Spirocerca lupi Proteomics and Its Role in Cancer Development: An Overview of Spirocercosis-Induced Sarcomas and Revision of Helminth-Induced Carcinomas
Abstract
1. Spirocerca lupi Overview
2. Spirocerca lupi Proteomics
3. Proteins Involved in Carcinogenesis in Other Helminthiases
3.1. Clonorchis sinensis
3.2. Opisthorchis viverrini
3.3. Schistosoma haematobium
3.4. Other Helminths Associated with Cancer in Humans and Animals
3.4.1. Taenia solium
3.4.2. Echinococcus granulosus
3.4.3. Strongyloides stercoralis
3.4.4. Heterakis gallinarum
3.4.5. Trichuris muris
4. Possible Model of Cancer Induction in Esophageal Spirocercosis
5. Future Directions in Spirocerca lupi Proteomic Studies
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gottlieb, Y.; Markovics, A.; Klement, E.; Naor, S.; Samish, M.; Aroch, I.; Lavy, E. Characterization of Onthophagus sellatus as the major intermediate host of the dog esophageal worm Spirocerca lupi in Israel. Veter. Parasitol. 2011, 180, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Dvir, E.; Baneth, G. Insights on Spirocerca lupi, the Carcinogenic Dog Nematode. Trends Parasitol. 2020, 36, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Mazaki-Tovi, M.; Baneth, G.; Aroch, I.; Harrus, S.; Kass, P.H.; Ben-Ari, T.; Zur, G.; Aizenberg, I.; Bark, H.; Lavy, E. Canine spirocercosis: Clinical, diagnostic, pathologic, and epidemiologic characteristics. Veter. Parasitol. 2002, 107, 235–250. [Google Scholar] [CrossRef]
- Psáder, R.; Balogh, M.; Pápa, K.; Sterczer, Á.; Lukács, Z.; Harnos, A. Occurrence of Spirocerca lupi Infection in Hungarian Dogs Referred for Gastroscopy. Parasitol. Res. 2017, 116, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, A.; Baldassarre, V.; Ramos, R.A.N.; Lia, R.P.; Furlanello, T.; Trotta, M.; Dantas-Torres, F.; Baneth, G.; Otranto, D. Spirocerca lupi infection in a dog from southern Italy: An “old fashioned” disease? Parasitol. Res. 2014, 113, 2391–2394. [Google Scholar] [CrossRef]
- Aroch, I.; Markovics, A.; Mazaki-Tovi, M.; Kuzi, S.; Harrus, S.; Yas, E.; Baneth, G.; Bar-El, M.; Bdolah-Abram, T.; Segev, G.; et al. Spirocercosis in dogs in Israel: A retrospective case-control study (2004–2009). Veter. Parasitol. 2015, 211, 234–240. [Google Scholar] [CrossRef]
- Van Der Merwe, L.L.; Kirberger, R.M.; Clift, S.J.; Williams, M.; Keller, N.; Naidoo, V. Spirocerca lupi infection in the dog: A review. Veter. J. 2008, 176, 294–309. [Google Scholar] [CrossRef]
- Rojas, A.; Freedberg, N.; Markovics, A.; Gottlieb, Y.; Baneth, G. Influence of physical and chemical factors on the embryonation, hatching and infectivity of Spirocerca lupi. Veter. Parasitol. 2017, 242, 71–78. [Google Scholar] [CrossRef]
- Sen, K.; Anantaraman, M. Some observations on the development of Spirocerca lupi in its intermediate and definitive hosts. J. Helminthol. 1971, 45, 123–131. [Google Scholar] [CrossRef]
- Chowdhury, N.; Sood, N.K.; Lal, S.; Gupta, K.; Singla, L.D. Development of Some Larval Nematodes in Experimental and Natural Animal Hosts: An Insight into Development of Pathological Lesions vis-a-vis Host-Parasite Interactions. Sci. World J. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Van der Merwe, L.L.; Christie, J.; Clift, S.J.; Dvir, E. Salivary gland enlargement and sialorrhoea in dogs with spirocercosis: A retrospective and prospective study of 298 cases. J. S. Afr. Vet. Assoc. 2012, 83, 920. