The Relevance of IL-1-Signaling in the Protection against Gram-Positive Bacteria
Abstract
1. Introduction
2. Results
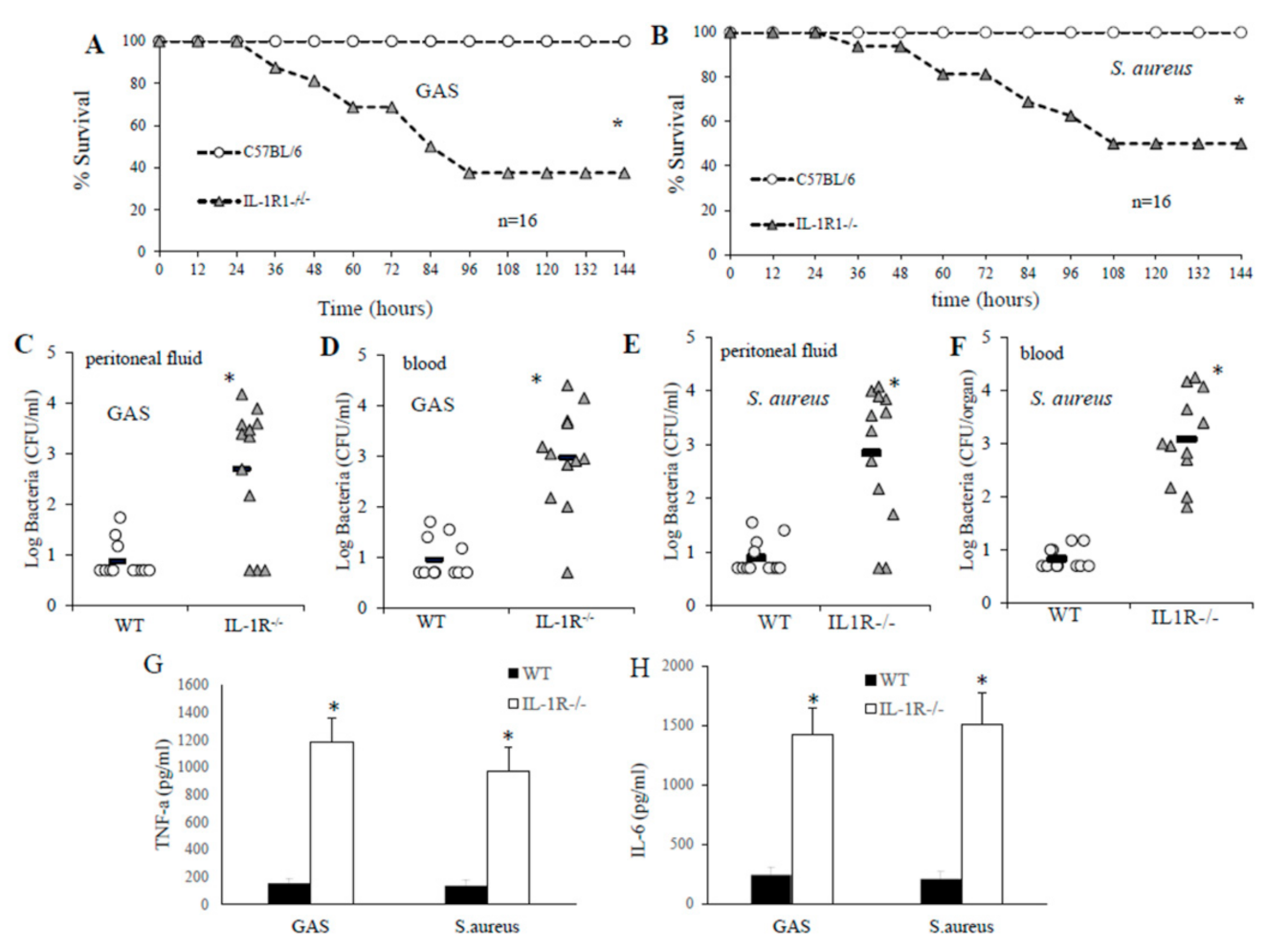
2.1. IL-1R-Deficient Mice Are Susceptible to GAS or S. aureus Infection
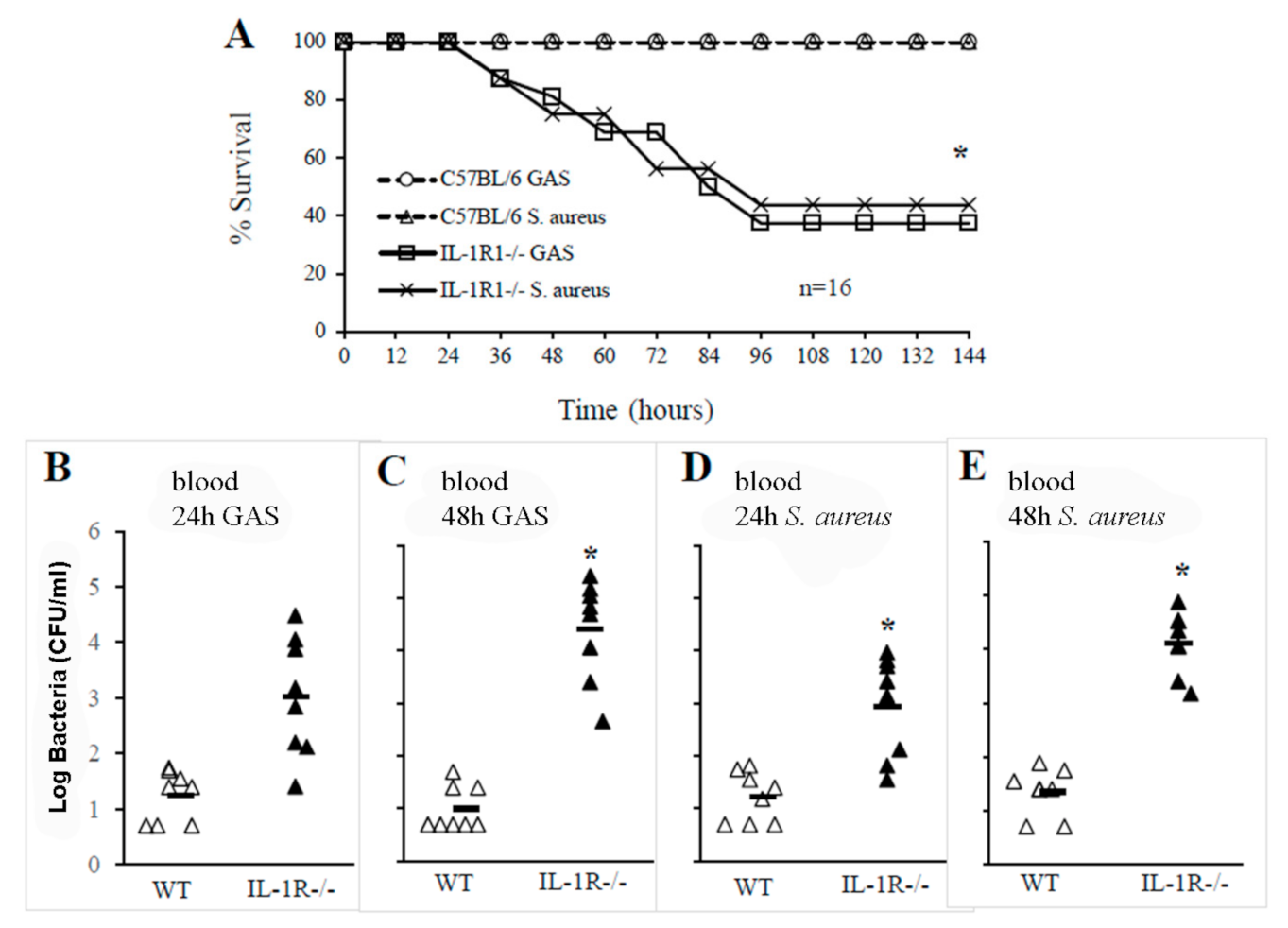
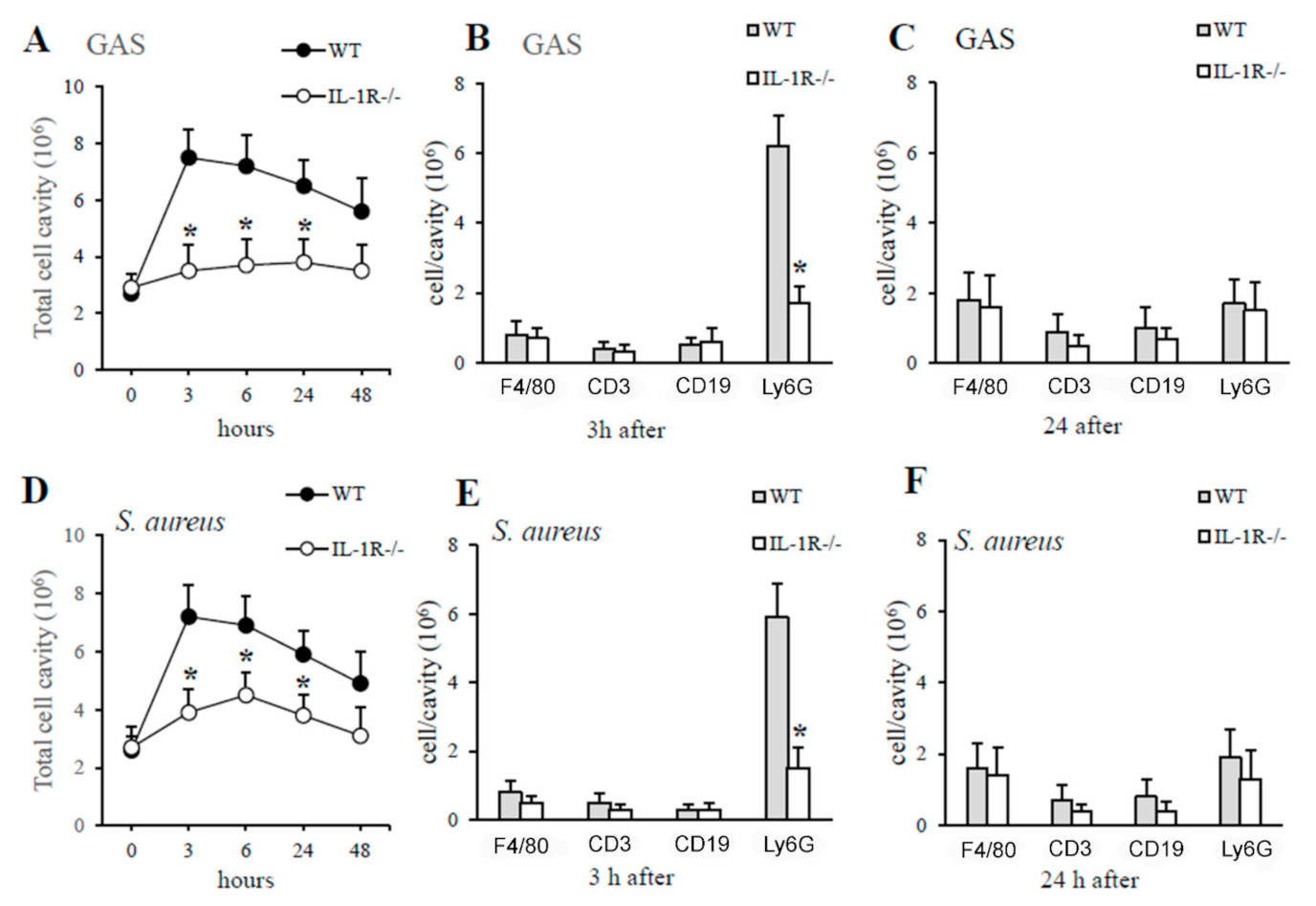
2.2. IL-1 Signaling Is Required for Host Resistance against GAS and S. aureus
2.3. The Lack of IL-1 Signaling Has No Effects on Phagocytosis and Bactericidal Activity
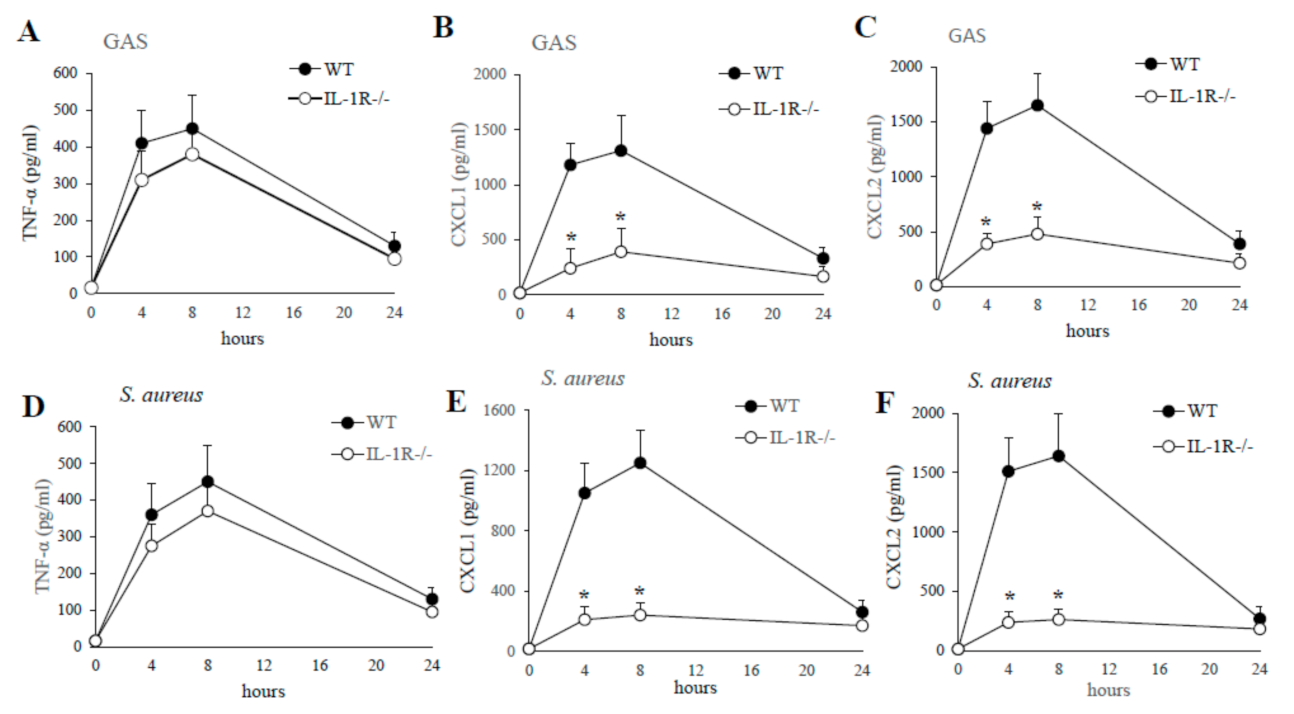
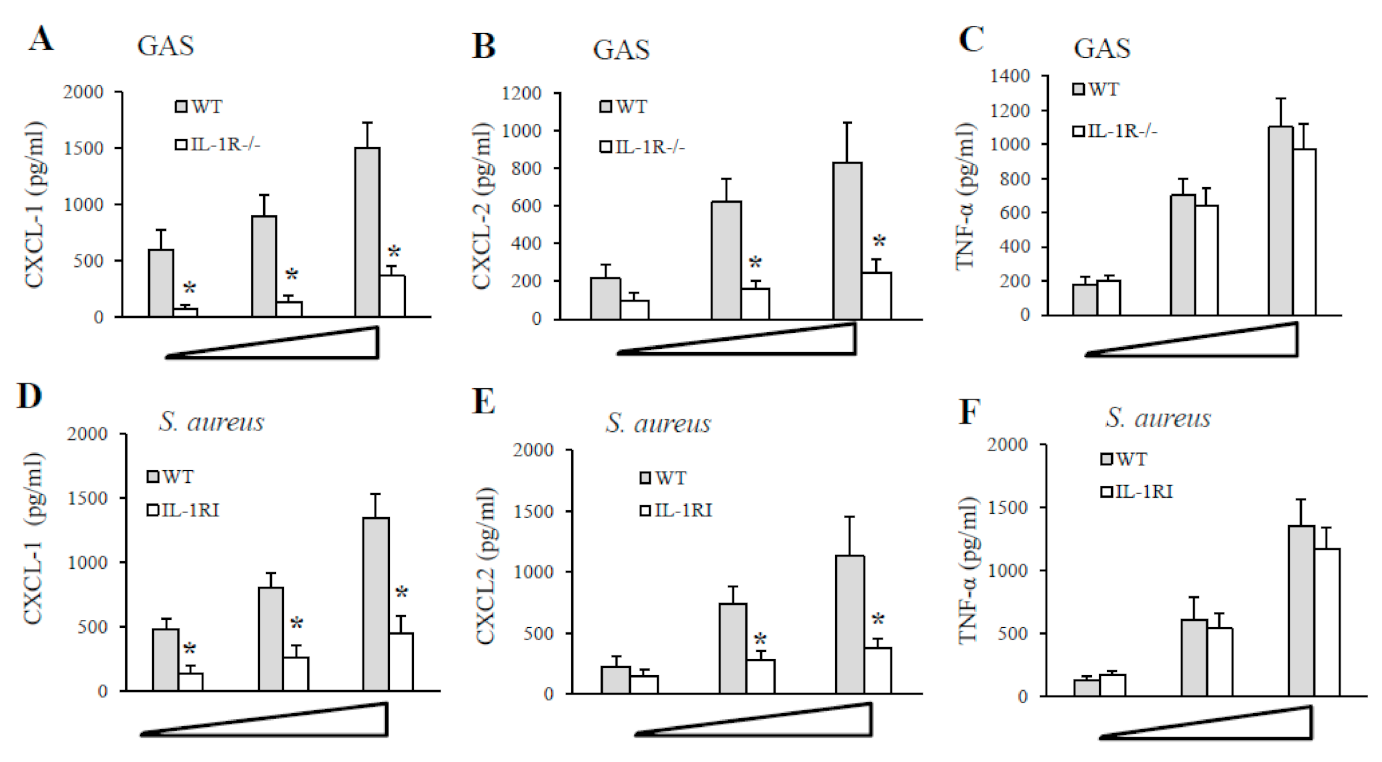
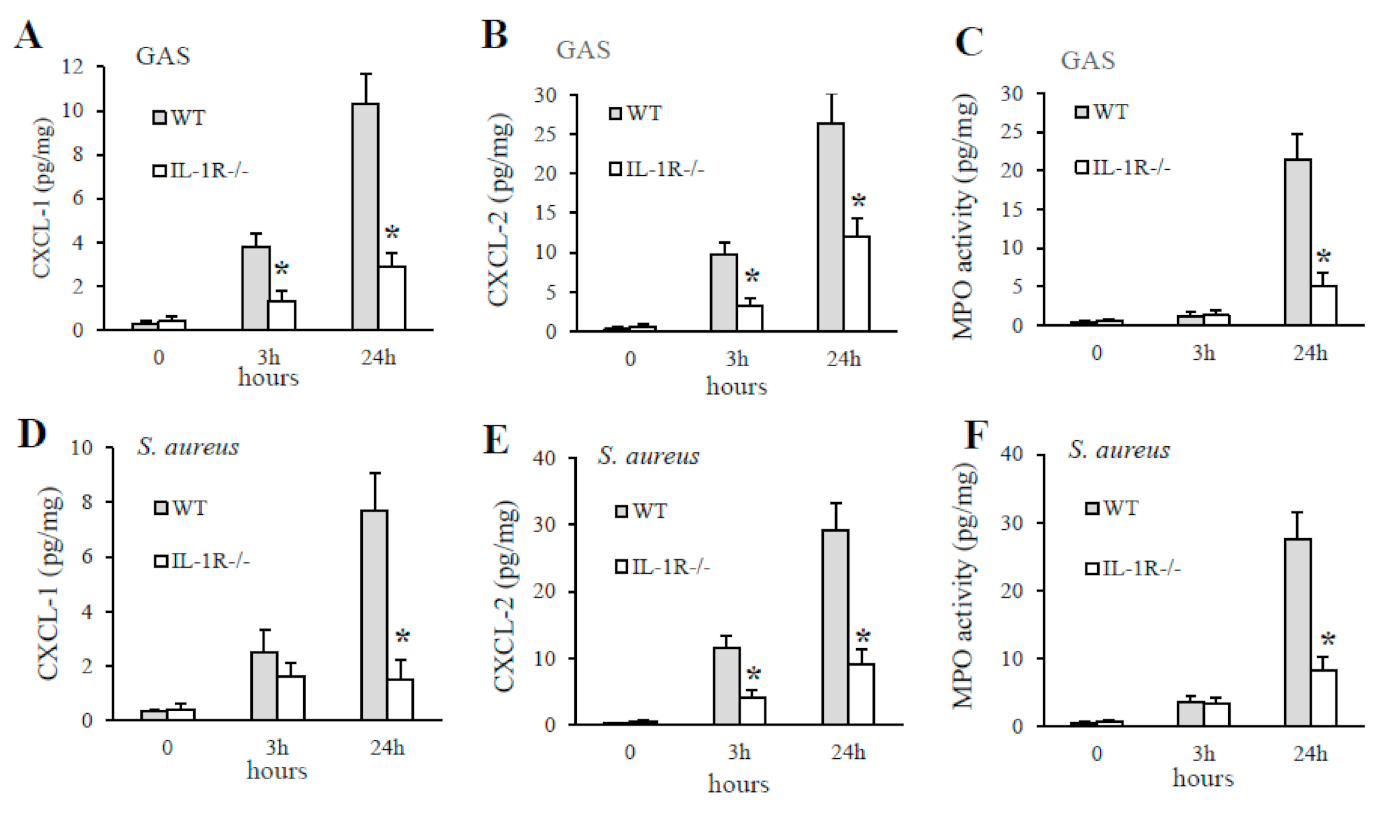
2.4. IL-1 Signaling Is Required for the Production of the CXCL1/2 Chemokines
2.5. The Lack of IL-1 Signaling Is Associated with Reduced Neutrophil Recruitment at Sites of GAS and S. aureus Infection
2.6. The Lack of IL-1 Signaling Is Associated with Decreased Production of Neutrophil Chemokines and Neutrophil Influx during GBS Infection
3. Discussion
4. Materials and Methods
4.1. Mice, Reagents and Bacterial Strains
4.2. Murine Infection Models
4.3. Organ Homogenates
4.4. Cytokine and MPO Measurement
4.5. Flow Cytometry
4.6. Growth Curves of Bacteria in Whole Blood
4.7. Macrophage Phagocytosis and Killing Assays
4.8. Stimulation of Peritoneal Macrophages
