Antitrypanosomal and Antileishmanial Activity of Chalcones and Flavanones from Polygonum salicifolium
Abstract
1. Introduction
2. Results
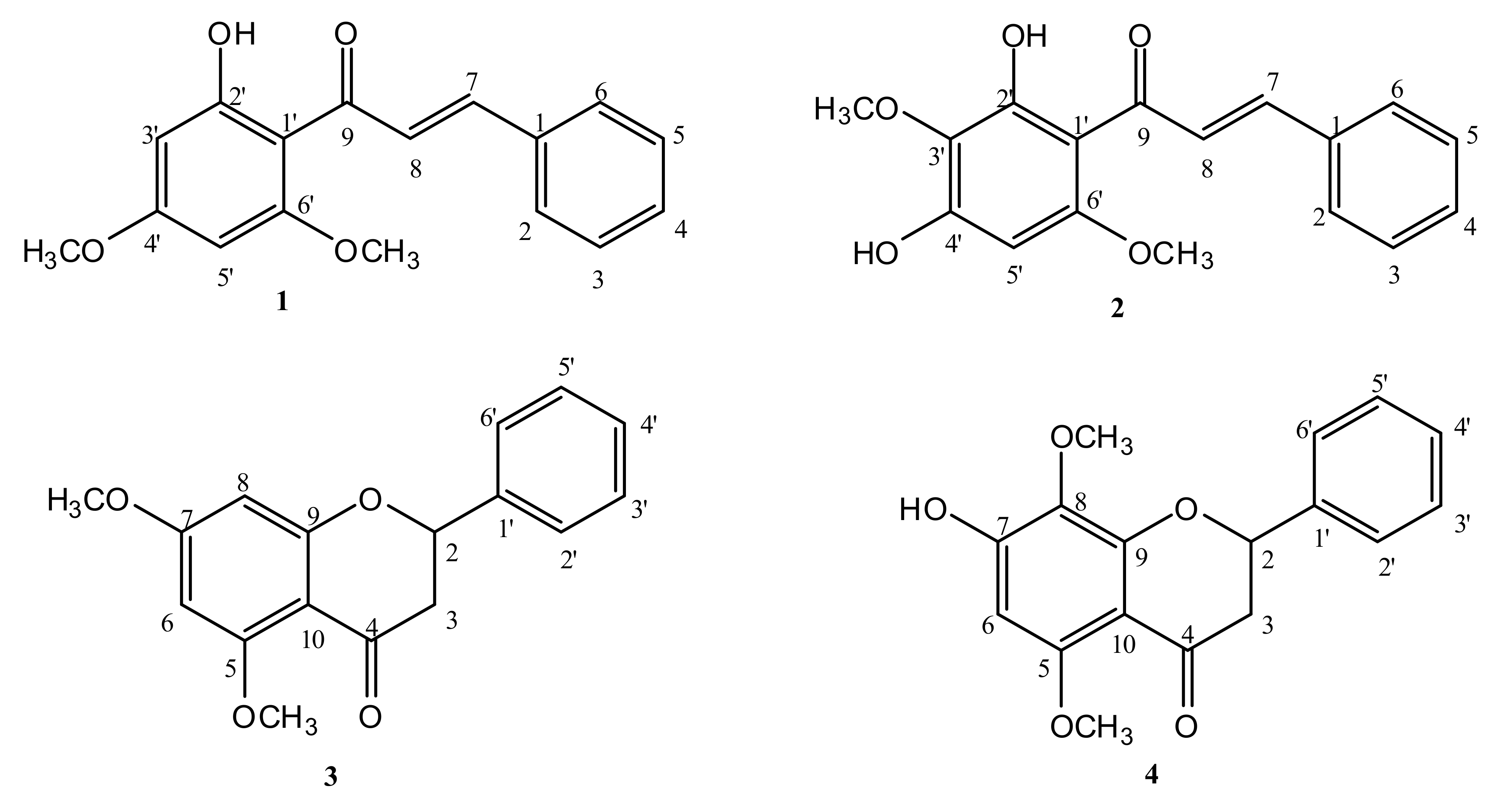
2.1. Isolation and Identification of Compounds
2.2. Anti-Kinetoplastid and Cytotoxic Activity of the Isolated Compounds
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Collection of Plant Material
4.3. Preparation of Extracts
4.4. Isolation and Identification of Compounds
4.5. Parasites and Cultures
4.6. Anti-Protozoal Drug Testing
4.7. Drug Toxicity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Al-Mudaffar Fawzi, N.; Goodwin, K.P.; Mahdi, B.A.; Stevens, M.L. Effects of Mesopotamian Marsh (Iraq) desiccation on the cultural knowledge and livelihood of Marsh Arab women. Ecosyst. Health Sustain. 2016, 2, e01207. [Google Scholar] [CrossRef]
- Hamdan, M.A.; Asada, T.; Hassan, F.M.; Warner, B.G.; Douabul, A.; Al-Hilli, M.R.; Alwan, A. Vegetation response to re-flooding in the Mesopotamian Wetlands, Southern Iraq. Wetlands 2010, 30, 177–188. [Google Scholar] [CrossRef]
- Calis, I.; Kuruüzüm, A.; Demirezer, L.Ö.; Sticher, O.; Ganci, W.; Rüedi, P. Phenylvaleric Acid and Flavonoid Glycosides from Polygonum salicifolium. J. Nat. Prod. 1999, 62, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.R.; Mohamed, A.A. Antioxidant activity and phenolic profiling of two Egyptian medicinal herbs Polygonum salicifolium Brouss ex Wild and Polygonum senegalense Meisn. An. Univ. din Oradea Fasc. Biol. 2013, 20, 59–63. [Google Scholar]
- Midiwo, J.O.; Yenesew, A.; Juma, B.; Derese, S.; Ayoo, J.; Aluoch, A.; Guchu, S. Bioactive compounds from some Kenyan ethnomedicinal plants: Myrsinaceae, Polygonaceae and Psiadia punctulata. Phytochem. Rev. 2002, 1, 311–323. [Google Scholar] [CrossRef]
- Kokwaro, J.O. Medicinal Plants of East Africa; East African Publishing Bureau: Nairobi, Kenya, 1976. [Google Scholar]
- Calderón, A.I.; Simithy-Williams, J.; Gupta, M.P. Antimalarial natural products drug discovery in Panama. Pharm. Biol. 2012, 50, 61–71. [Google Scholar] [CrossRef]
- Hu, Y.; Ji, J.; Ling, F.; Chen, Y.; Lu, L.; Zhang, Q.; Wang, G. Screening medicinal plants for use against Dactylogyrus intermedius (Monogenea) infection in goldfish. J. Aquat. Anim. Health 2014, 26, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Gürağaç Dereli, F.T.; Ilhan, M.; Kozan, E.; Küpeli Akkol, E. Effective eradication of pinworms (Syphacia obvelata and Aspiculuris tetraptera) with Polygonum cognatum Meissn. Exp. Parasitol. 2019, 196, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Liu, Y.M.; Zhang, Q.Z.; Fu, Y.W.; Lin, D.J. Evaluation of an antiparasitic compound extracted from Polygonum cuspidatum against Ichthyophthirius multifiliis in grass carp. Vet. Parasitol. 2018, 253, 22–25. [Google Scholar] [CrossRef]