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brenner, O.; Botero-Anug, A.M.; Rojas, A.; Hahn, S.; Baneth, G. Aberrant Mesenteric Migration of Spirocerca lupi Larvae Causing Necrotizing Eosinophilic Arteritis, Thrombosis, and Intestinal Infarction in Dogs. Veter. Pathol. 2019, 57, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Lerman, O.; Israeli, I.; Weingram, T.; Benzioni-Bar, H.; Milgram, J.; Shipov, A. Acute mesenteric ischemia-like syndrome associated with suspected Spirocerca lupi aberrant migration in dogs. J. Veter. Emerg. Crit. Care 2019, 29, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Chai, O.; Yas, E.; Brenner, O.; Rojas, A.; Konstantin, L.; Klainbart, S.; Shamir, M.H. Clinical characteristics of Spirocerca lupi migration in the spinal cord. Veter. Parasitol. 2018, 253, 16–21. [Google Scholar] [CrossRef]
- Merhavi, N.; Segev, G.; Dvir, E.; Peery, D. Ultrasonography is insensitive but specific for detecting aortic wall abnormalities in dogs infected with Spirocerca lupi. Veter. Rec. 2020, 187, e59. [Google Scholar] [CrossRef]
- Kirberger, R.M.; Clift, S.J.; Van Wilpe, E.; Dvir, E. Spirocerca lupi-associated vertebral changes: A radiologic-pathologic study. Veter. Parasitol. 2013, 195, 87–94. [Google Scholar] [CrossRef]
- Pazzi, P.; Kavkovsky, A.; Shipov, A.; Segev, G.; Dvir, E. Spirocerca lupi induced oesophageal neoplasia: Predictors of surgical outcome. Veter. Parasitol. 2018, 250, 71–77. [Google Scholar] [CrossRef]
- Dvir, E.; Kirberger, R.M.; Malleczek, D. Radiographic and computed tomographic changes and clinical presentation of Spirocercosis in the dog. Veter. Radiol. Ultrasound 2001, 42, 119–129. [Google Scholar] [CrossRef]
- Wijekoon, H.S.; Munasinghe, D.M.S.; Wijayawardhane, K.A.N.; Ariyarathna, H.M.H.S.; Horadagoda, N.; Rajapakse, J.; De Silva, N. Postmortem detection and histopathological features of canine spirocercosis-induced putative esophageal chondrosarcoma. Veter. World 2018, 11, 1376–1379. [Google Scholar] [CrossRef]
- Ranen, E.; Dank, G.; Lavy, E.; Perl, S.; Lahav, D.; Orgad, U. Oesophageal sarcomas in dogs: Histological and clinical evaluation. Veter. J. 2008, 178, 78–84. [Google Scholar] [CrossRef]
- Dvir, E.; Clift, S.J.; Williams, M. Proposed histological progression of the Spirocerca lupi-induced oesophageal lesion in dogs. Veter. Parasitol. 2010, 168, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Dvir, E.; Mellanby, R.; Kjelgaard-Hansen, M.; Schoeman, J.P. Plasma IL-8 concentrations are increased in dogs with spirocercosis. Veter. Parasitol. 2012, 190, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Ye, Y.; Zhang, L.; Liu, P.; Yu, W.; Wei, F.; Ren, X.; Yu, J. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways (Review). Int. J. Oncol. 2016, 48, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.; Weigert, A. IL-1 family cytokines in cancer immunity—A matter of life and death. Biol. Chem. 2016, 397, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Dvir, E.; Clift, S.J. Evaluation of selected growth factor expression in canine spirocercosis (Spirocerca lupi)-associated non-neoplastic nodules and sarcomas. Veter. Parasitol. 2010, 174, 257–266. [Google Scholar] [CrossRef]
- Mukorera, V.; Kirberger, R.M.; Mabeta, P.; Dvir, E. Vascular Endothelial Growth Factor Concentrations in Dogs with Spirocercosis. J. Veter. Intern. Med. 2013, 27, 1642–1645. [Google Scholar] [CrossRef]
