494. [Google Scholar] [CrossRef]
- Spada, C.S.; Nieves, A.L.; Krauss, A.H.-P.; Woodward, D.F. Comparison of leukotriene B4 and D4 effects on human eosinophil and neutrophil motility in vitro. J. Leukoc. Biol. 1994, 55, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Hamelmann, E.; Joetham, A.; Shultz, L.D.; Larsen, G.L.; Irvin, C.G.; Gelfand, E.W. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J. Exp. Med. 1997, 186, 449–454. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Canetti, C.; Mancuso, P.; Coffey, M.J. Leukotreienes: Underappreciated mediators of innate immune responses. J. Immunol. 2005, 174, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Aderem, A.A.; Cohen, D.S.; Wright, S.D.; Cohn, Z.A. Bacterial lipopolysaccharide prime macrophages for enhanced release of arachidonic acid metabolites. J. Exp. Med. 1986, 164, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Coffey, M.J.; Phare, S.M.; Peters-Golden, M. Prolonged exposure to lipopolysaccharide inhibits macrophages 5-lipoxygenase metabolism via induction of nitric oxide synthesis. J. Immunol. 2000, 165, 3592–3598. [Google Scholar] [CrossRef]
- Sun, G.; Liu, F.; Lin, T.-J. Identification of Pseudomonas aeruginosa-induced genes in human mast cells using suppression subtractive hybridization: Up-regulation of IL-8 and CCL4 production. Clin. Exp. Immunol. 2005, 142, 199–205. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents-How P. aeruginosa can escape antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; A critical review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Doener, F.; Michel, A.; Reuter, S.; Friedrich, P.; Böhm, L.; Relle, M.; Codarri, L.; Tenzer, S.; Klein, M.; Bopp, T.; et al. Mast cell-derived mediators promote murine neutrophil effector functions. Int. Immunol. 2013, 25, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ramos, B.F.; Jakschik, B.A. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science 1992, 258, 1957–1959. [Google Scholar] [CrossRef]
- Moffatt, J.H.; Harper, M.; Harrison, P.; Hale, J.D.; Vinogradov, E.; Seemann, T.; Henry, R.; Crane, B.; St. Michael, F.; Cox, A.D.; et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 2010, 54, 4971–4977. [Google Scholar] [CrossRef] [PubMed]
- Beceiro, A.; Moreno, A.; Fernández, N.; Vallejo, J.A.; Aranda, J.; Adler, B.; Harper, M.; Boyce, J.D.; Bou, G. Biological cost of different mechanisms of colistin resistance and their impact on virulence in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2014, 58, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Boll, J.M.; Crofts, A.A.; Peters, K.; Cattoir, V.; Vollmer, W.; Davies, B.W.; Trent, M.S. A penicillin-binding protein inhibits selection of colistin-resistant, lipopolioligosaccharide-deficient Acinetobacter baumannii. Proc. Natl. Acad. Sci. USA 2016, 113, E6228–E6237. [Google Scholar] [CrossRef]
- Okuda, S.; Sherman, D.J.; Silhavy, T.J.; Ruiz, N.; Kahne, D. Lipopolysaccharide transport and assembly at the outer membrane: The PEZ model. Nat. Rev. Microbiol. 2016, 14, 337–345. [Google Scholar] [CrossRef]
- Ubagai, T.; Nakano, R.; Nakano, A.; Kamoshida, G.; Ono, Y. Gene expression analysis in human polymorphonuclear leukocytes stimulated by LPSs from nosocomial opportunistic pathogens. Innate Immun. 2015, 21, 802–812. [Google Scholar] [CrossRef]
