Will Fly Repellency Using Deltamethrin Reduce Intramammary Infections, Stress and Fatigue Indicators of Dairy Ewes under Intensive Management?
Abstract
1. Introduction
2. Results
2.1. Meteorological Data
2.2. Fly Species Identification
2.3. Short and Long-Term Deltamethrin Repellency Effect
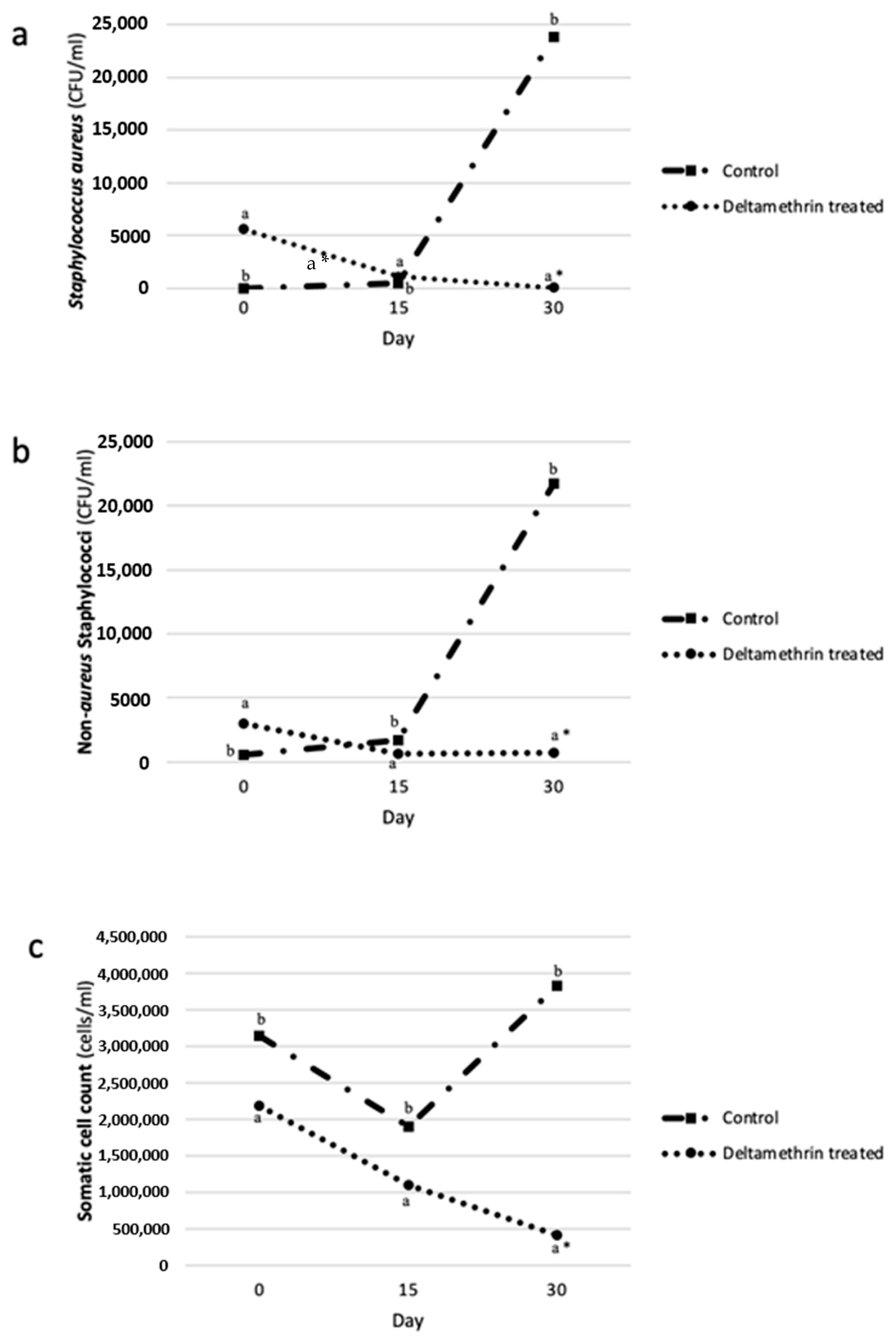
2.4. The Association between Deltamethrin Treatment, Intramammary Infections, and the Somatic Cell Counts of Milk
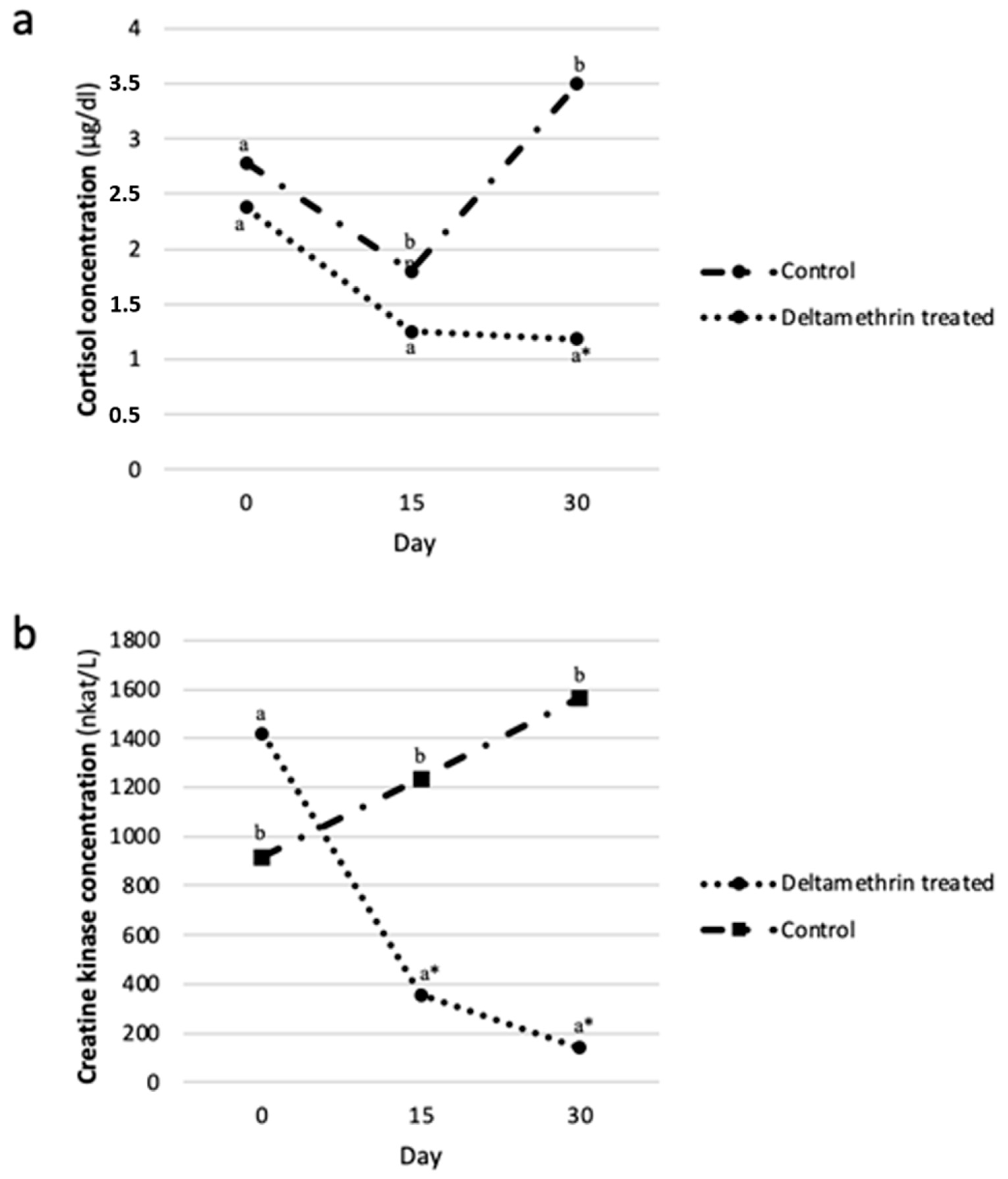
2.5. The Association between Deltamethrin Treatment, Serum Cortisol (SC) and Creatine Kinase (CK) Levels
3. Discussion
4. Materials and Methods
4.1. Flock History
4.2. Experimental Design
4.3. Milking Routine and Technical Information of the Milking Machine
4.4. Fly Monitoring, Trapping and Identification
4.5. Meteorological Data
4.6. Milk Sampling and Analyses
4.7. Blood Sampling and Analyses
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Lianou, D.T.; Fthenakis, G.C. Scientometrics Approach to Research in Ovine Mastitis from 1970 to 2019 (with a Complete List of Relevant Literature References). Pathogens 2020, 9, 585. [Google Scholar] [CrossRef]
- Gelasakis, A.I.; Mavrogianni, V.S.; Petridis, I.G.; Vasileiou, N.G.C.; Fthenakis, G.C. Mastitis in sheep – The last 10 years and the future of research. Vet. Microbiol. 2015, 181, 136–146. [Google Scholar] [CrossRef]
- Fthenakis, G.C.; Barbagianni, M.S.; Fragkou, I.A.; Gougoulis, D.A.; Katsafadou, A.I.; Mavrogianni, V.S.; Petridis, I.G.; Vasileiou, N.G.C. Elucidation of predisposing factors for ovine mastitis contributed to sustainable control of the disease. In Proceedings of the 9th International Sheep Congress, Harrogate, UK, 22–26 May 2017. [Google Scholar]
- EFSA Panel on Animal Health and Welfare (AHAW) Scientific Opinion on the welfare risks related to the farming of sheep for wool, meat and milk production. EFSA J. 2014, 12, 3933–4060. [CrossRef]
- Vasileiou, N.G.; Sarrou, S.; Papagiannitsis, C.; Chatzopoulos, D.C.; Malli, E.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Antimicrobial Agent Susceptibility and Typing of Staphylococcal Isolates from Subclinical Mastitis in Ewes. Microb. Drug Resist. 2019, 25, 1099–1110. [Google Scholar] [CrossRef]
- Poveda, J.M.; Jiménez, L.; Perea, J.M.; Arias, R.; Palop, M.L. Farming Practices Influence Antibiotic Resistance and Biogenic Amine Capacity of Staphylococci from Bulk Tank Ewe’s Milk. Animals 2020, 10, 1622. [Google Scholar] [CrossRef]
