A Neutralization Assay Based on Pseudo-Typed Lentivirus with SARS CoV-2 Spike Protein in ACE2-Expressing CRFK Cells
Abstract
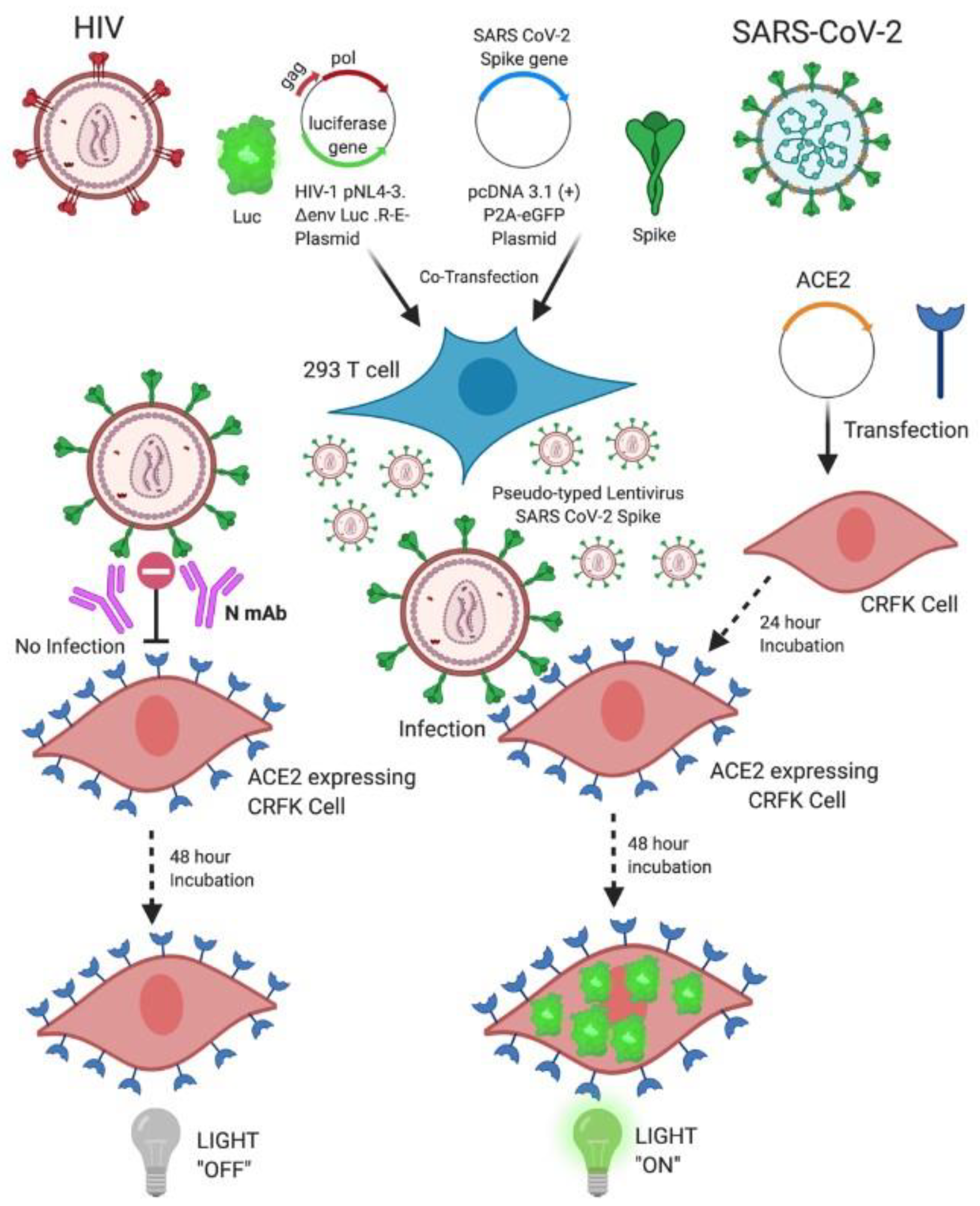
1. Introduction
2. Results
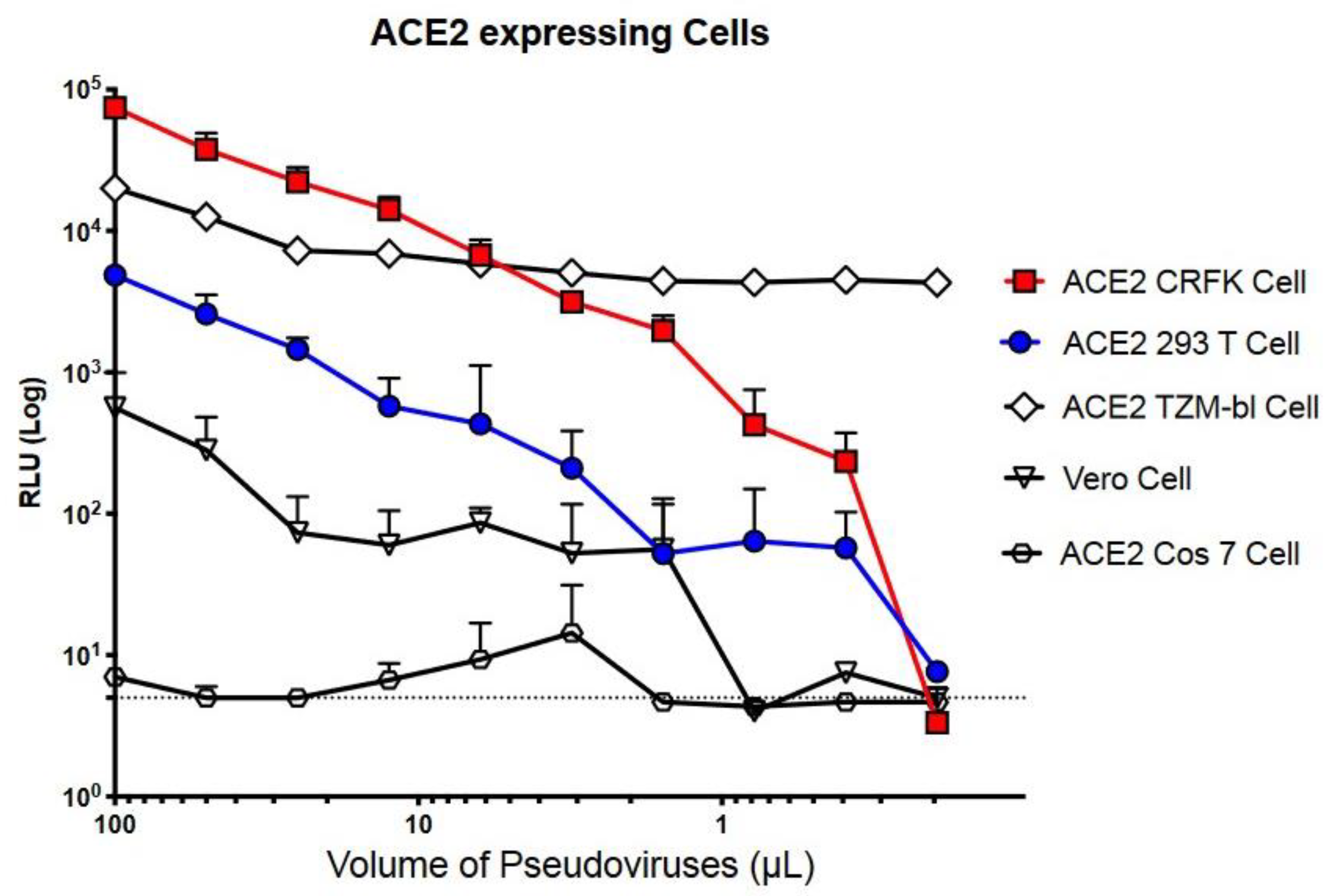
2.1. Measuring LpVspike(+) Infectivity in Various ACE2-Expressing Cell Types
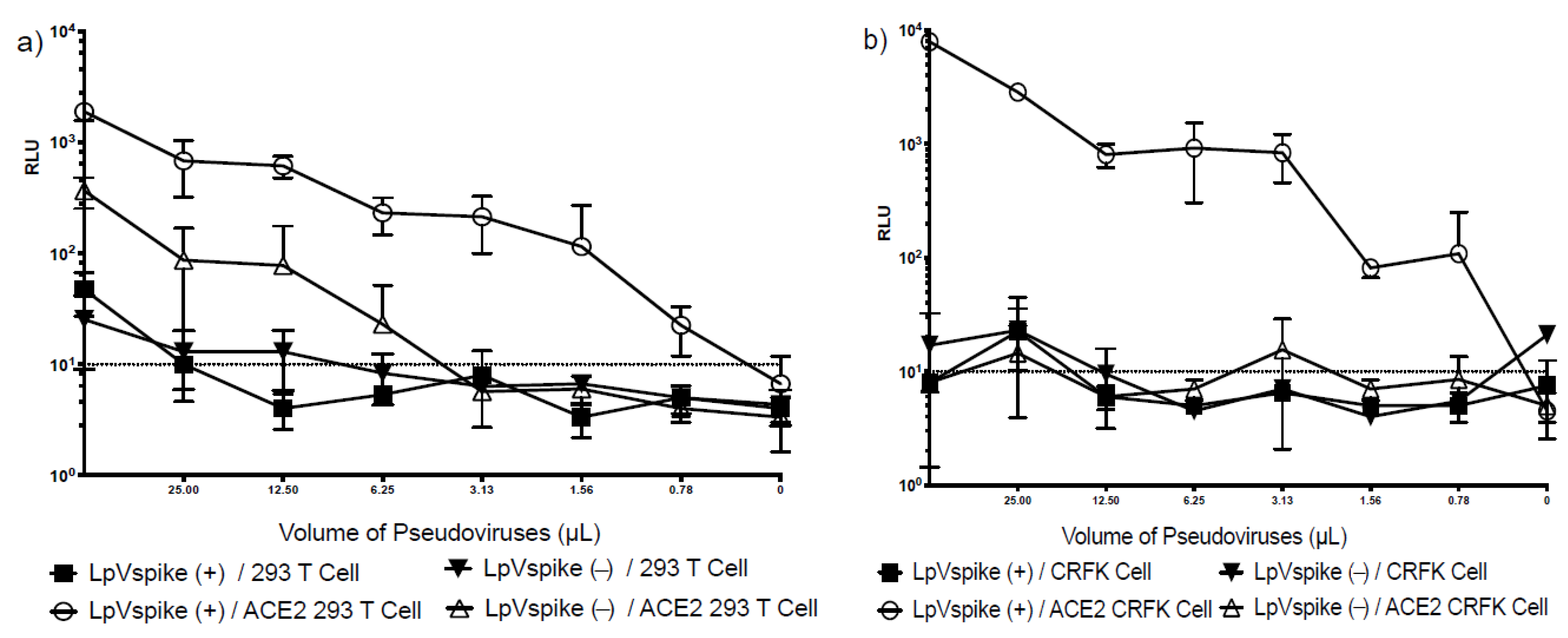
2.2. The Comparison of LpVspike(+) Infectivity in HEK293T and CRFK Cell Lines with/without ACE2 Transfection
2.3. Effect of Diethylaminoethyl-Dextran on Infectivity
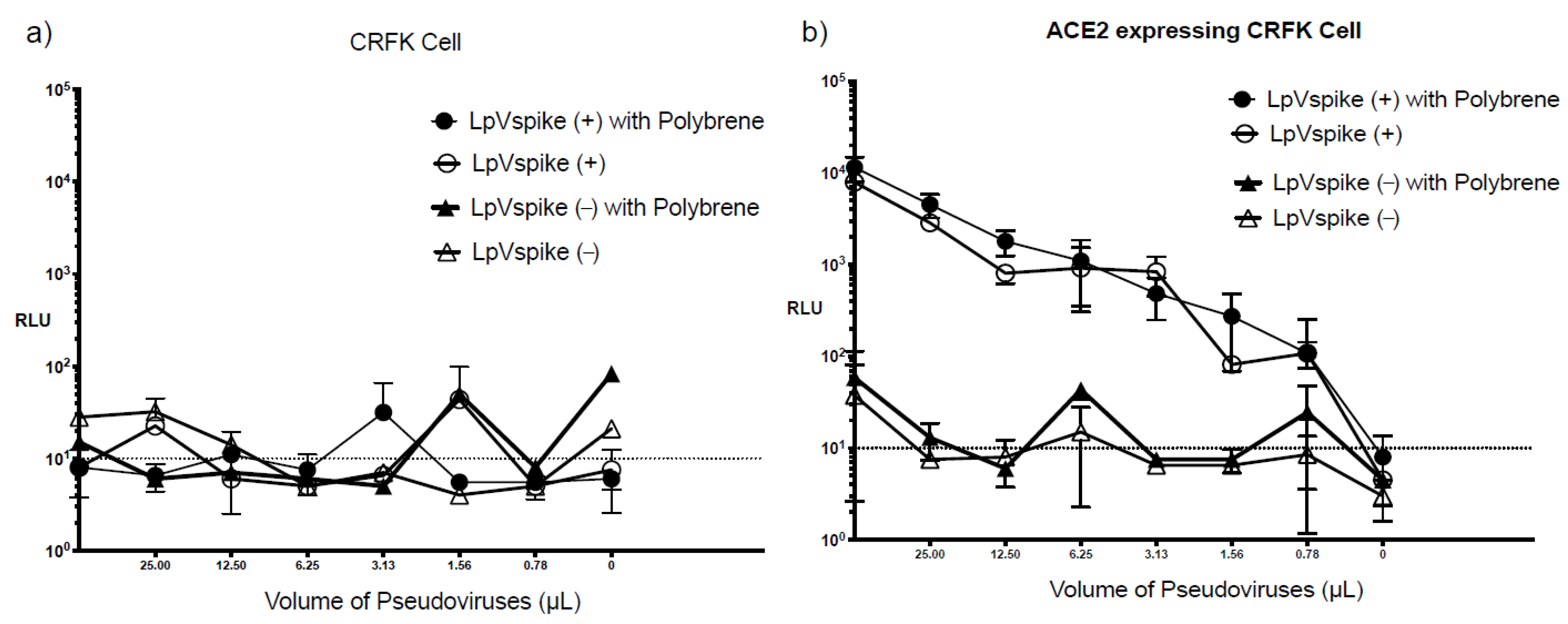
2.4. Effect of Polybrene on Infectivity
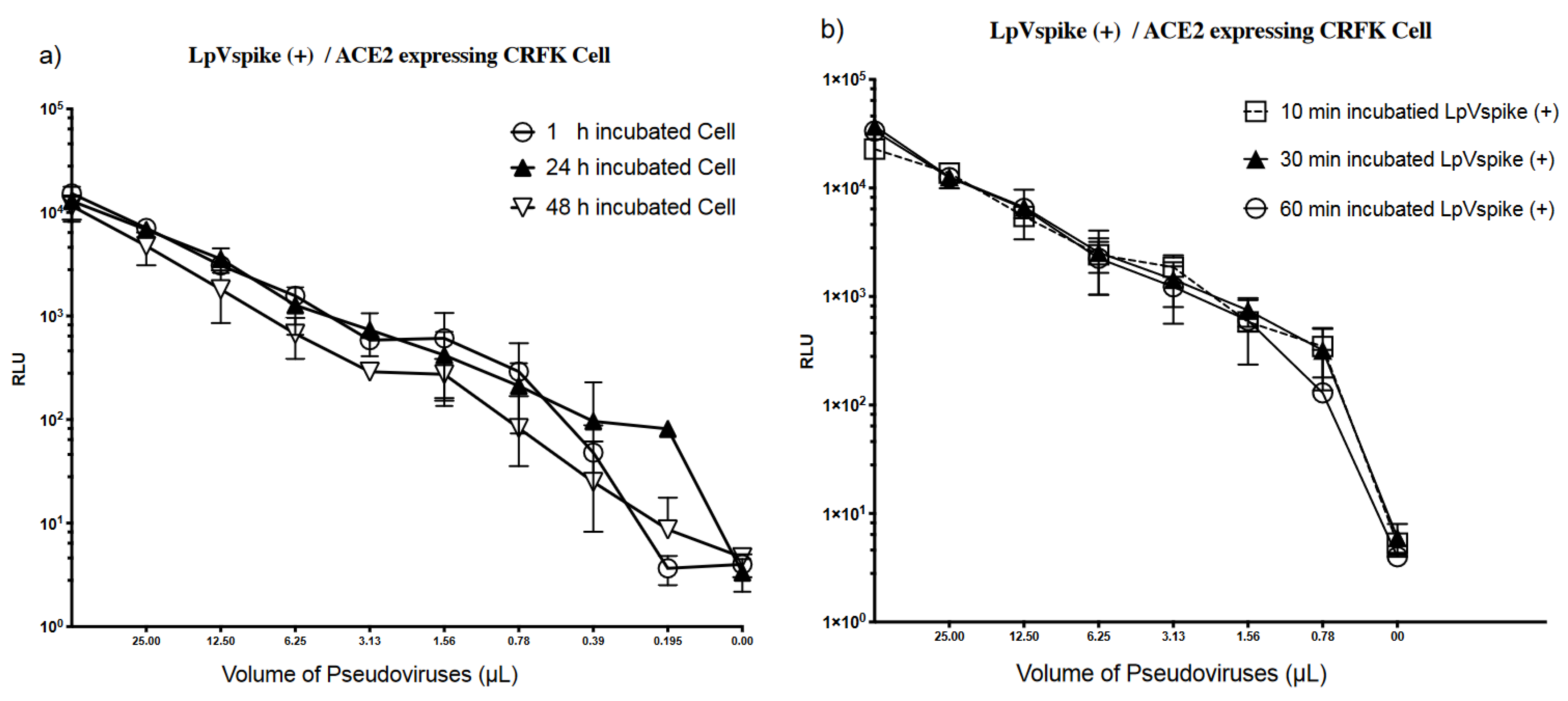
2.5. Stability of ACE2 Expression in CRFK Cells and LpVspike(+)
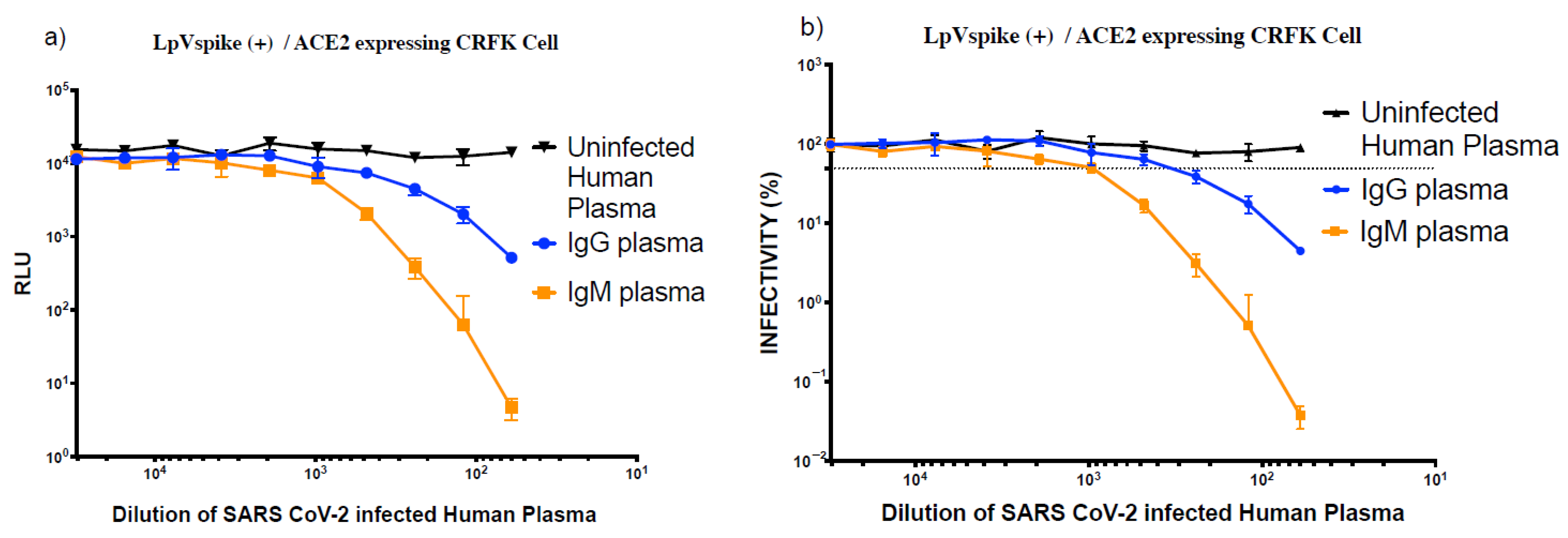
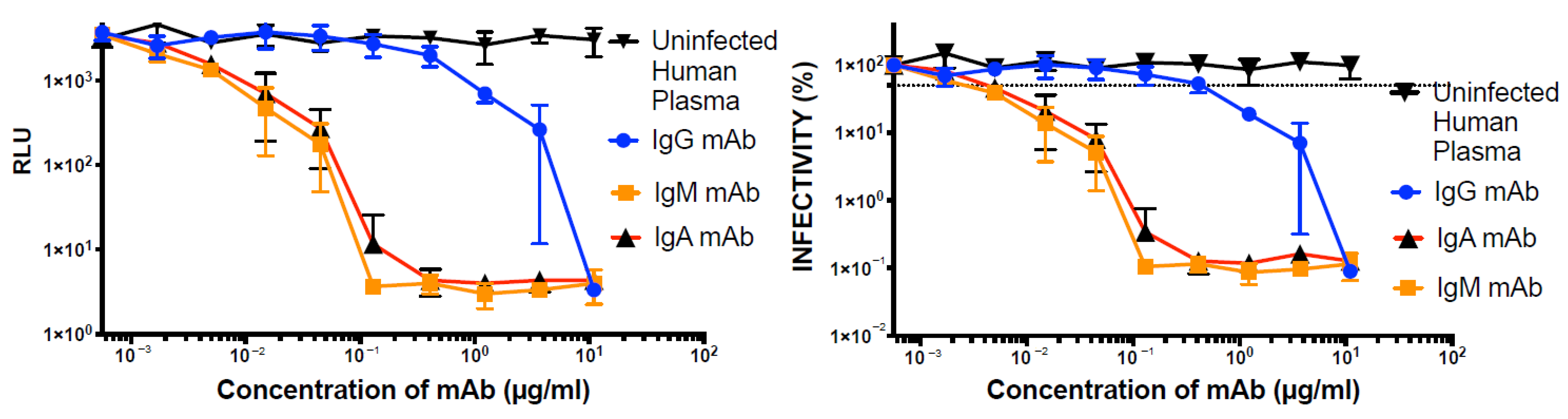
2.6. Inhibition of LpVspike(+) in ACE2-Expressing CRFK Cells with Plasma and Monoclonal Antibodies
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Generation of Pseudo-Typed Lentivirus with SARS-CoV-2 S Particles
4.3. Generation of Transient ACE2-Expressing Cell Lines
4.4. Neutralization Assays
