Interspecies Metabolic Complementation in Cystic Fibrosis Pathogens via Purine Exchange
Abstract
1. Introduction
2. Results and Discussion
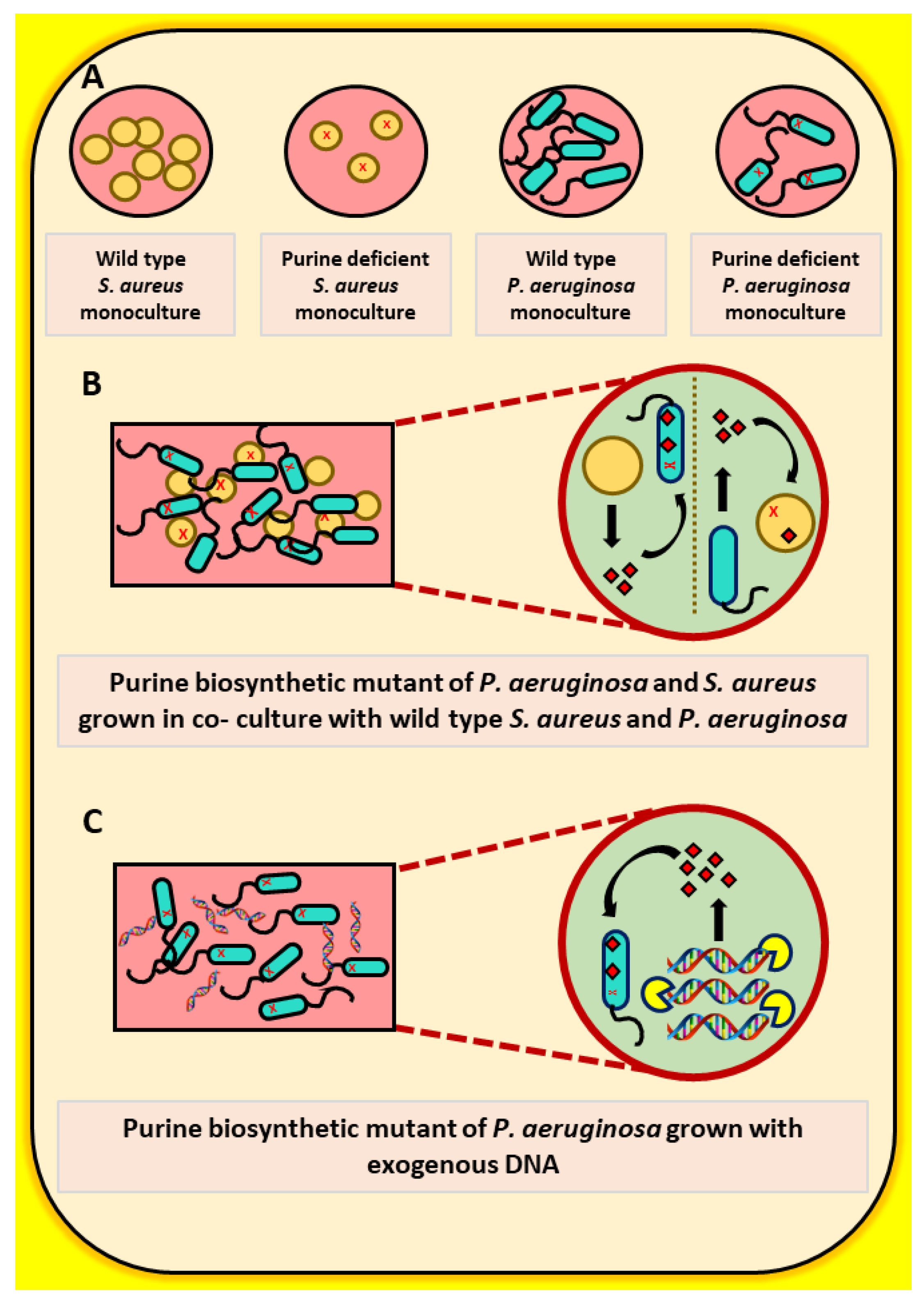
2.1. The Growth of a Purine-Deficient Mutant of P. aeruginosa Can Be Complemented by the Presence of S. aureus
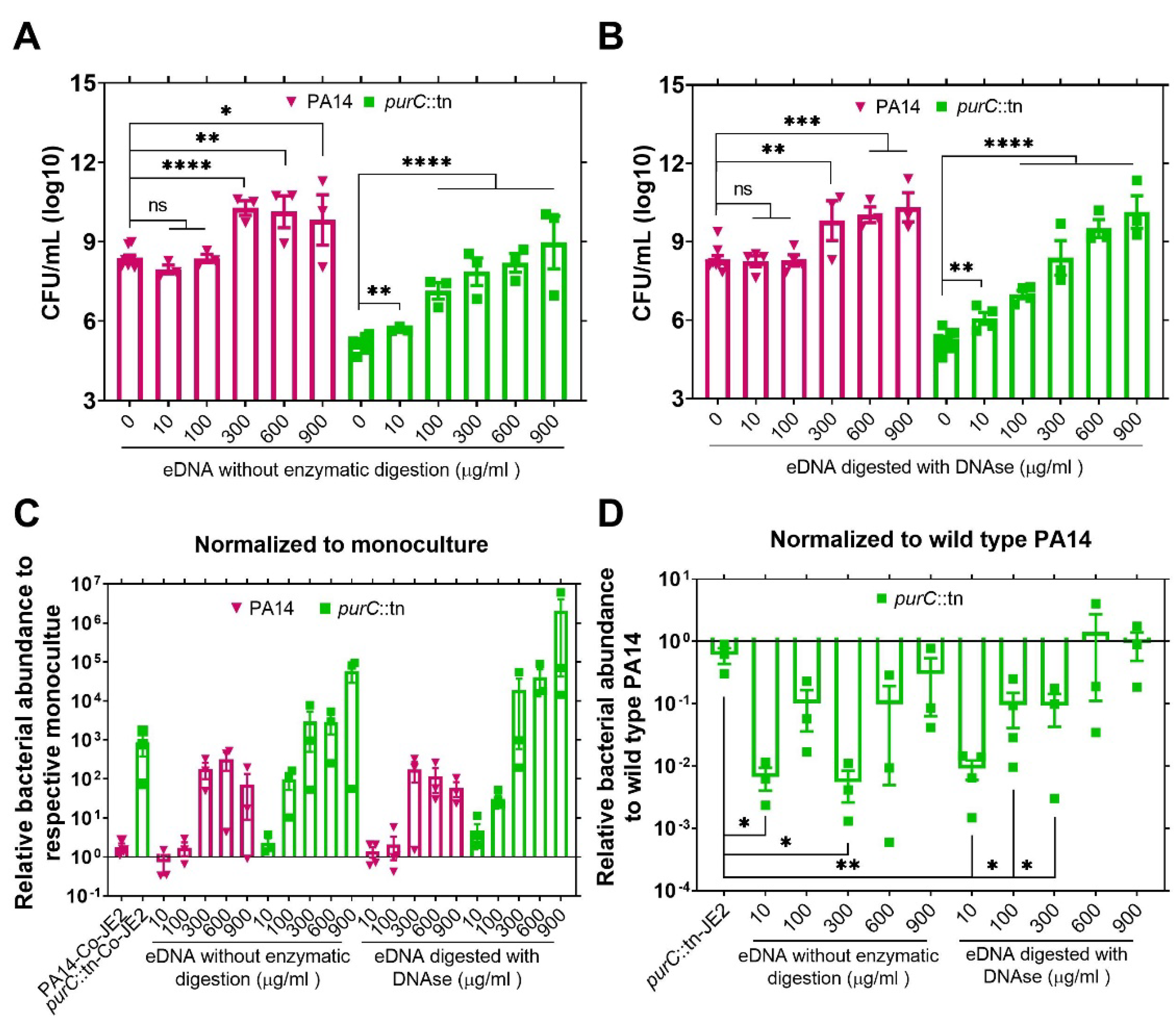
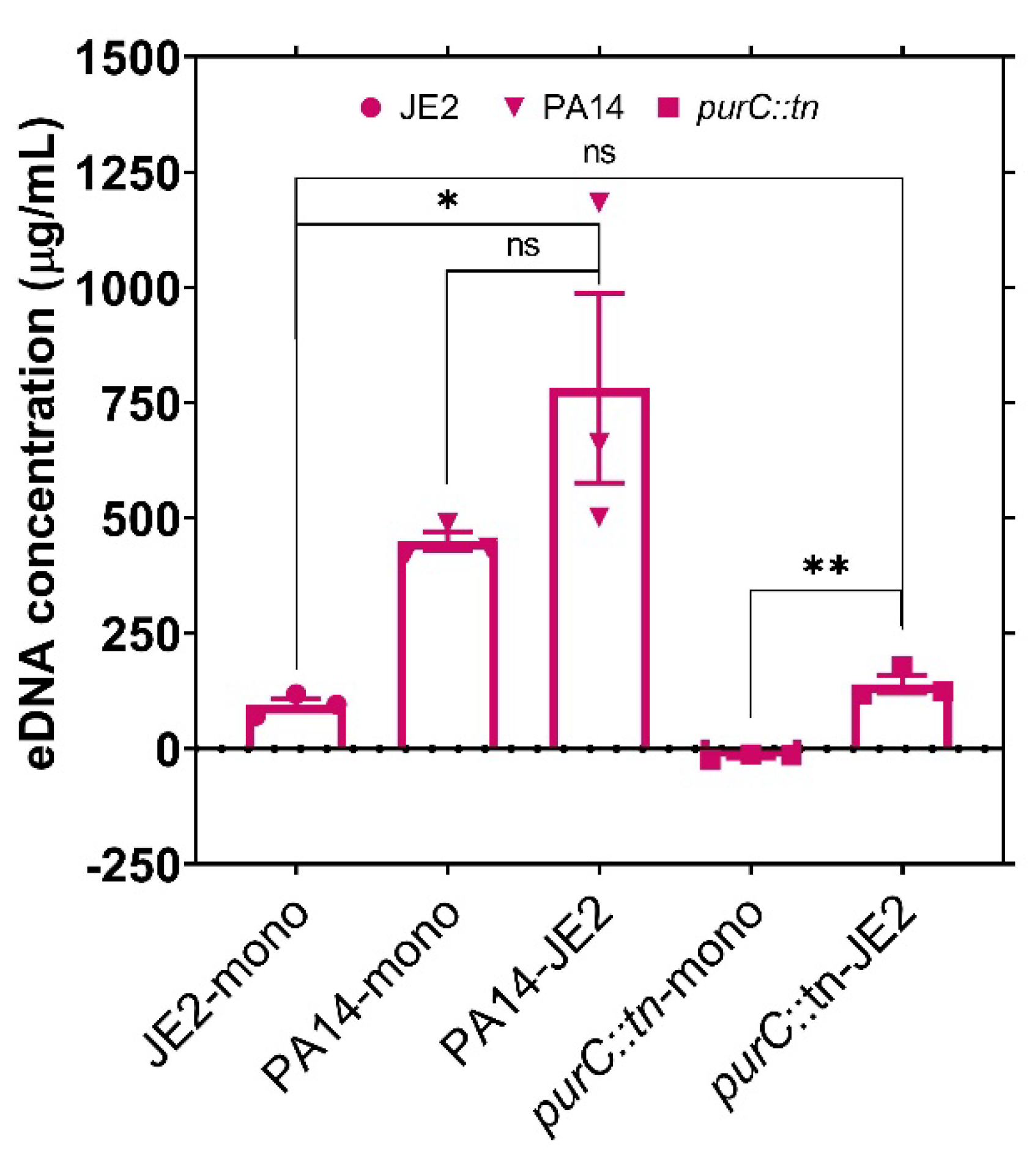
2.2. Exogenous DNA Complements the Growth of P. aeruginosa and May Contribute to the Rescue of Purine-Deficient P. aeruginosa by S. aureus
2.3. The Growth of a Purine-Deficient Mutant of S. aureus Can Be Complemented by the Presence of P. aeruginosa
3. Materials and Methods
3.1. Chemicals
3.2. Bacterial Strains
3.3. Co-Culture in Purine-Deficient Growth Medium
3.4. Purine Complementation of P. aeruginosa by Exogenous DNA
3.5. Measuring the Concentration of Exogenous DNA in Bacterial Culture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajan, S.; Saiman, L. Pulmonary infections in patients with cystic fibrosis. Semin. Respir. Infect. 2002, 17, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Beaume, M.; Köhler, T.; Fontana, T.; Tognon, M.; Renzoni, A.; van Delden, C. Metabolic pathways of Pseudomonas aeruginosa involved in competition with respiratory bacterial pathogens. Front. Microbiol. 2015, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Saiman, L.; Siegel, J. Infection Control in Cystic Fibrosis. Clin. Microbiol. Rev. 2004, 17, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.F.; Hodson, M.E.; Pitt, T.L. Auxotrophy of Pseudomonas aeruginosa in cystic fibrosis. FEMS microbiol. lett. 1992, 92, 243–246. [Google Scholar] [CrossRef][Green Version]
- Thomas, S.R.; Ray, A.; Hodson, M.E.; Pitt, T.L. Increased sputum amino acid concentrations and auxotrophy ofPseudomonas aeruginosa in severe cystic fibrosis lung disease. Thorax 2000, 55, 795–797. [Google Scholar] [CrossRef]
