In Vitro and In Planta Studies on Temperature Adaptation of Exserohilum turcicum Isolates from Maize in Europe and South America
Abstract
1. Introduction
2. Results
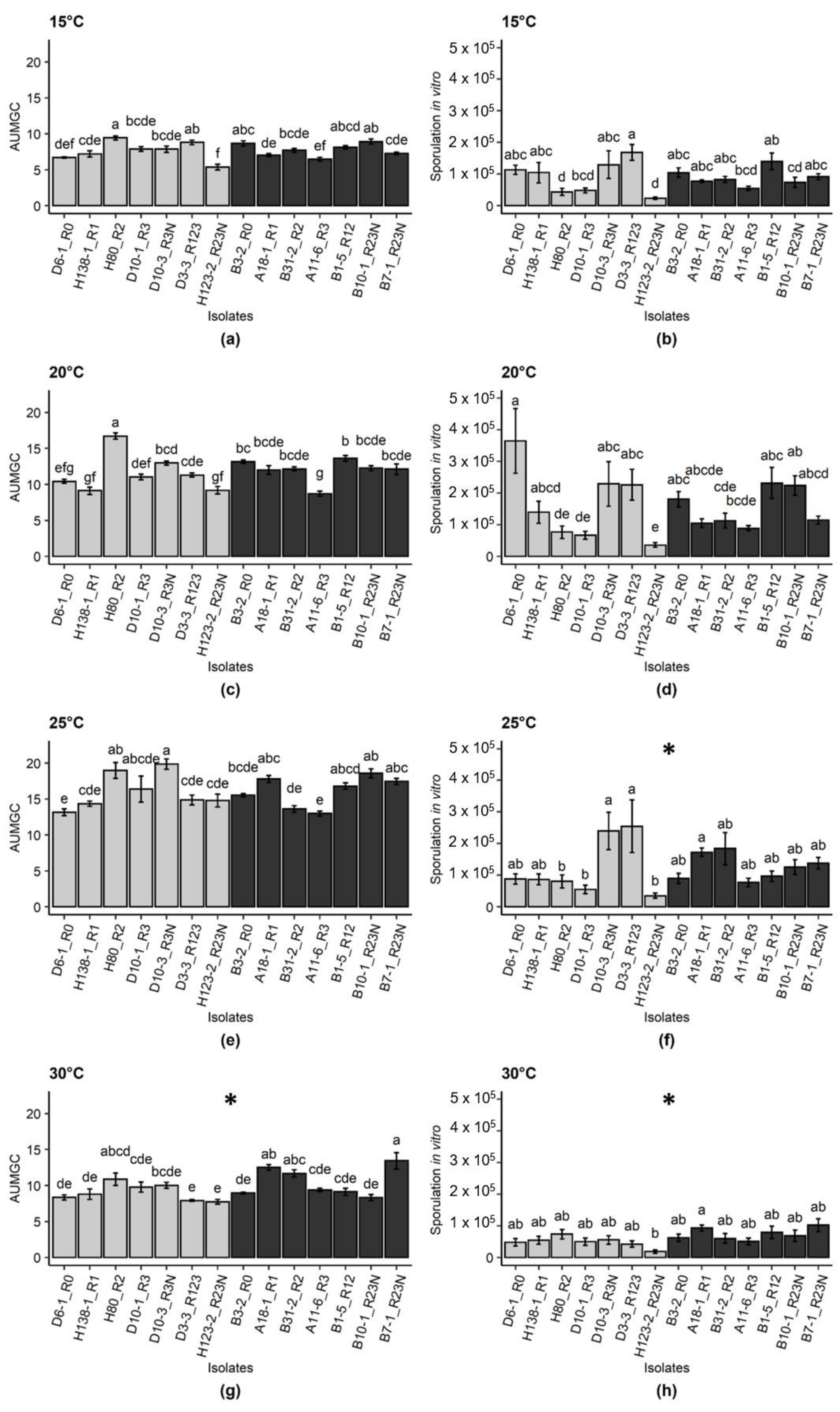
2.1. Effect of Temperature and Isolate Origin on Pathogen Development In Vitro
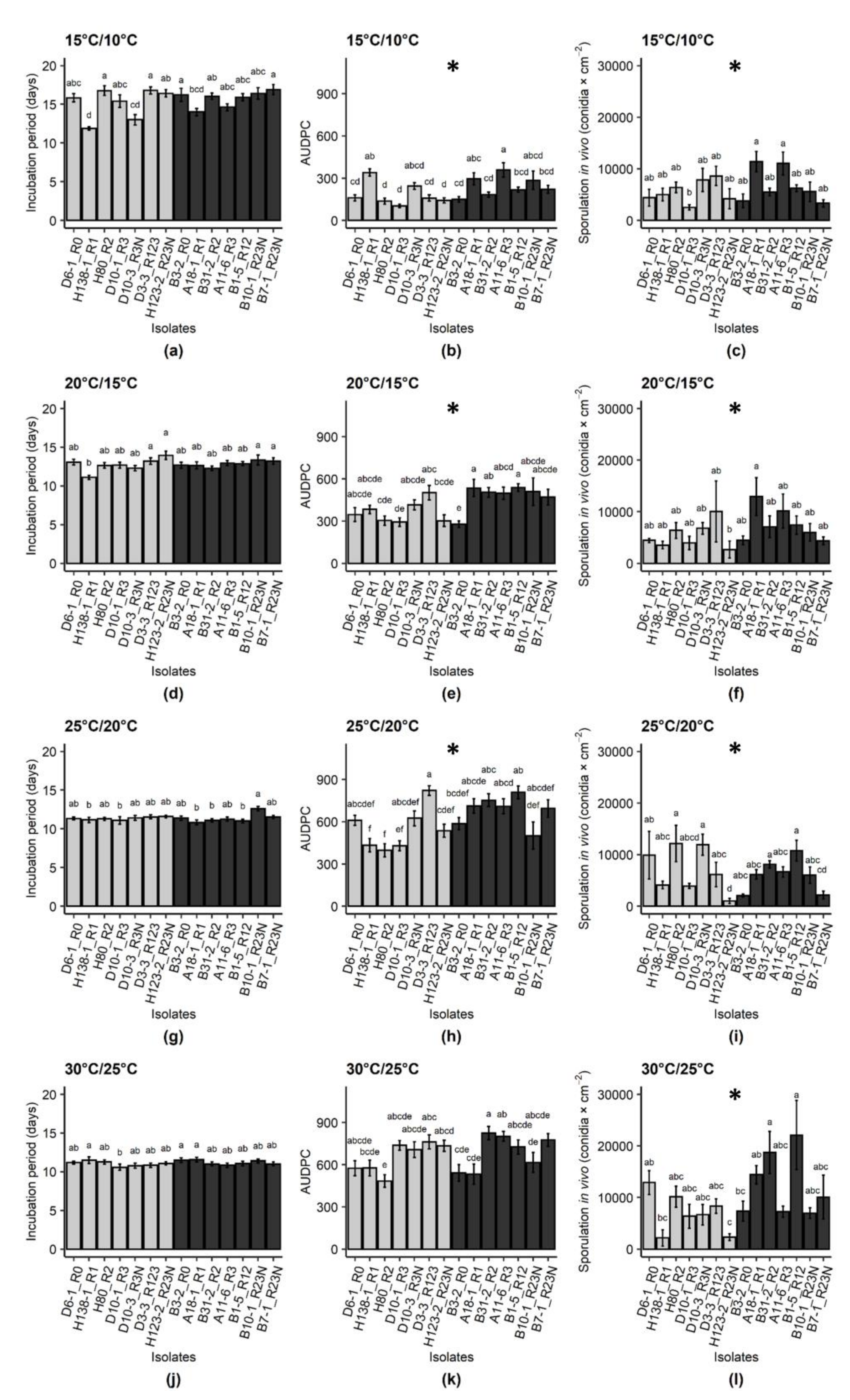