4.9. Data Expression and Statistical Significance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics Statement
References
- Ibrahim, J.; Eisen, J.A.; Jospin, G.; Coil, D.A.; Khazen, G.; Tokajian, S. Genome analysis of Streptococcus pyogenes associated with pharyngitis and skin infections. PLoS ONE 2016, 11, e0168177. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Park, B.G.; Wolf, A.J.; Brikos, C.; Goodridge, H.S.; Becker, C.A.; Reyes, C.N.; Miao, E.A.; Aderem, A.; Gotz, F.; et al. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell Host Microbe 2010, 7, 38–49. [Google Scholar] [CrossRef]
- Szeto, C.C.; Chow, K.M.; Kwan, B.C.; Law, M.C.; Chung, K.Y.; Yu, S.; Leung, C.B.; Li, P.K. Staphylococcus aureus peritonitis complicates peritoneal dialysis: Review of 245 consecutive cases. Clin. J. Am. Soc. Nephrol. CJASN 2007, 2, 245–251. [Google Scholar] [CrossRef]
- Sims Sanyahumbi, A.; Colquhoun, S.; Wyber, R.; Carapetis, J.R. Global disease burden of group A Streptococcus. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Science Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Bisno, A.L.; Stevens, D.L. Streptococcal infections of skin and soft tissues. N. Engl. J. Med. 1996, 334, 240–245. [Google Scholar] [CrossRef]