- Barth, A.; Pitt, T. The high amino-acid content of sputum from cystic fibrosis patients promotes growth of auxotrophic Pseudomonas aeruginosa. J. Med. Microbiol. 1996, 45, 110–119. [Google Scholar] [CrossRef]
- Qin, X.; Zerr, D.M.; McNutt, M.A.; Berry, J.E.; Burns, J.L.; Kapur, R.P. Pseudomonas aeruginosa syntrophy in chronically colonized airways of cystic fibrosis patients. Antimicrob. Agents Chemother. 2012, 56, 5971–5981. [Google Scholar] [CrossRef]
- Bisht, K.; Baishya, J.; Wakeman, C.A. Pseudomonas aeruginosa polymicrobial interactions during lung infection. Curr. Opin. Microbiol. 2020, 53, 1–8. [Google Scholar] [CrossRef]
- Hammer, N.D.; Cassat, J.E.; Noto, M.J.; Lojek, L.J.; Chadha, A.D.; Schmitz, J.E.; Creech, C.B.; Skaar, E.P. Inter-and intraspecies metabolite exchange promotes virulence of antibiotic-resistant Staphylococcus aureus. Cell Host Microbe. 2014, 16, 531–537. [Google Scholar] [CrossRef]
- Wakeman, C.A.; Moore, J.L.; Noto, M.J.; Zhang, Y.; Singleton, M.D.; Prentice, B.M.; Gilston, B.A.; Doster, R.S.; Gaddy, J.A.; Chazin, W.J. The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat. Commun. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Turner, K.H.; Wessel, A.K.; Palmer, G.C.; Murray, J.L.; Whiteley, M. Essential genome of Pseudomonas aeruginosa in cystic fibrosis sputum. Proc. Nat. Acad. Sci. USA 2015, 112, 4110–4115. [Google Scholar] [CrossRef] [PubMed]
- Neuhard, J. Purines and pyrimidines. Escherichia Coli Salmonella typhimurium. Cell. Mol. Biol. 1987, 445–473. [Google Scholar]