- De Koning, H.P. The drugs of sleeping sickness: Their mechanisms of action and resistance, and a brief history. Trop. Med. Infect. Dis. 2020, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; De Koning, H.P.; Barrett, M.P. The animal trypanosomiases and their chemotherapy: A review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [CrossRef]
- Tabbabi, A. Review of leishmaniasis in the Middle East and North Africa. Afr. Health Sci. 2019, 19, 1329–1337. [Google Scholar] [CrossRef]
- Monge-Maillo, B.; Norman, F.F.; Cruz, I.; Alvar, J.; Lopez-Velez, R. Visceral leishmaniasis and HIV coinfection in the Mediterranean region. PLoS Negl. Trop. Dis. 2014, 8, e3021. [Google Scholar] [CrossRef] [PubMed]
- Babuadze, G.; Alvar, J.; Argaw, D.; De Koning, H.P.; Iosava, M.; Kekelidze, M.; Tsertsvadze, N.; Tsereteli, D.; Chakhunashvili, G.; Mamatsashvili, T. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl. Trop. Dis. 2014, 8, e2725. [Google Scholar] [CrossRef]
- Babuadze, G.; Farlow, J.; De Koning, H.P.; Carrillo, E.; Chakhunashvili, G.; Murskvaladze, M.; Kekelidze, M.; Karseladze, I.; Kokaia, N.; Kalandadze, I. Seroepidemiology and molecular diversity of Leishmania donovani complex in Georgia. Parasites Vectors 2016, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Delespaux, V.; De Koning, H.P. Drugs and drug resistance in African trypanosomiasis. Drug Resist. Updates 2007, 10, 30–50. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis–Authors’ reply. Lancet 2019, 393, 872–873. [Google Scholar] [CrossRef]
- Elmahallawy, E.K.; Agil, A. Treatment of leishmaniasis: A review and assessment of recent research. Curr. Pharm. Des. 2015, 21, 2259–2275. [Google Scholar] [CrossRef]
- Dike, V.T.; Vihiior, B.; Bosha, J.A.; Yin, T.M.; Ebiloma, G.U.; De Koning, H.P.; Igoli, J.O.; Gray, A.I. Antitrypanosomal activity of a novel taccalonolide from the tubers of Tacca leontopetaloides. Phytochem. Anal. 2016, 27, 217–221. [Google Scholar] [CrossRef]
- Ebiloma, G.U.; Igoli, J.O.; Katsoulis, E.; Donachie, A.-M.; Eze, A.; Gray, A.I.; De Koning, H.P. Bioassay-guided isolation of active principles from Nigerian medicinal plants identifies new trypanocides with low toxicity and no cross-resistance to diamidines and arsenicals. J. Ethnopharmacol. 2017, 202, 256–264. [Google Scholar] [CrossRef]