- Arias-Constanti, V.; Trenchs-Sainz de la Maza, V.; Sanz-Marcos, N.E.; Guitart-Pardellans, C.; Gene-Giralt, A.; Luaces-Cubells, C. Invasive disease by Streptococcus pyogenes: Patients hospitalized for 6 years. Enferm. Infecc. Y Microbiol. Clin. 2018, 36, 352–356. [Google Scholar] [CrossRef]
- Gonzalez-Abad, M.J.; Alonso Sanz, M. Invasive Streptococcus pyogenes infections (2011–2018): EMM-type and clinical presentation. An. De Pediatr. 2020, 92, 351–358. [Google Scholar] [CrossRef]
- O’Reilly, D.; O’Connor, C.; McCallion, N.; Drew, R.J. A retrospective study (2001–2017) of acute and chronic morbidity and mortality associated with Staphylococcus aureus bacteraemia in a tertiary neonatal intensive care unit. Ir. J. Med. Sci. 2019, 188, 1297–1301. [Google Scholar] [CrossRef]
- Sauget, M.; Bouiller, K.; Richard, M.; Chagrot, J.; Cholley, P.; Hocquet, D.; Bertrand, X. Increasing incidence of bloodstream infections due to Staphylococcus aureus clonal complex 398 in a French hospital between 2010 and 2017. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2127–2132. [Google Scholar] [CrossRef]
- Shabayek, S.; Spellerberg, B. Group B streptococcal colonization, molecular characteristics, and epidemiology. Front. Microbiol. 2018, 9, 437. [Google Scholar] [CrossRef]
- Armistead, B.; Oler, E.; Adams Waldorf, K.; Rajagopal, L. The double life of group B streptococcus: Asymptomatic colonizer and potent pathogen. J. Mol. Biol. 2019, 431, 2914–2931. [Google Scholar] [CrossRef]