- Yudoh, K.; Kanamori, M.; Ohmori, K.; Yasuda, T.; Aoki, M.; Kimura, T. Concentration of vascular endothelial growth factor in the tumour tissue as a prognostic factor of soft tissue sarcomas. Br. J. Cancer 2001, 84, 1610–1615. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Ojha, U.; You, Q. Pathological angiogenesis and inflammation in tissues. Arch. Pharmacal Res. 2020, 1–15. [Google Scholar] [CrossRef]
- Dvir, E.; Schoeman, J.P.; Clift, S.J.; McNeilly, T.N.; Mellanby, R.J. Immunohistochemical characterization of lymphocyte and myeloid cell infiltrates in spirocercosis-induced oesophageal nodules. Parasite Immunol. 2011, 33, 545–553. [Google Scholar] [CrossRef]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 1–23. [Google Scholar] [CrossRef]
- Harnett, W. Secretory products of helminth parasites as immunomodulators. Mol. Biochem. Parasitol. 2014, 195, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, J.P.; Grainger, J.R.; Maizels, R.M. Helminth immunoregulation: The role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasitol. 2009, 167, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Astroz, Y.; De Oliveira, F.S.; Nahum, L.A.; Oliveira, G. Helminth secretomes reflect different lifestyles and parasitized hosts. Int. J. Parasitol. 2017, 47, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Sako, K.; Rensburg, I.J.; Clift, S.J.; Naidoo, V. The use of primary murine fibroblasts to ascertain if Spirocerca lupi secretory/excretory protein products are mitogenic ex vivo. BMC Veter. Res. 2017, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Baneth, G. Secretome of the carcinogenic helminth Spirocerca lupi reveals specific parasite proteins associated with its different life stages. Veter. Parasitol. 2019, 275, 108935. [Google Scholar] [CrossRef]
- I Lomnytska, M.; Becker, S.; Bodin, I.; Olsson, A.; Hellman, K.; Hellström, A.-C.; Mints, M.; Hellman, U.; Auer, G.; Andersson, S. Differential expression of ANXA6, HSP27, PRDX2, NCF2, and TPM4 during uterine cervix carcinogenesis: Diagnostic and prognostic value. Br. J. Cancer 2010, 104, 110–119. [Google Scholar] [CrossRef]
- Smith, D.L.; Evans, C.A.; Pierce, A.; Gaskell, S.J.; Whetton, A.D. Changes in the Proteome Associated with the Action of Bcr-Abl Tyrosine Kinase Are Not Related to Transcriptional Regulation. Mol. Cell. Proteom. 2002, 1, 876–884. [Google Scholar] [CrossRef]
- Tesniere, A.; Panaretakis, T.; Kepp, O.; Apetoh, L.; Ghiringhelli, F.; Zitvogel, L.; Kroemer, G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2007, 15, 3–12. [Google Scholar] [CrossRef]
- Shevtsov, M.; Multhoff, G. Heat Shock Protein–Peptide and HSP-Based Immunotherapies for the Treatment of Cancer. Front. Immunol. 2016, 7, 171. [Google Scholar] [CrossRef]
- Fan, X.; Cui, L.; Zeng, Y.; Song, W.; Gaur, U.; Yang, M. 14-3-3 Proteins Are on the Crossroads of Cancer, Aging, and Age-Related Neurodegenerative Disease. Int. J. Mol. Sci. 2019, 20, 3518. [Google Scholar] [CrossRef]
- Hassan, K.; Kumar, D.; Naik, M.; Dixit, M. The expression profile and prognostic significance of eukaryotic translation elongation factors in different cancers. PLoS ONE 2018, 13, e0191377. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Sripa, B.; Kaewkes, S.; Sithithaworn, P.; Mairiang, E.; Laha, T.; Smout, M.; Pairojkul, C.; Bhudhisawasdi, V.; Tesana, S.; Thinkamrop, B.; et al. Liver Fluke Induces Cholangiocarcinoma. PLoS Med. 2007, 4, e201. [Google Scholar] [CrossRef] [PubMed]
- Lun, Z.-R.; Gasser, R.B.; Lai, D.-H.; Li, A.; Zhu, X.-Q.; Yu, X.-B.; Fang, Y.-Y. Clonorchiasis: A key foodborne zoonosis in China. Lancet Infect. Dis. 2005, 5, 31–41. [Google Scholar] [CrossRef]