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, W.; Qu, X.; Li, W.; Han, Z.; Yu, M.; Zhou, P.; Zhang, S.-Y.; Wang, L.-F.; Deng, H.; Shi, Z. Difference in Receptor Usage between Severe Acute Respiratory Syndrome (SARS) Coronavirus and SARS-Like Coronavirus of Bat Origin. J. Virol. 2008, 82, 1899–1907. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Harcourt, J.; Tamin, A.; Lu, X.; Kamili, S.; Sakthivel, S.K.; Murray, J.; Queen, K.; Tao, Y.; Paden, C.R.; Zhang, J.; et al. Isolation and Characterization of SARS-CoV-2 from the First US COVID-19 Patient. bioRxiv 2020. [Google Scholar] [CrossRef]
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.; Tan, Y.-Y.; Chen, S.; Jin, H.; Tan, K.S.; Wang, D.Y.; Yan, Y. The Origin, Transmission and Clinical Therapies on Coronavirus Disease 2019 (COVID-19) Outbreak—An Update on the Status. Mil. Med. Res. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Khailany, R.A.; Safdar, M.; Ozaslan, M. Genomic Characterization of a Novel SARS-CoV-2. Gene Rep. 2020, 19, 100682. [Google Scholar] [CrossRef]
- Narayanan, K.; Huang, C.; Makino, S. SARS Coronavirus Accessory Proteins. Virus Res. 2008, 133, 113–121. [Google Scholar] [CrossRef]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Establishment and Validation of a Pseudovirus Neutralization Assay for SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 680–686. [Google Scholar] [CrossRef]
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; McLellan, J.S.; Crispin, M. Site-Specific Glycan Analysis of the SARS-CoV-2 spike. Science 2020, 369, 330–333. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Yang, R.; Huang, B.; Ruhan, A.; Li, W.; Wang, W.; Deng, Y.; Tan, W. Development and Effectiveness of Pseudotyped SARS-CoV-2 System as Determined by Neutralizing Efficiency and Entry Inhibition Test In Vitro. Biosaf. Health 2020, 2, 226–231. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.-F.; Xu, W.; Liu, S.-W. Structural and Functional Properties of SARS-CoV-2 Spike Protein: Potential Antivirus Drug Development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Chu, H.; Chan, J.F.-W.; Yuen, T.T.-T.; Shuai, H.; Yuan, S.; MPhil, Y.W.; MPhil, B.H.; Yip, C.C.-Y.; Tsang, Y.O.