- Zeppa, J.J.; Kasper, K.J.; Mohorovic, I.; Mazzuca, D.M.; Haeryfar, S.M.M.; McCormick, J.K. Nasopharyngeal infection by Streptococcus pyogenes requires superantigen-responsive Vbeta-specific T cells. Proc. Natl. Acad. Sci. USA 2017, 114, 10226–10231. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, S.; Barnett, T.C.; Rivera-Hernandez, T.; Rohde, M.; Walker, M.J. Streptococcus pyogenes adhesion and colonization. FEBS Lett. 2016, 590, 3739–3757. [Google Scholar] [CrossRef]
- Zecconi, A.; Scali, F. Staphylococcus aureus virulence factors in evasion from innate immune defenses in human and animal diseases. Immunol. Lett. 2013, 150, 12–22. [Google Scholar] [CrossRef]
- Goldmann, O.; Medina, E. Staphylococcus aureus strategies to evade the host acquired immune response. Int. J. Med. Microbiol. IJMM 2018, 308, 625–630. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int. Rev. Immunol. 1998, 16, 457–499. [Google Scholar] [CrossRef]
- Van de Veerdonk, F.L.; Netea, M.G. New insights in the immunobiology of IL-1 family members. Front. Immunol. 2013, 4, 167. [Google Scholar] [CrossRef]