- Kirshenbaum, A.S.; Akin, C.; Wu, Y.; Rottem, M.; Goff, J.P.; Beaven, M.A.; Rao, V.K.; Metcalf, D.D. Characterization of novel stem cell factor responsive human mast cell lines LAD1 and LAD 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcεRI or FcγRI. Leuk. Res. 2003, 27, 677–682. [Google Scholar] [CrossRef]
- Akuta, T.; Kikuchi-Ueda, T.; Imaizumi, K.; Oshikane, H.; Nakaki, T.; Okada, Y.; Sultana, S.; Kobayashi, K.; Kiyokawa, N.; Ono, Y. Expression of bioactive soluble human stem cell factor (SCF) from recombinant Escherichia coli by coproduction of thioredoxin and efficient purification using arginine in affinity chromatography. Protein Expr. Purif. 2015, 105, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Galanos, C.; Lüderitz, O.; Westphal, O. A new method for the extraction of R lipopolysaccharides. Eur. J. Biochem. 1969, 9, 245–249. [Google Scholar] [CrossRef]
- Rajeevan, M.S.; Vernon, S.D.; Taysavang, N.; Unger, E.R. Validation of array-based gene expression profiles by real-time (kinetic) RT-PCR. J. Mol. Diagn. 2001, 3, 26–31. [Google Scholar] [CrossRef]
- Elshal, M.F.; McCoy, J.P., Jr. Multiplex bead array assays: Performance evaluation and comparison of sensitivity to ELISA. Methods 2006, 38, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Boyden, S. The chemotactic effect of mixture of antibody and antigen on polymorphonuclear leukocytes. J. Exp. Med. 1962, 115, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Kamoshida, G.; Matsuda, A.; Sekine, W.; Mizuno, H.; Oku, T.; Itoh, S.; Irimura, T.; Tsuji, T. Monocyte differentiation induced by co-culture with tumor cells involves RGD-dependent cell adhesion to extracellular matrix. Cancer Lett. 2012, 315, 145–152. [Google Scholar] [CrossRef]

| Gene | Sequence | Amplicon Length (bp) |
|---|---|---|
| TNF-a | F: 5′-AGACCAAGGTCAACCTCCT-3′ R: 5′-AAAGTAGACCTGCCCAGAC-3′ | 194 |
| IL-8 | F: 5′-ATCCACAAGTCCTTGTTCCA-3′ R: 5′-AAGTGCTTCCACATGTCCTC-3′ | 113 |
| CCL4 | F: 5′-GCCTGCTGCTTTTCTTACAC-3′ R: 5′-CTTGCTTCTTTTGGTTTGGA-3′ | 117 |
| LTC4S | F: 5′-AGGTGGGCTGGTTCCTATCTA-3′ R: 5′-CCCATGGCTATCCTACCATTT-3′ | 220 |
| TLR4 | F: 5′-ATTTCAGCTCTGCCTTCACTA-3′ R: 5′-CTTCTGCAGGACAATGAAGAT-3′ | 212 |
| β-actin | F: 5′-TTAAGGAGAAGCTGTGCTACG-3′ R: 5′-TTGAAGGTAGTTTCGTGGATG-3′ | 205 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikuchi-Ueda, T.; Ubagai, T.; Kamoshida, G.; Nakano, R.; Nakano, A.; Ono, Y. Acinetobacter baumannii LOS Regulate the Expression of Inflammatory Cytokine Genes and Proteins in Human Mast Cells. Pathogens 2021, 10, 290. https://doi.org/10.3390/pathogens10030290
Kikuchi-Ueda T, Ubagai T, Kamoshida G, Nakano R, Nakano A, Ono Y. Acinetobacter baumannii LOS Regulate the Expression of Inflammatory Cytokine Genes and Proteins in Human Mast Cells. Pathogens. 2021; 10(3):290. https://doi.org/10.3390/pathogens10030290
Chicago/Turabian StyleKikuchi-Ueda, Takane, Tsuneyuki Ubagai, Go Kamoshida, Ryuichi Nakano, Akiyo Nakano, and Yasuo Ono. 2021. "Acinetobacter baumannii LOS Regulate the Expression of Inflammatory Cytokine Genes and Proteins in Human Mast Cells" Pathogens 10, no. 3: 290. https://doi.org/10.3390/pathogens10030290
APA StyleKikuchi-Ueda, T., Ubagai, T., Kamoshida, G., Nakano, R., Nakano, A., & Ono, Y. (2021). Acinetobacter baumannii LOS Regulate the Expression of Inflammatory Cytokine Genes and Proteins in Human Mast Cells. Pathogens, 10(3), 290. https://doi.org/10.3390/pathogens10030290