-L.; Huang, X.; et al. Comparative Tropism, Replication Kinetics, and Cell Damage Profiling of SARS-CoV-2 and SARS-CoV with Implications for Clinical Manifestations, Transmissibility, and Laboratory Studies of COVID-19: An Observational Study. Lancet Microbe 2020, 1, e14–e23. [Google Scholar] [CrossRef]
- Takayama, K. In Vitro and Animal Models for SARS-CoV-2 Research. Trends Pharmacol. Sci. 2020, 41, 513–517. [Google Scholar] [CrossRef]
- National Institutes of Health; Richmond, J.Y.; McKinney, R.W. Biosafety in Microbiological and Biomedical Laboratories; U.S. Department of Health and Human Services: Washington, DC, USA, 2009.
- Crawford, K.H.D.; Eguia, R.; Dingens, A.S.; Loes, A.N.; Malone, K.D.; Wolf, C.R.; Chu, H.Y.; Tortorici, M.A.; Veesler, D.; Murphy, M.; et al. Protocol and Reagents for Pseudotyping Lentiviral Particles with SARS-CoV-2 Spike Protein for Neutralization Assays. Viruses 2020, 12, 513. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nao, N.; Shirato, K.; Miyuki, K.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced Isolation of SARS-CoV-2 by TMPRSS2-Expressing Cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef]