- Ebiloma, G.U.; Katsoulis, E.; Igoli, J.O.; Gray, A.I.; De Koning, H.P. Multi-target mode of action of a Clerodane-type diterpenoid from Polyalthia longifolia targeting African trypanosomes. Sci. Rep. 2018, 8, 4613. [Google Scholar] [CrossRef] [PubMed]
- Ebiloma, G.U.; Ayuga, T.D.; Balogun, E.O.; Gil, L.A.; Donachie, A.; Kaiser, M.; Herraiz, T.; Inaoka, D.K.; Shiba, T.; Harada, S. Inhibition of trypanosome alternative oxidase without its N-terminal mitochondrial targeting signal (ΔMTS-TAO) by cationic and non-cationic 4-hydroxybenzoate and 4-alkoxybenzaldehyde derivatives active against T. brucei and T. congolense. Eur. J. Med. Chem. 2018, 150, 385–402. [Google Scholar] [CrossRef]
- Omar, R.; Igoli, J.O.; Zhang, T.; Gray, A.I.; Ebiloma, G.U.; Clements, C.J.; Fearnley, J.; Ebel, R.E.; Paget, T.; De Koning, H.P. The chemical characterization of Nigerian propolis samples and their activity against Trypanosoma brucei. Sci. Rep. 2017, 7, 923. [Google Scholar] [CrossRef]
- Siheri, W.; Ebiloma, G.U.; Igoli, J.O.; Gray, A.I.; Biddau, M.; Akrachalanont, P.; Alenezi, S.; Alwashih, M.A.; Edrada-Ebel, R.; Muller, S. Isolation of a novel flavanonol and an alkylresorcinol with highly potent anti-trypanosomal activity from Libyan propolis. Molecules 2019, 24, 1041. [Google Scholar] [CrossRef] [PubMed]
- Siheri, W.; Zhang, T.; Ebiloma, G.U.; Biddau, M.; Woods, N.; Hussain, M.Y.; Clements, C.J.; Fearnley, J.; Ebel, R.E.; Paget, T. Chemical and antimicrobial profiling of propolis from different regions within Libya. PLoS ONE 2016, 11, e0155355. [Google Scholar] [CrossRef] [PubMed]
- Cerone, M.; Uliassi, E.; Prati, F.; Ebiloma, G.U.; Lemgruber, L.; Bergamini, C.; Watson, D.G.; de AM Ferreira, T.; Roth Cardoso, G.S.H.; Soares Romeiro, L.A. Discovery of sustainable drugs for neglected tropical diseases: Cashew nut shell liquid (CNSL)-based hybrids target mitochondrial function and ATP production in Trypanosoma brucei. ChemMedChem 2019, 14, 621–635. [Google Scholar] [CrossRef]
- Jhoo, J.; Freeman, J.P.; Heinze, T.M.; Moody, J.D.; Schnackenberg, R.K.; Berger, R.D.; Dragull, K.; Tang, C.; Ang, C.Y.W. In vitro cytotoxicity of nononpolar constituents from different parts of kava plant (Piper methysticum). J. Agric. Food Chem. 2006, 54, 3157–3162. [Google Scholar] [CrossRef] [PubMed]
- Dzoyem, J.P.; NKuete, A.H.; Kuete, V.; Tala, M.F.; Wabo, H.K.; Guru, S.K.; Rajput, V.S.; Sharma, A.; Tane, P.; Khan, I.A. Cytotoxicity and antimicrobial activity of the methanol extract and compounds from Polyg. Limbatum. Planta Med. 2012, 78, 787–792. [Google Scholar] [PubMed]