- Bergonier, D.; Rupp, R.; Lagriffoul, G.; Berthelot, X. Mastitis of dairy small ruminants. Veter. Res. 2003, 34, 689–716. [Google Scholar] [CrossRef]
- Pugh, D.G.; Baird, A.N. Diseases of the mammary gland. In Sheep and Goat Medicine, 2nd ed.; Plummer, P.J., Plummer, C., Eds.; Elsevier: St. Louis, MI, USA, 2011; pp. 462–465. [Google Scholar]
- Selvaggi, M.; D’Alessandro, A.G.; Dario, C. Environmental and genetic factors affecting milk yield and quality in three Italian sheep breeds. J. Dairy Res. 2016, 84, 27–31. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Chatzopoulos, D.C.; Sarrou, S.; Fragkou, I.A.; Katsafadou, A.I.; Mavrogianni, V.S.; Petinaki, E.; Fthenakis, G.C. Role of staphylococci in mastitis in sheep. J. Dairy Res. 2019, 86, 254–266. [Google Scholar] [CrossRef]
- Ruegg, P.L. New Perspectives in Udder Health Management. Veter. Clin. North. Am. Food Anim. Pr. 2012, 28, 149–163. [Google Scholar] [CrossRef]
- Fthenakis, G.C.; Mavrogianni, V.S.; Fragkou, I.A. Mastitis. In Small Ruminants’ Reproduction, 1st ed.; Fthenakis, G.C., Ed.; Tziolas Publications: Thesaloniki, Greece, 2011; pp. 331–351. [Google Scholar]
- Bisdorff, B.; Milnes, A.; Wall, R. Prevalence and regional distribution of scab, lice and blowfly strike in Great Britain. Veter. Rec. 2006, 158, 749–752. [Google Scholar] [CrossRef]
- Taylor, D.B.; Moon, R.D.; Mark, D.R. Economic Impact of Stable Flies (Diptera: Muscidae) on Dairy and Beef Cattle Production. J. Med. Èntomol. 2012, 49, 198–209. [Google Scholar] [CrossRef]
- Farkas, R.; Hall, M.J.; Kelemen, F. Wound myiasis of sheep in Hungary. Veter. Parasitol. 1997, 69, 133–144. [Google Scholar] [CrossRef]
- Pugh, D.G.; Baird, A.N. Diseases of the integumentary system. In Sheep and Goat Medicine, 2nd ed.; Roberson, J.R., Baird, A.N., Pugh, D.G., Eds.; Elsevier: St. Louis Missouri, USA, 2011; pp. 274–279. [Google Scholar]
- Moberg, G.P. Biological response to stress: Implications for animal welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; CABI Publishing: Wallingford, UK, 2009; pp. 1–21. [Google Scholar]
- Brancaccio, P.; Lippi, G.; Maffulli, N. Biochemical markers of muscular damage. Clin. Chem. Lab. Med. 2010, 48, 757–767. [Google Scholar] [CrossRef]