2.2. Effect of Isolate Origin, Temperature and Host Genotype on Incubation Period, Disease Severity and Sporulation
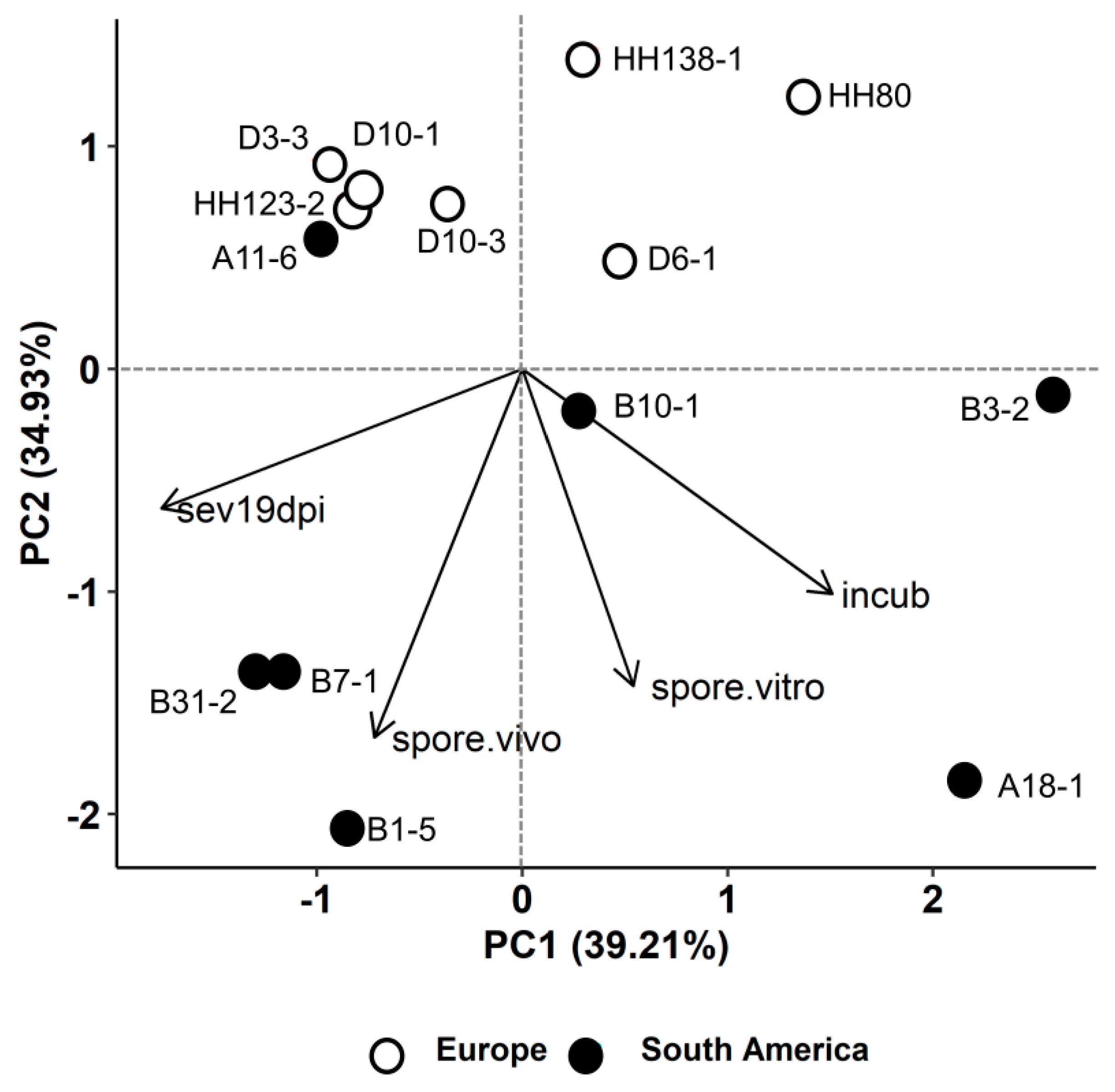
2.3. Relation between Aggressiveness Components and Isolate Groups
3. Discussion
4. Materials and Methods
4.1. Exserohilum turcicum Isolates
4.2. In Vitro Tests
4.3. In Vivo Tests
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Additional Information
Appendix A

References
- CABI. Crop Protection Compendium: Setosphaeria turcica (Maize Leaf Blight). Available online: https://www.cabi.org/cpc/datasheet/49783 (accessed on 12 May 2020).