- Waters, C.M.; Hirt, H.; McCormick, J.K.; Schlievert, P.M.; Wells, C.L.; Dunny, G.M. An amino-terminal domain of Enterococcus faecalis aggregation substance is required for aggregation, bacterial internalization by epithelial cells and binding to lipoteichoic acid. Mol. Microbiol. 2004, 52, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Newell, P.D. Disruption of de novo purine biosynthesis in Pseudomonas fluorescens Pf0-1 leads to reduced biofilm formation and a reduction in cell size of surface-attached but not planktonic cells. Peer J. 2016, 4, 1543. [Google Scholar] [CrossRef] [PubMed]
- Thammavongsa, V.; Kern, J.W.; Missiakas, D.M.; Schneewind, O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J. Exp. Med. 2009, 206, 2417–2427. [Google Scholar] [CrossRef]
- Machan, Z.A.; Taylor, G.W.; Pitt, T.L.; Cole, P.J.; Wilson, R. 2-Heptyl-4-hydroxyquinoline N-oxide, an antistaphylococcal agent produced by Pseudomonas aeruginosa. J. Antimicrob. Chemother. 1992, 30, 615–623. [Google Scholar] [CrossRef]
- Mei, J.M.; Nourbakhsh, F.; Ford, C.W.; Holden, D.W. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol. Microbiol. 1997, 26, 399–407. [Google Scholar] [CrossRef]
- Samant, S.; Lee, H.; Ghassemi, M.; Chen, J.; Cook, J.L.; Mankin, A.S.; Neyfakh, A.A. Nucleotide biosynthesis is critical for growth of bacteria in human blood. PLoS Pathog. 2008, 4, e37. [Google Scholar] [CrossRef]
- Klarsfeld, A.D.; Goossens, P.L.; Cossart, P. Five Listeria monocytogenes genes preferentially expressed in infected mammalian cells: plcA, purH, purD, pyrE and an arginine ABC transporter gene, arpJ. Mol. Microbiol. 1994, 13, 585–597. [Google Scholar] [CrossRef]
- Jenkins, A.; Cote, C.; Twenhafel, N.; Merkel, T.; Bozue, J.; Welkos, S. Role of purine biosynthesis in Bacillus anthracis pathogenesis and virulence. Infect. Immun. 2011, 79, 153–166. [Google Scholar] [CrossRef]