- Sarzotti-Kelsoe, M.; Bailer, R.T.; Turk, E.; Lin, C.-L.; Bilska, M.; Greene, K.M.; Gao, H.; Todd, C.A.; Ozaki, D.A.; Seaman, M.S.; et al. Optimization and Validation of the TZM-bl Assay for Standardized Assessments of Neutralizing Antibodies against HIV-1. J. Immunol. Methods 2014, 409, 131–146. [Google Scholar] [CrossRef]
- Easterhoff, D.; DiMaio, J.T.; Doran, T.M.; Dewhurst, S.; Nilsson, B.L. Enhancement of HIV-1 Infectivity by Simple, Self-Assembling Modular Peptides. Biophys. J. 2011, 100, 1325–1334. [Google Scholar] [CrossRef]
- Ng, K.W.; Faulkner, N.; Cornish, G.H.; Rosa, A.; Harvey, R.; Hussain, S.; Ulferts, R.; Earl, C.; Wrobel, A.G.; Benton, D.J.; et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020, 370, 1339–1343. [Google Scholar] [CrossRef]
- Calado, M.; Matoso, P.; Santos-Costa, Q.; Espirito-Santo, M.; Machado, J.; Rosado, L.; Antunes, F.; Mansinho, K.; Lopes, M.M.; Maltez, F.; et al. Coreceptor Usage by HIV-1 and HIV-2 Primary Isolates: The Relevance of CCR8 Chemokine Receptor as an Alternative Coreceptor. Virology 2010, 408, 174–182. [Google Scholar] [CrossRef]