- Levy, Y.; Pataky, J.K. Epidemiology of northern leaf blight on sweet corn. Phytoparasistica 1992, 20, 53–66. [Google Scholar] [CrossRef]
- Levy, Y.; Cohen, Y. Biotic and environmental factors affecting infection of sweet corn with Exserohilum turcicum. Phytopathology 1983, 73, 722–725. [Google Scholar] [CrossRef]
- Levy, Y.; Leonard, K.J. Yield loss in sweet corn in response to defoliation or infection by Exserohilum turcicum. J. Phytopathol. 1990, 128, 161–171. [Google Scholar] [CrossRef]
- Rossi, R.; Plazas, M.; Brucher, E.; Ducasse, D.; Guerra, G. El Tizón del Maíz (Exserohilum turcicum): Presencia e Impacto en el Centro Norte de Córdoba Durante Tres Campañas Agrícolas; Actas IX Congreso Nacional de Maíz: Rosario, Argentina, 2010; pp. 17–19. [Google Scholar]
- Cota, L.V.; Silva, D.D.; Costa, R.V. Helmintosporiose causada por Exserohilum turcicum na culturado milho. Embrapa Circ. Téc. 2013, 195, 1–8. [Google Scholar]
- BVL. Federal Office of Consumer Protection and Food Safety (Bundesamts für Verbraucherschutz und Lebensmittelsicherheit, BVL). Zugelassener Pflanzenschutzmittel. Available online: https://apps2.bvl.bund.de/psm/jsp/ListeMain.jsp?page=1&ts=1589917920513 (accessed on 19 May 2020).
- Peel, M.C.; Finlayson, B.L.; Mcmahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- MAPA. Ministry of Agriculture, Livestock, and Supply (Ministério da Agricultura, Pecuária e Abastecimento, MAPA). Agrofit. Available online: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 25 May 2020).