- Owens, W.E.; Oliver, S.P.; E Gillespie, B.; Ray, C.H.; Nickerson, S.C. Role of horn flies (Haematobia irritans) in Staphylococcus aureus-induced mastitis in dairy heifers. Am. J. Veter. Res. 1998, 59, 1122–1124. [Google Scholar]
- Oliver, S.P.; Gillespie, B.E.; Headrick, S.J.; Lewis, M.J.; Dowlen, H.H. Prevalence, risk factors, and strategies for controlling mastitis in heifers during the periparturient period. Int. J. Appl. Res. Vet. Med. 2005, 3, 150–162. [Google Scholar]
- Förster, M.; Klimpel, S.; Mehlhorn, H.; Sievert, K.; Messler, S.; Pfeffer, K. Pilot study on synanthropic flies (e.g., Musca, Sarcophaga, Calliphora, Fannia, Lucilia, Stomoxys) as vectors of pathogenic microorganisms. Parasitol. Res. 2007, 101, 243–246. [Google Scholar] [CrossRef]
- Anderson, K.; Lyman, R.; Moury, K.; Ray, D.; Watson, D.; Correa, M. Molecular epidemiology of Staphylococcus aureus mastitis in dairy heifers. J. Dairy Sci. 2012, 95, 4921–4930. [Google Scholar] [CrossRef]
- Sajid, M.S.; Iqbal, A.; Khan, M.N.; Iqbal, Z.; Siddique, F. Descriptive epidemiology of insects infesting domestic sheep (Ovis aries) of district Toba Tek Singh, Punjab, Pakistan. Pak. J. Agric. Sci. 2013, 50, 117–122. [Google Scholar]
- Plant, J.W. A survey of chemical application techniques for fly and lice control. Pr. Aus. Sheep Vet. Soc. 1993, 107–115. [Google Scholar]
- Mehlhorn, H.; Al-Rasheid, K.A.S.; Abdel-Ghaffar, F.; Klimpel, S.; Pohle, H. Life cycle and attacks of ectoparasites on ruminants during the year in Central Europe: Recommendations for treatment with insecticides (e.g., Butox®). Parasitol. Res. 2010, 107, 425–431. [Google Scholar] [CrossRef]
- Bergonier, D.; Berthelot, X. New advances in epizootiology and control of ewe mastitis. Livest. Prod. Sci. 2003, 79, 1–16. [Google Scholar] [CrossRef]
- Mørk, T.; Waage, S.; Tollersrud, T.; Kvitle, B.; Sviland, S. Clinical mastitis in ewes; bacteriology, epidemiology and clinical features. Acta Veter. Scand. 2007, 49, 23. [Google Scholar] [CrossRef]
- Al-Majali, A.M.; Jawabreh, S. Period prevalence and aetiology of subclinical mastitis in Awassi sheep in Southern Jordan. Small Rumin. Res. 2003, 47, 243–248. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Cripps, P.J.; Ioannidi, K.S.; Chatzopoulos, D.C.; Gougoulis, D.A.; Sarrou, S.; Orfanou, D.C.; Politis, A.P.; Gonzalez-Valerio, T.C.; Argyros, S.; et al. Extensive countrywide field investigation of subclinical mastitis in sheep in Greece. J. Dairy Sci. 2018, 101, 7297–7310. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureusInfections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Murray, R.J.; Pearson, J.C.; Coombs, G.W.; Flexman, J.P.; Golledge, C.L.; Speers, D.J.; Dyer, J.R.; McLellan, D.G.; Reilly, M.; Bell, J.M.; et al. Outbreak of Invasive Methicillin-Resistant Staphylococcus aureusInfection Associated with Acupuncture and Joint Injection. Infect. Control. Hosp. Epidemiol. 2008, 29, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Piepers, S.; Peeters, K.; Opsomer, G.; Barkema, H.; Frankena, K.; De Vliegher, S. Pathogen group specific risk factors at herd, heifer and quarter levels for intramammary infections in early lactating dairy heifers. Prev. Veter. Med. 2011, 99, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Ryman, V.E.; Nickerson, S.C.; Hurley, D.J.; Berghaus, R.D.; Kautz, F.M. Influence of horn flies (Haematobia irritans) on teat skin condition, intramammary infection, and serum anti-S. aureus antibody titres in holstein heifers. Res. Vet. Sci. 2013, 95, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.N.; Abdel-Latef, G.K.; Abdel-Azeem, N.M.; El-Dakhly, K.M. Ecological study on antimicrobial-resistant zoonotic bacteria transmitted by flies in cattle farms. Parasitol. Res. 2016, 115, 3889–3896. [Google Scholar] [CrossRef]
- Lafi, S.Q.; Al-Majali, A.M.; Rousan, M.D.; Alawneh, J.M. Epidemiological studies of clinical and subclinical ovine mastitis in Awassi sheep in northern Jordan. Prev. Veter. Med. 1998, 33, 171–181. [Google Scholar] [CrossRef]
- Fragkou, I.A.; Boscos, C.M.; Fthenakis, G.C. Diagnosis of clinical or subclinical mastitis in ewes. Small Rumin. Res. 2014, 118, 86–92. [Google Scholar] [CrossRef]
- Derakhshani, H.; Fehr, K.B.; Sepehri, S.; Francoz, D.; De Buck, J.; Barkema, H.W.; Plaizier, J.C.; Khafipour, E. Invited review: Microbiota of the bovine udder: Contributing factors and potential implications for udder health and mastitis susceptibility. J. Dairy Sci. 2018, 101, 10605–10625. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M. Factors affecting mammary gland immunity and mastitis susceptibility. Livest. Prod. Sci. 2005, 98, 89–99. [Google Scholar] [CrossRef]