- Nomaguchi, M.; Yokoyama, M.; Kono, K.; Nakayama, E.E.; Shioda, T.; Doi, N.; Fujiwara, S.; Saito, A.; Akari, H.; Miyakawa, K.; et al. Generation of Rhesus Macaque-Tropic HIV-1 Clones That Are Resistant to Major Anti-HIV-1 Restriction Factors. J. Virol. 2013, 87, 11447–11461. [Google Scholar] [CrossRef]
- Zhang, X.; Kondo, M.; Chen, J.; Miyoshi, H.; Suzuki, H.; Ohashi, T.; Shida, H. Inhibitory Effect of Human TRIM5α on HIV-1 Production. Microbes Infect. 2010, 12, 768–777. [Google Scholar] [CrossRef]
- Al-Allaf, F.A.; Tolmachov, O.E.; Zambetti, L.P.; Tchetchelnitski, V.; Mehmet, H. Remarkable Stability of an Instability-Prone Lentiviral Vector Plasmid in Escherichia coli Stbl3. 3 Biotech 2013, 3, 61–70. [Google Scholar] [CrossRef]
- Cronin, J.; Zhang, X.-Y.; Reiser, J. Altering the Tropism of Lentiviral Vectors through Pseudotyping. Curr. Gene Ther. 2005, 5, 387–398. [Google Scholar] [CrossRef]
- Chen, B.K.; Saksela, K.; Andino, R.; Baltimore, D. Distinct Modes of Human Immunodeficiency Virus Type 1 Proviral Latency Revealed by Superinfection of Nonproductively Infected Cell Lines with Recombinant Luciferase-Encoding Viruses. J. Virol. 1994, 68, 654–660. [Google Scholar] [CrossRef]