- Fried, B.; Reddy, A.; Mayer, D. Helminths in human carcinogenesis. Cancer Lett. 2011, 305, 239–249. [Google Scholar] [CrossRef]
- Hu, F.; Hu, X.; Ma, C.; Zhao, J.; Xu, J.; Yu, X. Molecular characterization of a novel Clonorchis sinensis secretory phospholipase A2 and investigation of its potential contribution to hepatic fibrosis. Mol. Biochem. Parasitol. 2009, 167, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liang, P.; Chen, W.; Wang, X.; Hu, Y.; Liang, C.; Sun, J.; Huang, Y.; Li, R.; Li, X.; et al. Stage-specific expression, immunolocalization of Clonorchis sinensis lysophospholipase and its potential role in hepatic fibrosis. Parasitol. Res. 2012, 112, 737–749. [Google Scholar] [CrossRef]
- Liang, P.; Sun, J.; Huang, Y.; Zhang, F.; Zhou, J.; Hu, Y.; Wang, X.; Liang, C.; Zheng, M.; Xu, Y.; et al. Biochemical characterization and functional analysis of fructose-1,6-bisphosphatase from Clonorchis sinensis. Mol. Biol. Rep. 2013, 40, 4371–4382. [Google Scholar] [CrossRef]
- Mao, Q.; Xie, Z.; Wang, X.; Chen, W.; Ren, M.; Shang, M.; Lei, H.; Tian, Y.; Li, S.; Liang, P.; et al. Clonorchis sinensis ferritin heavy chain triggers free radicals and mediates inflammation signaling in human hepatic stellate cells. Parasitol. Res. 2014, 114, 659–670. [Google Scholar] [CrossRef]
- Wang, X.; Hu, F.; Hu, X.; Chen, W.; Huang, Y.; Yu, X. Proteomic identification of potential Clonorchis sinensis excretory/secretory products capable of binding and activating human hepatic stellate cells. Parasitol. Res. 2014, 113, 3063–3071. [Google Scholar] [CrossRef]
- Tang, Z.-L.; Huang, Y.; Yu, X. Current status and perspectives of Clonorchis sinensis and clonorchiasis: Epidemiology, pathogenesis, omics, prevention and control. Infect. Dis. Poverty 2016, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-S.; Pak, J.H.; Kim, J.-B.; Bahk, Y.Y. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: A brief review. BMB Rep. 2016, 49, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yu, K.; Liang, A.; Huang, Y.; Ou, F.; Wei, H.; Wan, X.; Yang, Y.; Zhang, W.; Jiang, Z. Identification and Analysis of the Tegument Protein and Excretory-Secretory Products of the Carcinogenic Liver Fluke Clonorchis sinensis. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhu, Y.; Zhao, Z.; Wu, Z.; Kamolnetr, O.; Lv, Z. Liver fluke infection and cholangiocarcinoma: A review. Parasitol. Res. 2016, 116, 11–19. [Google Scholar] [CrossRef]
- Pak, J.H.; Lee, J.-Y.; Jeon, B.Y.; Dai, F.; Yoo, W.G.; Hong, S.-J. Cytokine Production in Cholangiocarcinoma Cells in Response to Clonorchis sinensis Excretory-Secretory Products and Their Putative Protein Components. Korean J. Parasitol. 2019, 57, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Bahk, Y.Y.; Pak, J.H. Toll-Like Receptor-Mediated Free Radical Generation in Clonorchis sinensis Excretory-Secretory Product-Treated Cholangiocarcinoma Cells. Korean J. Parasitol. 2016, 54, 679–684. [Google Scholar] [CrossRef]
- Yan, C.; Wang, Y.-H.; Yu, Q.; Cheng, X.-D.; Zhang, B.; Li, B.; Zhang, B.; Tang, R.; Zheng, K.-Y. Clonorchis sinensis excretory/secretory products promote the secretion of TNF-alpha in the mouse intrahepatic biliary epithelial cells via Toll-like receptor 4. Parasites Vectors 2015, 8, 559. [Google Scholar] [CrossRef]
- He, L.; Ren, M.; Chen, X.; Wang, X.; Li, S.; Lin, J.; Liang, C.; Liang, P.; Hu, Y.; Lei, H.; et al. Biochemical and immunological characterization of annexin B30 from Clonorchis sinensis excretory/secretory products. Parasitol. Res. 2014, 113, 2743–2755. [Google Scholar] [CrossRef]