- Alcantara, R.B.; Read, R.D.; Valderas, M.W.; Brown, T.D.; Roop, R.M. Intact purine biosynthesis pathways are required for wild-type virulence of Brucella abortus 2308 in the BALB/c mouse model. Infect. Immun. 2004, 72, 4911–4917. [Google Scholar] [CrossRef] [PubMed]
- Marcos, V.; Zhou-Suckow, Z.; Önder Yildirim, A.; Bohla, A.; Hector, A.; Vitkov, L.; Krautgartner, W.D.; Stoiber, W.; Griese, M.; Eickelberg, O. Free DNA in cystic fibrosis airway fluids correlates with airflow obstruction. Mediat. Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.; Wakeman, C.A. Discovery and therapeutic targeting of differentiated biofilm subpopulations. Front. Microbiol. 2019, 10, 1908. [Google Scholar] [CrossRef] [PubMed]
- Lewenza, S. Extracellular DNA-induced antimicrobial peptide resistance mechanisms in Pseudomonas aeruginosa. Front. Microbiol. 2013, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Vorkapic, D.; Pressler, K.; Schild, S. Multifaceted roles of extracellular DNA in bacterial physiology. Curr. Gen. 2016, 62, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Alhede, M.; Alhede, M.; Qvortrup, K.; Kragh, K.N.; Jensen, P.Ø.; Stewart, P.S.; Bjarnsholt, T. The origin of extracellular DNA in bacterial biofilm infections in vivo. Pathog. Dis. 2020, 78, ftaa018. [Google Scholar] [CrossRef]
- Brandt, T.; Breitenstein, S.; von der Hardt, H.; Tümmler, B. DNA concentration and length in sputum of patients with cystic fibrosis during inhalation with recombinant human DNase. Thorax 1995, 50, 880–882. [Google Scholar] [CrossRef]
- DeFrancesco, A.S.; Masloboeva, N.; Syed, A.K.; DeLoughery, A.; Bradshaw, N.; Li, G.W.; Gilmore, M.S.; Walker, S.; Losick, R. Genome-wide screen for genes involved in eDNA release during biofilm formation by Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2017, 114, 5969–5978. [Google Scholar] [CrossRef]