- Ishida, Y.; Yoneda, M.; Otsuki, H.; Watanabe, Y.; Kato, F.; Matsuura, K.; Kikukawa, M.; Matsushita, S.; Hishiki, T.; Igarashi, T.; et al. Generation of a Neutralization-Resistant CCR5 Tropic Simian/Human Immunodeficiency Virus (SHIV-MK38) Molecular Clone, a Derivative of SHIV-89.6. J. Gen. Virol. 2016, 97, 1249–1260. [Google Scholar] [CrossRef]
- Matsuda, K.; Inaba, K.; Fukazawa, Y.; Matsuyama, M.; Ibuki, K.; Horiike, M.; Saito, N.; Hayami, M.; Igarashi, T.; Miura, T. In Vivo Analysis of a New R5 Tropic SHIV Generated from the Highly Pathogenic SHIV-KS661, a Derivative of SHIV-89.6. Virology 2010, 399, 134–143. [Google Scholar] [CrossRef]
- Seaman, M.S.; Janes, H.; Hawkins, N.; Grandpre, L.E.; Devoy, C.; Giri, A.; Coffey, R.T.; Harris, L.; Wood, B.; Daniels, M.G.; et al. Tiered Categorization of a Diverse Panel of HIV-1 Env Pseudoviruses for Assessment of Neutralizing Antibodies. J. Virol. 2009, 84, 1439–1452. [Google Scholar] [CrossRef]