- Hanekamp, H. Europäisches Rassen-Monitoring und Pathogenesestudien zur Turcicum-Blattdürre (Exserohilum turcicum) an Mais (Zea mays L.); Georg-August Universität Göttingen: Göttingen, Germany, 2016. [Google Scholar]
- Navarro, B.L.; Romero, L.R.; Kistner, M.B.; Iglesias, J.; von Tiedemann, A. Monitoring of physiological races of Exserohilum turcicum isolates from maize in Argentina and Brazil. Trop. Plant Pathol. 2021. manuscript accepted for publication. [Google Scholar]
- Gianasi, L.; Castro, H.A.; Silva, H.D. Raças fisiológicas de Exserohilum turcicum identificadas em regiões produtoras de milho no Brasil, Safra 93/94. Summa Phytopathol. 1996, 22, 214–217. [Google Scholar]
- Ogliari, J.B.; Guimarães, M.A.; Geraldi, I.O.; Camargo, L.E.A. New resistance genes in the Zea mays—Exserohilum turcicum pathosystem. Genet. Mol. Biol. 2005, 28, 435–439. [Google Scholar] [CrossRef]
- Welz, H.G.; Geiger, H.H. Genes for resistance to northern corn leaf blight in diverse maize populations. Plant Breed. 2000, 119, 1–14. [Google Scholar] [CrossRef]
- Hilu, H.M.; Hooker, A.L. Host-pathogen relationship of Helminthosporium turcicum in resistant and susceptible corn seedlings. Phytopathology 1964, 54, 570–575. [Google Scholar]
- Levy, Y.; Cohen, Y. Differential effect of light on spore germination of Exserohilum turcicum on corn leaves and corn leaf impressions. Phytopathology 1983, 73, 249–252. [Google Scholar] [CrossRef]
- Borchardt, D.S.; Welz, H.G.; Geiger, H.H. Genetic structure of Setosphaeria turcica populations in tropical and temperate climates. Phytopathology 1998, 88, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Welz, H.G. Genetics and Epidemiology of the Pathosystem Zea mays/Setosphaeria turcica. Habilitation Thesis, Stuttgart, Düren/Maastricht, Germany, 1998. [Google Scholar]
- Vanderplank, J.E. Disease Resistance in Plants; Academic Press: New York, NY, USA, 1968. [Google Scholar]
- Andrivon, D. Nomenclature for pathogenicity and virulence: The need for precision. Phytopathology 1993, 83, 889–890. [Google Scholar] [CrossRef]
- Delmas, C.E.L.; Fabre, F.; Jolivet, J.; Mazet, I.D.; Cervera, S.R.; Delière, L.; Delmotte, F. Adaptation of a plant pathogen to partial host resistance: Selection for greater aggressiveness in grapevine downy mildew. Evol. Appl. 2016, 9, 709–725. [Google Scholar] [CrossRef]
- Lannou, C. Variation and selection of quantitative traits in plant pathogens. Annu. Rev. Phytopathol. 2012, 50, 319–338. [Google Scholar] [CrossRef]
- Kranz, J. Comparative Epidemiology of Plant Diseases; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Pariaud, B.; Ravigné, V.; Halkett, F.; Goyeau, H.; Carlier, J.; Lannou, C. Aggressiveness and its role in the adaptation of plant pathogens. Plant Pathol. 2009, 58, 409–424. [Google Scholar] [CrossRef]
- Bergamin Filho, A.; Amorim, L. Doenças de Plantas Tropicais: Epidemiologia e Controle Econômico; Editora Agronômica Ceres Lda.: São Paulo, Brazil, 1996. [Google Scholar]
- Vitti, A.J.; Filho, A.B.; Amorim, L.; Fegies, N.C. Comparative epidemiology of common maize rust and northern corn leaf blight: II. Development under natural conditions. Summa Phytopathol. 1995, 21, 131–133. [Google Scholar]
- Carson, M.L. Response of maize synthetic to selection for components of partial resistance to Exserohilum turcicum. Plant Dis. 2006, 90, 910–914. [Google Scholar] [CrossRef]
- Uloth, M.B.; You, M.P.; Cawthray, G.; Barbetti, M.J. Temperature adaptation in isolates of Sclerotinia sclerotiorum affects their ability to infect Brassica carinata. Plant Pathol. 2015, 64, 1140–1148. [Google Scholar] [CrossRef]