- Mori, M.; Ponce-de-Leon, M.; Pereto, J.; Montero, F. Metabolic Complementation in Bacterial Communities: Necessary Conditions and Optimality. Front. Microbiol. 2016, 7, 1553. [Google Scholar] [CrossRef]
- Baishya, J.; Wakeman, C.A. Selective pressures during chronic infection drive microbial competition and cooperation. NPJ Biofilms. Microbiomes. 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Liberati, N.T.; Urbach, J.M.; Miyata, S.; Lee, D.G.; Drenkard, E.; Wu, G.; Villanueva, J.; Wei, T.; Ausubel, F.M. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc. Nat. Acad. Sci. USA 2006, 103, 2833–2838. [Google Scholar] [CrossRef] [PubMed]
- Fey, P.D.; Endres, J.L.; Yajjala, V.K.; Widhelm, T.J.; Boissy, R.J.; Bose, J.L.; Bayles, K.W. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 2013, 4, 512–537. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Mahmud, H.; Baishya, J.; Wakeman, C.A. Interspecies Metabolic Complementation in Cystic Fibrosis Pathogens via Purine Exchange. Pathogens 2021, 10, 146. https://doi.org/10.3390/pathogens10020146
Al Mahmud H, Baishya J, Wakeman CA. Interspecies Metabolic Complementation in Cystic Fibrosis Pathogens via Purine Exchange. Pathogens. 2021; 10(2):146. https://doi.org/10.3390/pathogens10020146
Chicago/Turabian StyleAl Mahmud, Hafij, Jiwasmika Baishya, and Catherine A. Wakeman. 2021. "Interspecies Metabolic Complementation in Cystic Fibrosis Pathogens via Purine Exchange" Pathogens 10, no. 2: 146. https://doi.org/10.3390/pathogens10020146
APA StyleAl Mahmud, H., Baishya, J., & Wakeman, C. A. (2021). Interspecies Metabolic Complementation in Cystic Fibrosis Pathogens via Purine Exchange. Pathogens, 10(2), 146. https://doi.org/10.3390/pathogens10020146