- Raynal-Ljutovac, K.; Pirisi, A.; De Crémoux, R.; Gonzalo, C. Somatic cells of goat and sheep milk: Analytical, sanitary, productive and technological aspects. Small Rumin. Res. 2007, 68, 126–144. [Google Scholar] [CrossRef]
- Berthelot, X.; Lagriffoul, G.; Concordet, D.; Barillet, F.; Bergonier, D. Physiological and pathological thresholds of somatic cell counts in ewe milk. Small Rumin. Res. 2006, 62, 27–31. [Google Scholar] [CrossRef]
- Fthenakis, G.; Jones, J. The effect of inoculation of coagulase-negative Staphylococci into the ovine mammary gland. J. Comp. Pathol. 1990, 102, 211–219. [Google Scholar] [CrossRef]
- Plant, J. Sheep ectoparasite control and animal welfare. Small Rumin. Res. 2006, 62, 109–112. [Google Scholar] [CrossRef]
- Eicher, S.; Morrow-Tesch, J.; Albright, J.; Williams, R. Tail-Docking Alters Fly Numbers, Fly-avoidance behaviors, and Cleanliness, but not Physiological Measures. J. Dairy Sci. 2001, 84, 1822–1828. [Google Scholar] [CrossRef]
- Mullens, B.A.; Lii, K.-S.; Mao, Y.; Meyer, J.A.; Peterson, N.G.; Szijj, C.E. Behavioural responses of dairy cattle to the stable fly, Stomoxys calcitrans, in an open field environment. Med. Vet. Èntomol. 2006, 20, 122–137. [Google Scholar] [CrossRef]
- Vitela-Mendoza, I.; Cruz-Vázquez, C.; Solano-Vergara, J.; Orihuela-Trujillo, A. Short communication: Relationship between serum cortisol concentration and defensive behavioral responses of dairy cows exposed to natural infestation by stable fly, Stomoxys calcitrans. J. Dairy Sci. 2016, 99, 9912–9916. [Google Scholar] [CrossRef]
- Möstl, E.; Palme, R. Hormones as indicators of stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Saco, Y.; Fina, M.; Giménez, M.; Pato, R.; Piedrafita, J.; Bassols, A. Evaluation of serum cortisol, metabolic parameters, acute phase proteins and faecal corticosterone as indicators of stress in cows. Veter. J. 2008, 177, 439–441. [Google Scholar] [CrossRef]
- Earley, B.; McDonnell, B.; Murray, M.; Prendiville, D.; Crowe, M. The effect of sea transport from Ireland to the Lebanon on inflammatory, adrenocortical, metabolic and behavioural responses of bulls. Res. Veter. Sci. 2011, 91, 454–464. [Google Scholar] [CrossRef]