- Platt, E.J.; Bilska, M.; Kozak, S.L.; Kabat, D.; Montefiori, D.C. Evidence That Ecotropic Murine Leukemia Virus Contamination in TZM-bl Cells Does Not Affect the Outcome of Neutralizing Antibody Assays with Human Immunodeficiency Virus Type 1. J. Virol. 2009, 83, 8289–8292. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pısıl, Y.; Shida, H.; Miura, T. A Neutralization Assay Based on Pseudo-Typed Lentivirus with SARS CoV-2 Spike Protein in ACE2-Expressing CRFK Cells. Pathogens 2021, 10, 153. https://doi.org/10.3390/pathogens10020153
Pısıl Y, Shida H, Miura T. A Neutralization Assay Based on Pseudo-Typed Lentivirus with SARS CoV-2 Spike Protein in ACE2-Expressing CRFK Cells. Pathogens. 2021; 10(2):153. https://doi.org/10.3390/pathogens10020153
Chicago/Turabian StylePısıl, Yalçın, Hisatoshi Shida, and Tomoyuki Miura. 2021. "A Neutralization Assay Based on Pseudo-Typed Lentivirus with SARS CoV-2 Spike Protein in ACE2-Expressing CRFK Cells" Pathogens 10, no. 2: 153. https://doi.org/10.3390/pathogens10020153
APA StylePısıl, Y., Shida, H., & Miura, T. (2021). A Neutralization Assay Based on Pseudo-Typed Lentivirus with SARS CoV-2 Spike Protein in ACE2-Expressing CRFK Cells. Pathogens, 10(2), 153. https://doi.org/10.3390/pathogens10020153