- Wang, C.; Lei, H.; Tian, Y.; Shang, M.; Wu, Y.; Li, Y.; Zhao, L.; Shi, M.; Tang, X.; Chen, T.; et al. Clonorchis sinensis granulin: Identification, immunolocalization, and function in promoting the metastasis of cholangiocarcinoma and hepatocellular carcinoma. Parasites Vectors 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, X.; Wang, X.; Lu, S.; Yin, J.; Zhang, J. Employing Parasite Against Cancer: A Lesson From the Canine Tapeworm Echinococcus Granulocus. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Chaiyadet, S.; Smout, M.; Laha, T.; Sripa, B.; Loukas, A.; Sotillo, J. Proteomic characterization of the internalization of Opisthorchis viverrini excretory/secretory products in human cells. Parasitol. Int. 2017, 66, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Smout, M.J.; Sripa, B.; Laha, T.; Mulvenna, J.; Gasser, R.B.; Young, N.D.; Bethony, J.M.; Brindley, P.J.; Loukas, A. Infection with the carcinogenic human liver fluke, Opisthorchis viverrini. Mol. BioSyst. 2011, 7, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Brindley, P.J.; Mulvenna, J.; Laha, T.; Smout, M.J.; Mairiang, E.; Bethony, J.M.; Loukas, A. The tumorigenic liver fluke Opisthorchis viverrine—Multiple pathways to cancer. Trends Parasitol. 2012, 28, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Suttiprapa, S.; Sotillo, J.; Smout, M.; Suyapoh, W.; Chaiyadet, S.; Tripathi, T.; Laha, T.; Loukas, A. Opisthorchis viverrini Proteome and Host–Parasite Interactions. Adv. Parasitol. 2018, 102, 45–72. [Google Scholar] [CrossRef] [PubMed]
- Brindley, P.J.; Loukas, A. Helminth infection–induced malignancy. PLoS Pathog. 2017, 13, e1006393. [Google Scholar] [CrossRef]
- Chaiyadet, S.; Smout, M.; Johnson, M.; Whitchurch, C.; Turnbull, L.; Kaewkes, S.; Sotillo, J.; Loukas, A.; Sripa, B. Excretory/secretory products of the carcinogenic liver fluke are endocytosed by human cholangiocytes and drive cell proliferation and IL6 production. Int. J. Parasitol. 2015, 45, 773–781. [Google Scholar] [CrossRef]
- Prum, S.; Plumworasawat, S.; Chaiyadet, S.; Saichua, P.; Thanan, R.; Laha, T.; Laohaviroj, M.; Sripa, B.; Suttiprapa, S. Characterization and in vitro functional analysis of thioredoxin glutathione reductase from the liver fluke Opisthorchis viverrini. Acta Trop. 2020, 210, 105621. [Google Scholar] [CrossRef]
- Matchimakul, P.; Rinaldi, G.; Suttiprapa, S.; Mann, V.H.; Popratiloff, A.; Laha, T.; Pimenta, R.N.; Cochran, C.J.; Kaewkes, S.; Sripa, B.; et al. Apoptosis of cholangiocytes modulated by thioredoxin of carcinogenic liver fluke. Int. J. Biochem. Cell Biol. 2015, 65, 72–80. [Google Scholar] [CrossRef]
- Nguyen, P.; Awwad, R.T.; Smart, D.D.K.; Spitz, D.R.; Gius, D. Thioredoxin reductase as a novel molecular target for cancer therapy. Cancer Lett. 2006, 236, 164–174. [Google Scholar] [CrossRef]
- Pennington, J.D.; Jacobs, K.M.; Sun, L.; Bar-Sela, G.; Mishra, M.; Gius, D. Thioredoxin and Thioredoxin Reductase as Redox-Sensitive Molecular Targets for Cancer Therapy. Curr. Pharm. Des. 2007, 13, 3368–3377. [Google Scholar] [CrossRef]