- Biondo, C.; Mancuso, G.; Midiri, A.; Signorino, G.; Domina, M.; Lanza Cariccio, V.; Venza, M.; Venza, I.; Teti, G.; Beninati, C. Essential role of interleukin-1 signaling in host defenses against group B streptococcus. mBio 2014, 5, e01428-14. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Martin, M.U.; Falk, W. The interleukin-1 receptor complex and interleukin-1 signal transduction. Eur. Cytokine Netw. 1997, 8, 5–17. [Google Scholar]
- Fields, J.K.; Gunther, S.; Sundberg, E.J. Structural basis of IL-1 family cytokine signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, N.C.; Baldwin, L.K.; Irons, E.E.; Papayannopoulou, T.; Tomlinson, S.; Shayakhmetov, D.M. IL-1alpha and complement cooperate in triggering local neutrophilic inflammation in response to adenovirus and eliminating virus-containing cells. PLoS Pathog. 2014, 10, e1004035. [Google Scholar] [CrossRef] [PubMed]
- LaRock, C.N.; Todd, J.; LaRock, D.L.; Olson, J.; O’Donoghue, A.J.; Robertson, A.A.; Cooper, M.A.; Hoffman, H.M.; Nizet, V. IL-1beta is an innate immune sensor of microbial proteolysis. Sci. Immunol. 2016, 1, eaah3539. [Google Scholar] [CrossRef]
- LaRock, D.L.; Russell, R.; Johnson, A.F.; Wilde, S.; LaRock, C.N. Group A streptococcus infection of the nasopharynx requires proinflammatory signaling through the Interleukin-1 receptor. Infect. Immun. 2020, 88, e00356-20. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S.; Pietras, E.M.; Uricchio, L.H.; Hirano, K.; Rao, S.; Lin, H.; O’Connell, R.M.; Iwakura, Y.; Cheung, A.L.; Cheng, G.; et al. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J. Immunol. 2007, 179, 6933–6942. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S.; O’Connell, R.M.; Gutierrez, M.A.; Pietras, E.M.; Shahangian, A.; Gross, C.E.; Thirumala, A.; Cheung, A.L.; Cheng, G.; Modlin, R.L. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 2006, 24, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Randad, P.R.; Dillen, C.A.; Ortines, R.V.; Mohr, D.; Aziz, M.; Price, L.B.; Kaya, H.; Larsen, J.; Carroll, K.C.; Smith, T.C.; et al. Comparison of livestock-associated and community-associated Staphylococcus aureus pathogenicity in a mouse model of skin and soft tissue infection. Sci. Rep. 2019, 9, 6774. [Google Scholar] [CrossRef]
- Hultgren, O.H.; Svensson, L.; Tarkowski, A. Critical role of signaling through IL-1 receptor for development of arthritis and sepsis during Staphylococcus aureus infection. J. Immunol. 2002, 168, 5207–5212. [Google Scholar] [CrossRef]
- Kielian, T.; Bearden, E.D.; Baldwin, A.C.; Esen, N. IL-1 and TNF-alpha play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J. Neuropathol. Exp. Neurol. 2004, 63, 381–396. [Google Scholar] [CrossRef]
- Verdrengh, M.; Thomas, J.A.; Hultgren, O.H. IL-1 receptor-associated kinase 1 mediates protection against Staphylococcus aureus infection. Microbes Infect. 2004, 6, 1268–1272. [Google Scholar] [CrossRef]