- Schwinghammer, K.A.; Knapp, F.W.; Boling, J.A.; Schillo, K.K. Physiological and Nutritional Response of Beef Steers to Infestations of the Stable Fly (Diptera: Muscidae). J. Econ. Èntomol. 1986, 79, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Heffron, J.; Mitchell, G.; Dreyer, J. Muscle Fibre Type, Fibre Diameter and pH 1 Values of M. Longissimus Dorsi of Normal, Malignant Hyperthermia- and Pse-Susceptible Pigs. Br. Veter. J. 1982, 138, 45–50. [Google Scholar] [CrossRef]
- Mitchell, G.; Hattingh, J.; Ganhao, M. Stress in cattle assessed after handling, after transport and after slaughter. Veter. Rec. 1988, 123, 201–205. [Google Scholar] [CrossRef]
- Forcados, G.E.; Lohlum, A.S.; Usman, Y.; Tondo, B.K.; Atiku, A.A. Changes in serum biochemical parameters and oxidative stress biomarkers in grazing cattle. Comp. Haematol. Int. 2016, 25, 1013–1016. [Google Scholar] [CrossRef]
- Castro, E.; Gil, A.; Solari, M.A.; Farias, N.A. Validation of a subjective counting method for a horn flies (Haematobia irritans irritans) (Diptera: Muscidae) population in a cattle herd. Veter. Parasitol. 2005, 133, 363–367. [Google Scholar] [CrossRef]
- Mullens, B.A.; Soto, D.; Gerry, A.C. Estimating Field Densities ofHaematobia irritans(Diptera: Muscidae) Using Direct Visual Field Counts Versus Photographic Assessments. J. Med. Èntomol. 2016, 53, 703–706. [Google Scholar] [CrossRef]
- Mullens, B.A.; Watson, D.W.; Gerry, A.C.; Sandelin, B.A.; Soto, D.; Rawls, D.; Denning, S.; Guisewite, L.; Cammack, J. Field trials of fatty acids and geraniol applied to cattle for suppression of horn flies, Haematobia irritans (Diptera: Muscidae), with observations on fly defensive behaviors. Veter. Parasitol. 2017, 245, 14–28. [Google Scholar] [CrossRef]
- Wall, R.; Shearer, D. Adult Flies (Diptera). In Veterinary Ectoparasites: Biology, Pathology, and Control, 2nd ed.; Wall, R., Shearer, D., Eds.; Blackwell Science Ltd.: Oxford Malden, USA, 2001; pp. 83–113. [Google Scholar]
- Couri, M.S.; Pont, A.C.; Penny, N.D. Muscidae (Diptera) from Madagascar: Identification keys, descriptions of new species and new records. Proc. Calif. Acad. Sci. 2006, 57, 799–923. [Google Scholar]
- National Mastitis Council (NMC). Laboratory Handbook on Bovine Mastitis, 3rd ed.; National Mastitis Council: New Prague, Minnesota, 1999; pp. 1–148. [Google Scholar]
- Quin, P.J.; Markey, B.K.; Carter, M.E.; Carter, G.R. Clinical Veterinary Microbiology, 2nd ed.; Mosby: St. Louis, MI, USA, 2013; pp. 105–433. [Google Scholar]