- Muiru, W.M.; Koopmann, B.; von Tiedemann, A.; Mutitu, E.W.; Kimenju, J.W. Race typing and evaluation of aggressiveness of Exserohilum turcicum isolates of Kenyan, german and Austrian Origin. World J. Agric. Sci. 2010, 6, 277–284. [Google Scholar]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Scholthof, K.B.G. The disease triangle: Pathogens, the environment and society. Nat. Ecol. Evol. 2007, 5, 152–156. [Google Scholar] [CrossRef]
- Santini, A.; Ghelardini, L. Plant pathogen evolution and climate change. CABI Rev. 2015, 10. [Google Scholar] [CrossRef]
- Chen, F.; Duan, G.-H.; Li, D.-L.; Zhan, J. Host resistance and temperature-dependent evolution of aggressiveness in the plant pathogen Zymoseptoria tritici. Front. Microbiol. 2017, 8, 1217. [Google Scholar] [CrossRef]
- Mariette, N.; Kröner, A.; Mabon, R.; Montarry, J.; Marquer, B.; Corbière, R.; Androdias, A.; Andrivon, D. A trade-off between sporangia size and number exists in the potato late blight pathogen Phytophthora infestans, and is not altered by biotic and abiotic factors. Front. Plant Sci. 2018, 9, 1841. [Google Scholar] [CrossRef] [PubMed]
- Mundt, C.C. Durable resistance: A key to sustainable management of pathogens and pests. Infect. Genet. Evol. 2014, 27, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Loladze, A.; Druml, T.; Wellings, C.R. Temperature adaptation in Australasian populations of Puccinia striiformis f. sp. tritici. Plant Pathol. 2014, 63, 572–580. [Google Scholar] [CrossRef]
- INMET. Instituto Nacional de Meteorologia. Normais Climatológicas do Brasil. Available online: http://www.inmet.gov.br/portal/index.php?r=clima/normaisClimatologicas (accessed on 11 May 2020).
- Wang, H.; Li, H.; Zhu, Z.; Wang, X. Expression of Ht2-related genes in response to the HT-toxin of Exserohilum turcicum in maize. Ann. Appl. Biol. 2010, 156, 111–120. [Google Scholar] [CrossRef]
- Levy, Y. Analysis in epidemics of northern neaf blight on sweet corn in Israel. Phytopathology 1989, 79, 1243–1245. [Google Scholar] [CrossRef]
- Aliaga, V.S.; Ferrelli, F.; Piccolo, M.C. Regionalization of climate over the Argentine Pampas. Int. J. Climatol. 2017, 37, 1237–1247. [Google Scholar] [CrossRef]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate change effects on plant disease: Genomes and ecosystems. Annu. Rev. Phytopathol. 2006, 44, 490–509. [Google Scholar] [CrossRef] [PubMed]
- Robeson, D.J.; Strobel, G.A. Monocerin, a phytotoxin from Exserohilum turcicum (≡ Drechslera turcica). Agric. Biol. Chem. 1982, 46, 2681–2683. [Google Scholar] [CrossRef]
- Coakley, S.M.; Scherm, H.; Chakraborty, S. Climate change and plant disease management. Annu. Rev. Phytopathol. 1999, 37, 399–426. [Google Scholar] [CrossRef] [PubMed]
- Tagle, A.G.; Chuma, I.; Tosa, Y. Rmg7, a new gene for resistance to triticum isolates of Pyricularia oryzae identified in tetraploid wheat. Phytopathology 2015, 105, 495–499. [Google Scholar] [CrossRef]
- Pedras, M.S.; Zaharia, I.L.; Gai, Y.; Zhou, Y.; Ward, D.E. In planta sequential hydroxylation and glycosylation of fungal phytotoxin: Avoiding cell death and overcoming the fungal invader. Proc. Natl. Acad. Sci. USA 2001, 98, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, A.; Willocquet, L.; Savary, S.; Kumar, J. Variability in aggressiveness of rice blast (Magnaporthe oryzae) isolates originating from rice leaves and necks: A case of pathogen specialization? PLoS ONE 2013, 8, e66180. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Yang, L.; Zhu, W.; Shang, L.; Newton, A.C. Pathogen populations evolve to greater race complexity in agricultural systems—Evidence from analysis of Rhynchosporium secalis virulence data. PLoS ONE 2012, 7, e38611. [Google Scholar] [CrossRef][Green Version]