- Biondo, C.; Mancuso, G.; Midiri, A.; Signorino, G.; Domina, M.; Lanza Cariccio, V.; Mohammadi, N.; Venza, M.; Venza, I.; Teti, G.; et al. The interleukin-1beta/CXCL1/2/neutrophil axis mediates host protection against group B streptococcal infection. Infect. Immun. 2014, 82, 4508–4517. [Google Scholar] [CrossRef] [PubMed]
- Kafka, D.; Ling, E.; Feldman, G.; Benharroch, D.; Voronov, E.; Givon-Lavi, N.; Iwakura, Y.; Dagan, R.; Apte, R.N.; Mizrachi-Nebenzahl, Y. Contribution of IL-1 to resistance to Streptococcus pneumoniae infection. Int. Immunol. 2008, 20, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.T.; Briggs, W.H.; Cheng, G.C.; Rossiter, H.B.; Libby, P.; Kupper, T. Mechanical deformation promotes secretion of IL-1 alpha and IL-1 receptor antagonist. J. Immunol. 1997, 159, 5084–5088. [Google Scholar] [PubMed]
- Verdrengh, M.; Tarkowski, A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect. Immun. 1997, 65, 2517–2521. [Google Scholar] [CrossRef] [PubMed]
- Cuzzola, M.; Mancuso, G.; Beninati, C.; Biondo, C.; Genovese, F.; Tomasello, F.; Flo, T.H.; Espevik, T.; Teti, G. Beta 2 integrins are involved in cytokine responses to whole Gram-positive bacteria. J. Immunol. 2000, 164, 5871–5876. [Google Scholar] [CrossRef] [PubMed]