- Nicholas, R.; Baker, S. Recovery of Mycoplasmas from Animals. Methods Mol. Biol. 1998, 104, 37–43. [Google Scholar] [CrossRef] [PubMed]
| Day | Group | ||
|---|---|---|---|
| FLY-REP | CON | ||
| 0 | (Pre-Treatment) | 45.0 a (± 11.04) | 53.0 a (± 6.01) |
| (6 Hours Post-Treatment) | 7.4 a (± 4.12) | 78.0 b (±1 9.83) | |
| 15 | 4.4 a (± 1.34) | 66.5 b (± 28.22) | |
| 30 | 7.3 a (± 2.52) | 69.8 b (± 12.34) | |
| Days | AUC FLY-REP | AUC CON |
|---|---|---|
| Staphylococcus aureus (CFU × 103/mL) | ||
| 0–15 | 50.2 a (± 4.99) | 3.7 b (± 0.69) |
| 15–30 | 8.8 a* (± 3.50) | 182.2 b (± 34.67) |
| Total | 59.9 a* (± 2.93) | 185.9 b (± 126.22) |
| Non-aureus Staphylococci (CFU × 103/mL) | ||
| 0–15 | 27.2 a (± 11.44) | 17.2 a (± 5.44) |
| 15–30 | 10.0 a* (± 1.20) | 175.9 b (± 12.46) |
| Total | 37.2 a* (± 12.17) | 193.1 b (± 112.19) |
| Somatic Cells Count (Cells×106/mL) | ||
| 0–15 | 24.7 a (± 16.60) | 37.9 b (± 12.22) |
| 15–30 | 11.4 a (± 0.59) | 43.0 b (± 21.06) |
| Total | 36.1 a (± 9.39) | 80.8 b (± 23.60) |
| Parameter | Category Level | B | SE | p-Value | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Logarithm of Staphylococcus aureus Colony-Forming Units (Mixed Linear Regression) | ||||||
| Deltamethrin Treatment | Yes | 0.13 | 0.176 | 0.459 | −0.23 | 0.49 |
| No | “Ref” | |||||
| Sampling Occasion | Day 0 | −0.32 | 0.210 | 0.135 | −0.74 | 0.10 |
| Day 15 | −0.88 | 0.198 | 0.000 | −1.28 | −0.49 | |
| Day 30 | “Ref” | |||||
| Intercept | Continuous | 3.68 | 0.144 | 0.000 | 3.39 | 3.97 |
| Logarithm of Non-aureus Staphylococci Colony-Forming Units (Mixed Linear Regression) | ||||||
| Deltamethrin Treatment | Yes | −0.44 | 0.171 | 0.014 | −0.79 | −0.10 |
| No | “Ref” | |||||
| Sampling Occasion | Day 0 | −0.60 | 0.151 | 0.000 | −0.90 | −0.30 |
| Day 15 | −0.58 | 0.127 | 0.000 | −0.83 | −0.32 | |
| Day 30 | “Ref” | |||||
| Intercept | Continuous | 3.59 | 0.139 | 0.000 | 3.31 | 3.87 |
| Logarithm of Milk Somatic Cell Counts (Mixed Linear Regression) | ||||||
| Deltamethrin Treatment | Yes | −0.53 | 0.107 | 0.003 | −0.80 | −0.26 |
| No | “Ref” | |||||
| Sampling Occasion | Day 0 | 0.36 | 0.080 | 0.000 | 0.20 | 0.51 |
| Day 15 | 0.09 | 0.069 | 0.200 | −0.05 | 0.23 | |
| Day 30 | “Ref” | |||||
| Intercept | Continuous | 6.16 | 0.150 | 0.012 | 4.55 | 7.78 |
| Serum Cortisol Level (Mixed Linear Regression) | ||||||