- Bansal, P.S.; Smout, M.; Wilson, D.; Caceres, C.C.; Dastpeyman, M.; Sotillo, J.; Seifert, J.; Brindley, P.J.; Loukas, A.; Daly, N.L. Development of a Potent Wound Healing Agent Based on the Liver Fluke Granulin Structural Fold. J. Med. Chem. 2017, 60, 4258–4266. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.; Karinshak, S.E.; Mann, V.H.; Popratiloff, A.; Loukas, A.; Brindley, P.J.; Smout, M. Granulin Secreted by the Food-Borne Liver Fluke Opisthorchis viverrini Promotes Angiogenesis in Human Endothelial Cells. Front. Med. 2018, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Smout, M.; Sotillo, J.; Laha, T.; Papatpremsiri, A.; Rinaldi, G.; Pimenta, R.N.; Chan, L.Y.; Johnson, M.S.; Turnbull, L.; Whitchurch, C.B.; et al. Carcinogenic Parasite Secretes Growth Factor That Accelerates Wound Healing and Potentially Promotes Neoplasia. PLoS Pathog. 2015, 11, e1005209. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.S.; Fedotova, M.M.; Zvonareva, O.I.; Mazeina, S.V.; Kovshirina, Y.V.; Sokolova, T.S.; Golovach, E.A.; Kovshirina, A.E.; Konovalova, U.V.; Kolomeets, I.L.; et al. Opisthorchis felineus infection, risks, and morbidity in rural Western Siberia, Russian Federation. PLoS Negl. Trop. Dis. 2020, 14, e0008421. [Google Scholar] [CrossRef] [PubMed]
- Pakharukova, M.Y.; Mordvinov, V.A. The liver flukeOpisthorchis felineus: Biology, epidemiology and carcinogenic potential. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 28–36. [Google Scholar] [CrossRef]
- Ishida, K.; Hsieh, M.H. Understanding Urogenital Schistosomiasis-Related Bladder Cancer: An Update. Front. Med. 2018, 5, 223. [Google Scholar] [CrossRef]
- Makhyoun, N.A.; El-Kashlan, K.M.; Al-Ghorab, M.M.; Mokhles, A.S. Aetiological factors in bilharzial bladder cancer. J. Trop. Med. Hyg. 1971, 74, 73–78. [Google Scholar]
- Mbanefo, E.C.; Agbo, C.T.; Zhao, Y.; Lamanna, O.K.; Thai, K.H.; Karinshak, S.E.; Khan, M.A.; Fu, C.-L.; Odegaard, J.I.; Saltikova, I.V.; et al. IPSE, an abundant egg-secreted protein of the carcinogenic helminth Schistosoma haematobium, promotes proliferation of bladder cancer cells and angiogenesis. Infect. Agents Cancer 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Botros, S.; Hammam, O.; El-Lakkany, N.; El-Din, S.H.S.; Ebeid, F.A. Schistosoma haematobium (Egyptian Strain): Rate of Development and Effect of Praziquantel Treatment. J. Parasitol. 2008, 94, 386–394. [Google Scholar] [CrossRef]
- Sotillo, J.; Pearson, M.S.; Becker, L.; Mekonnen, G.G.; Amoah, A.S.; Van Dam, G.J.; Corstjens, P.L.; Murray, J.; Mduluza, T.; Mutapi, F.; et al. In-depth proteomic characterization of Schistosoma haematobium: Towards the development of new tools for elimination. PLoS Negl. Trop. Dis. 2019, 13, e0007362. [Google Scholar] [CrossRef]
- Botelho, M.C.; Ferreira, A.C.; Oliveira, M.J.; Domingues, A.; Machado, J.C.; Da Costa, J.M.C. Schistosoma haematobium total antigen induces increased proliferation, migration and invasion, and decreases apoptosis of normal epithelial cells. Int. J. Parasitol. 2009, 39, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.C.; Oliveira, P.A.; Gomes, J.; Gärtner, F.; Lopes, C.; Da Costa, J.M.C.; Machado, J.C. Tumourigenic effect ofSchistosoma haematobiumtotal antigen in mammalian cells. Int. J. Exp. Pathol. 2009, 90, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.C.; Oliveira, P.A.; Lopes, C.; Da Costa, J.M.C.; Machado, J.C.; Da Costa, J.M.C. Urothelial dysplasia and inflammation induced by Schistosoma haematobium total antigen instillation in mice normal urothelium. Urol. Oncol. Semin. Orig. Investig. 2011, 29, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Pennington, L.F.; Alouffi, A.; Mbanefo, E.C.; Ray, D.; Heery, D.M.; Jardetzky, T.S.; Hsieh, M.H.; Falcone, F.H. H-IPSE Is a Pathogen-Secreted Host Nucleus-Infiltrating Protein (Infiltrin) Expressed Exclusively by the Schistosoma haematobium Egg Stage. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef]