- Dzoyem, J.P.; Nkuete, A.H.; Ngameni, B.; Eloff, J.N. Anti-inflammatory and anticholinesterase activity of six flavonoids isolated from Polygonum and Dorstenia species. Arch. Pharmacal. Res. 2017, 40, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.D.; Fukuta, M.; Wei, A.C.; Elzaawely, A.A.; Khanh, T.D.; Tawata, S. Efficacy of extracting solvents to chemical components of kava (Piper methysticum) roots. J. Nat. Med. 2008, 62, 188. [Google Scholar] [CrossRef]
- Mitra, A.K.; Mawson, A.R. Neglected tropical diseases: Epidemiology and global burden. Trop. Med. Infect. Dis. 2017, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.P.; MacLeod, A.; Barrett, M.P.; Cover, B.; Jarvis, S.M. Further evidence for a link between melarsoprol resistance and P2 transporter function in African trypanosomes. Mol. Biochem. Parasitol. 2000, 106, 181–185. [Google Scholar] [CrossRef]
- Matovu, E.; Stewart, M.L.; Geiser, F.; Brun, R.; Mäser, P.; Wallace, L.J.; Burchmore, R.J.; Enyaru, J.C.; Barrett, M.P.; Kaminsky, R. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell 2003, 2, 1003–1008. [Google Scholar] [CrossRef]
- Coustou, V.; Guegan, F.; Plazolles, N.; Baltz, T. Complete in vitro life cycle of Trypanosoma congolense: Development of genetic tools. PLoS Negl. Trop. Dis. 2010, 4, e618. [Google Scholar] [CrossRef] [PubMed]
- Al-Salabi, M.I.; Wallace, L.J.; De Koning, H.P. A Leishmania major nucleobase transporter responsible for allopurinol uptake is a functional homolog of the Trypanosoma brucei H2 transporter. Mol. Pharmacol. 2003, 63, 814–820. [Google Scholar] [CrossRef]
- Alzahrani, K.J.; Matyugina, E.S.; Khandazhinskaya, A.L.; Kochetkov, S.N.; Seley-Radtke, K.L.; De Koning, H.P. Evaluation of the antiprotozoan properties of 5′-norcarbocyclic pyrimidine nucleosides. Bioorg. Med. Chem. Lett. 2017, 27, 3081–3086. [Google Scholar] [CrossRef] [PubMed]
- Eze, A.A.; Igoli, J.; Gray, A.I.; Skellern, G.G.; De Koning, H.P. The individual components of commercial isometamidium do not possess stronger trypanocidal activity than the mixture, nor bypass isometamidium resistance. Int. J. Parasitol. Drugs Drug Resist. 2019, 9, 54–58. [Google Scholar] [CrossRef]
- Gould, M.K.; Vu, X.L.; Seebeck, T.; De Koning, H.P. Propidium iodide-based methods for monitoring drug action in the kinetoplastidae: Comparison with the Alamar Blue assay. Anal. Biochem. 2008, 382, 87–93. [Google Scholar] [CrossRef]
- Alzahrani, K.J.; Ali, J.A.; Eze, A.A.; Looi, W.L.; Tagoe, D.N.; Creek, D.J.; Barrett, M.P.; De Koning, H.P. Functional and genetic evidence that nucleoside transport is highly conserved in Leishmania species: Implications for pyrimidine-based chemotherapy. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 206–226. [Google Scholar] [CrossRef]