| Deltamethrin Treatment | Yes | −0.30 | 0.033 | 0.000 | −0.36 | −0.23 |
| No | “Ref” | |||||
| Sampling Occasion | Day 0 | 0.12 | 0.030 | 0.000 | 0.07 | 0.18 |
| Day 15 | −0.08 | 0.026 | 0.003 | −0.13 | −0.03 | |
| Day 30 | “Ref” | |||||
| Intercept | Continuous | 0.43 | 0.028 | 0.000 | 0.37 | 0.48 |
| Creatine Kinase Level (Inverse Gaussian Linear Regression) | ||||||
| Deltamethrin Treatment | Yes | −0.58 | 0.037 | 0.000 | −0.65 | −0.51 |
| No | “Ref” | |||||
| Sampling Occasion | Day 0 | 0.53 | 0.079 | 0.000 | 0.37 | 0.68 |
| Day 15 | 0.16 | 0.054 | 0.003 | 0.05 | 0.26 | |
| Day 30 | “Ref” | |||||
| Intercept | Continuous | 2.88 | 0.046 | 0.000 | 2.79 | 2.97 |
| Days | AUC FLY-REP | AUC CON |
|---|---|---|
| Cortisol (μg/dL) | ||
| 0–15 | 27.2a (± 4.55) | 34.4a (± 18.05) |
| 15–30 | 18.2a (± 2.34) | 32.8b (± 11.57) |
| Total | 45.4a (± 6.36) | 67.1b (± 18.13) |
| Creatine Kinase (× 103 nkat/L) | ||
| 0–15 | 13.3a (± 2.30) | 16.1a (± 0.45) |
| 15–30 | 3.7a (± 0.67) | 21.0b (± 8.67) |
| Total | 17.1a (± 6.79) | 37.1b (± 3.44) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arsenopoulos, K.V.; Sioutas, G.; Triantafillou, E.; Gelasakis, A.I.; Papadopoulos, E. Will Fly Repellency Using Deltamethrin Reduce Intramammary Infections, Stress and Fatigue Indicators of Dairy Ewes under Intensive Management? Pathogens 2021, 10, 232. https://doi.org/10.3390/pathogens10020232
Arsenopoulos KV, Sioutas G, Triantafillou E, Gelasakis AI, Papadopoulos E. Will Fly Repellency Using Deltamethrin Reduce Intramammary Infections, Stress and Fatigue Indicators of Dairy Ewes under Intensive Management? Pathogens. 2021; 10(2):232. https://doi.org/10.3390/pathogens10020232
Chicago/Turabian StyleArsenopoulos, Konstantinos V., Georgios Sioutas, Eleutherios Triantafillou, Athanasios I. Gelasakis, and Elias Papadopoulos. 2021. "Will Fly Repellency Using Deltamethrin Reduce Intramammary Infections, Stress and Fatigue Indicators of Dairy Ewes under Intensive Management?" Pathogens 10, no. 2: 232. https://doi.org/10.3390/pathogens10020232
APA StyleArsenopoulos, K. V., Sioutas, G., Triantafillou, E., Gelasakis, A. I., & Papadopoulos, E. (2021). Will Fly Repellency Using Deltamethrin Reduce Intramammary Infections, Stress and Fatigue Indicators of Dairy Ewes under Intensive Management? Pathogens, 10(2), 232. https://doi.org/10.3390/pathogens10020232