- Biondo, C.; Midiri, A.; Gambuzza, M.; Gerace, E.; Falduto, M.; Galbo, R.; Bellantoni, A.; Beninati, C.; Teti, G.; Leanderson, T.; et al. IFN-alpha/beta signaling is required for polarization of cytokine responses toward a protective type 1 pattern during experimental cryptococcosis. J. Immunol. 2008, 181, 566–573. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Midiri, A.; Mancuso, G.; Beninati, C.; Gerace, E.; Biondo, C. The Relevance of IL-1-Signaling in the Protection against Gram-Positive Bacteria. Pathogens 2021, 10, 132. https://doi.org/10.3390/pathogens10020132
Midiri A, Mancuso G, Beninati C, Gerace E, Biondo C. The Relevance of IL-1-Signaling in the Protection against Gram-Positive Bacteria. Pathogens. 2021; 10(2):132. https://doi.org/10.3390/pathogens10020132
Chicago/Turabian StyleMidiri, Angelina, Giuseppe Mancuso, Concetta Beninati, Elisabetta Gerace, and Carmelo Biondo. 2021. "The Relevance of IL-1-Signaling in the Protection against Gram-Positive Bacteria" Pathogens 10, no. 2: 132. https://doi.org/10.3390/pathogens10020132
APA StyleMidiri, A., Mancuso, G., Beninati, C., Gerace, E., & Biondo, C. (2021). The Relevance of IL-1-Signaling in the Protection against Gram-Positive Bacteria. Pathogens, 10(2), 132. https://doi.org/10.3390/pathogens10020132