- Rodenko, B.; Wanner, M.J.; Alkhaldi, A.A.; Ebiloma, G.U.; Barnes, R.L.; Kaiser, M.; Brun, R.; McCulloch, R.; Koomen, G.-J.; De Koning, H.P. Targeting the parasite’s DNA with methyltriazenyl purine analogs is a safe, selective, and efficacious antitrypanosomal strategy. Antimicrob. Agents Chemother. 2015, 59, 6708–6716. [Google Scholar] [CrossRef]
| T. b. brucei EC50 (µM) | T. congolense EC50 (µM) | L. mexicana EC50 (µM) | HFF | |||||
|---|---|---|---|---|---|---|---|---|
| Compound | MW | 427-WT | B48 | IL3000-WT | DA-Res | WT | EC50 (µM) | SI (Tbb) |
| 1 | 284.3 | 2.04 ± 0.07 | 1.80 ± 011 | 8.8 ± 0.39 | 8.8 ± 0.28 | 18.2 ± 1.0 | >350 | >172 |
| 2 | 300.3 | 14.6 ± 0.80 | 13.9 ± 0.60 | 34.0 ± 1.2 | 28.6 ± 1.4 * | 83.6 ± 0.7 | >330 | >22.8 |
| 3 | 284.3 | 30.1 ± 0.95 | 30.3 ± 1.1 | 137 ± 10 | 106 ± 15 | 271 ± 31 | >350 | >11.7 |
| 4 | 300.3 | 55.3 ± 1.6 | 51.9 ± 1.6 | 63.3 ± 7.9 | 48.0 ± 6.2 | 338 ± 9 | >330 | >6.0 |
| Diminazene | 0.15 ± 0.01 | 2.4 ± 0.36 ** | 0.15 ± 0.01 | 1.43 ± 0.03 *** | ND | ND | -- | |
| Pentamidine | 0.0034 ± 0.0004 | 0.72 ± 0.03 *** | 0.72 ± 0.07 | ND | 0.56 ± 0.07 | ND | -- | |
| PAO | ND | ND | ND | ND | ND | 1.31 ± 0.08 | -- | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheoat, A.M.; Alenezi, S.; Elmahallawy, E.K.; Ungogo, M.A.; Alghamdi, A.H.; Watson, D.G.; Igoli, J.O.; Gray, A.I.; de Koning, H.P.; Ferro, V.A. Antitrypanosomal and Antileishmanial Activity of Chalcones and Flavanones from Polygonum salicifolium. Pathogens 2021, 10, 175. https://doi.org/10.3390/pathogens10020175
Zheoat AM, Alenezi S, Elmahallawy EK, Ungogo MA, Alghamdi AH, Watson DG, Igoli JO, Gray AI, de Koning HP, Ferro VA. Antitrypanosomal and Antileishmanial Activity of Chalcones and Flavanones from Polygonum salicifolium. Pathogens. 2021; 10(2):175. https://doi.org/10.3390/pathogens10020175
Chicago/Turabian StyleZheoat, Ahmed M., Samya Alenezi, Ehab Kotb Elmahallawy, Marzuq A. Ungogo, Ali H. Alghamdi, David G. Watson, John O. Igoli, Alexander I. Gray, Harry P. de Koning, and Valerie A. Ferro. 2021. "Antitrypanosomal and Antileishmanial Activity of Chalcones and Flavanones from Polygonum salicifolium" Pathogens 10, no. 2: 175. https://doi.org/10.3390/pathogens10020175
APA StyleZheoat, A. M., Alenezi, S., Elmahallawy, E. K., Ungogo, M. A., Alghamdi, A. H., Watson, D. G., Igoli, J. O., Gray, A. I., de Koning, H. P., & Ferro, V. A. (2021). Antitrypanosomal and Antileishmanial Activity of Chalcones and Flavanones from Polygonum salicifolium. Pathogens, 10(2), 175. https://doi.org/10.3390/pathogens10020175