- Bigirwa, G.; Julian, A.M.; Adipala, E. Characterization of Ugandan isolates of Exserohilum turcicum from maize. Afr. Crop Sci. J. 1993, 1, 69–72. [Google Scholar]
- Weems, J.D.; Bradley, C.A. Exserohilum turcicum race population distribution in the north central United States. Plant Dis. 2018, 102, 292–299. [Google Scholar] [CrossRef]
- Berger, R.D. The analysis of effects of control measures on the development of epidemics. In Experimental Techniques in Plant Disease Epidemiology; Kranz, J., Rotem, J., Eds.; Springer: Berlin, Germany, 1988; pp. 137–151. [Google Scholar]
- Pataky, J.K. Relationships between yield of sweet corn and northern leaf blight caused by Exserohilum turcicum. Phytopathology 1992, 82, 370–375. [Google Scholar] [CrossRef]


| Isolate | Continent | Country | Region | City | Race | Rc 1 | Climate 2 |
|---|---|---|---|---|---|---|---|
| D6-1 | Europe | Germany | Bayern | Regensburg | Race 0 | 0 | Dfb |
| HH138-1 | Europe | France | Oberhein Region | Fessenheim | Race 1 | 1 | Cfb |
| HH80 | Europe | Belgium | East Flanders | Beervelde | Race 2 | 1 | Cfb |
| D10-1 | Europe | Germany | Niedersachsen | Meppen | Race 3 | 1 | Dfb |
| D10-3 | Europe | Germany | Niedersachsen | Meppen | Race 3N | 2 | Dfb |
| D3-3 | Europe | Germany | Bayern | Regensburg | Race 123 | 3 | Dfb |
| HH123-2 | Europe | Turkey | Adana | Adana | Race 23N | 3 | Csa |
| B3-2 | South America | Brazil | Paraná | Castro | Race 0 | 0 | Cfb |
| A18-1 | South America | Argentina | Entre Ríos | Victoria | Race 1 | 1 | Cfa |
| B31-2 | South America | Brazil | Rio Grande do Sul | Horizontina | Race 2 | 1 | Cfa |
| A11-6 | South America | Argentina | Missiones | Pergamino | Race 3 | 1 | Cfa |
| B1-5 | South America | Brazil | Paraná | Campo Largo | Race 12 | 2 | Cfb |
| B10-1 | South America | Brazil | Minas Gerais | Florestal | Race 23N | 3 | Cwa |
| B7-1 | South America | Brazil | Minas Gerais | Sete Lagoas | Race 23N | 3 | Cwa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, B.L.; Campos, R.d.A.; Gasparoto, M.C.d.G.; von Tiedemann, A. In Vitro and In Planta Studies on Temperature Adaptation of Exserohilum turcicum Isolates from Maize in Europe and South America. Pathogens 2021, 10, 154. https://doi.org/10.3390/pathogens10020154
Navarro BL, Campos RdA, Gasparoto MCdG, von Tiedemann A. In Vitro and In Planta Studies on Temperature Adaptation of Exserohilum turcicum Isolates from Maize in Europe and South America. Pathogens. 2021; 10(2):154. https://doi.org/10.3390/pathogens10020154
Chicago/Turabian StyleNavarro, Barbara Ludwig, Raphael de Araújo Campos, Maria Cândida de Godoy Gasparoto, and Andreas von Tiedemann. 2021. "In Vitro and In Planta Studies on Temperature Adaptation of Exserohilum turcicum Isolates from Maize in Europe and South America" Pathogens 10, no. 2: 154. https://doi.org/10.3390/pathogens10020154
APA StyleNavarro, B. L., Campos, R. d. A., Gasparoto, M. C. d. G., & von Tiedemann, A. (2021). In Vitro and In Planta Studies on Temperature Adaptation of Exserohilum turcicum Isolates from Maize in Europe and South America. Pathogens, 10(2), 154. https://doi.org/10.3390/pathogens10020154