- Garcia, H.H.; Rodriguez, S.; Friedland, J.S.; Cysticercosis Working Group in Peru. Immunology of Taenia solium taeniasis and human cysticercosis. Parasite Immunol. 2014, 36, 388–396. [Google Scholar] [CrossRef]
- Herrera, L.A.; Ramírez, T.; Rodríguez, U.; Corona, T.; Sotelo, J.; Lorenzo, M.; Ramos, F.; Verdorfer, I.; Gebhart, E.; Ostrosky-Wegman, P.; et al. Possible association between Taenia solium cysticercosis and cancer: Increased frequency of DNA damage in peripheral lymphocytes from neurocysticercosis patients. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 61–65. [Google Scholar] [CrossRef]
- Gomez, S.; Adalid-Peralta, L.; Palafox-Fonseca, H.; Cantu-Robles, V.A.; Soberón, X.; Sciutto, E.; Fragoso, G.; Bobes, R.J.; Laclette, J.P.; Yauner, L.D.P.; et al. Genome analysis of Excretory/Secretory proteins in Taenia solium reveals their Abundance of Antigenic Regions (AAR). Sci. Rep. 2015, 5, 9683. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Castillo, P.; Mena, I.X.; Freire, A.X. Neurocysticercosis among Patients with Cerebral Gliomas. Arch. Neurol. 1997, 54, 1125–1128. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Dolezal, M.; Castillo, P.R.; García, H.H. Neurocysticercosis and Oncogenesis. Arch. Med. Res. 2000, 31, 151–155. [Google Scholar] [CrossRef]
- Herrera, L.A.; Santiago, P.; Rojas, G.; Salazar, P.M.; Tato, P.; Molinari, J.; Schiffmann, D.; Ostrosky-Wegman, P. Immune response impairment, genotoxicity and morphological transformation induced by Taenia solium metacestode. Mutat. Res. Mol. Mech. Mutagen. 1994, 305, 223–228. [Google Scholar] [CrossRef]
- Herrera, L.A.; Benita-Bordes, A.; Sotelo, J.; Chávez, L.; Olvera, J.; Rascón, A.; López, M.; Ostrosky-Wegman, P. Possible relationship between neurocysticercosis and hematological malignancies. Arch. Med Res. 1999, 30, 154–158. [Google Scholar] [CrossRef]
- Laclette, J.P.; Shoemaker, C.B.; Richter, D.; Arcos, L.; Pante, N.; Cohen, C.; Bing, D.; Nicholson-Weller, A. Paramyosin inhibits complement C1. J. Immunol. 1992, 148, 124–128. [Google Scholar] [PubMed]
- Hammerberg, B.; Williams, J.F. Interaction between Taenia taeniaeformis and the complement system. J. Immunol. 1978, 120, 1033–1038. [Google Scholar] [PubMed]
- Arechavaleta, F.; Molinari, J.L.; Tato, P. A Taenia solium metacestode factor nonspecifically inhibits cytokine production. Parasitol. Res. 1997, 84, 117–122. [Google Scholar] [CrossRef]
- Cui, S.-J.; Xu, L.-L.; Zhang, T.; Xu, M.; Yao, J.; Fang, C.-Y.; Feng, Z.; Yang, P.; Hu, W.; Liu, F. Proteomic characterization of larval and adult developmental stages in Echinococcus granulosus reveals novel insight into host–parasite interactions. J. Proteom. 2013, 84, 158–175. [Google Scholar] [CrossRef]
- Oikonomopoulou, K.; Yu, H.; Wang, Z.; Vasiliou, S.K.; Brinc, D.; Christofi, G.; Theodorou, M.; Pavlou, P.; Hadjisavvas, A.; Christodoulou, C.C.; et al. Association between Echinococcus granulosus infection and cancer risk—A pilot study in Cyprus. Clin. Chem. Lab. Med. 2016, 54, 1955–1961. [Google Scholar] [CrossRef]
- Turhan, N.; Esendagli, G.; Ozkayar, O.; Tunali, G.; Sokmensuer, C.; Abbasoglu, O. Co-existence ofEchinococcus granulosusinfection and cancer metastasis in the liver correlates with reduced Th1 immune responses. Parasite Immunol. 2015, 37, 16–22. [Google Scholar] [CrossRef]
- Gundogdu, S.B.; Saylam, B.; Tez, M. Cyst hydatid and cancer: The myth continues. Clin. Chem. Lab. Med. 2017, 55, e150–e151. [Google Scholar] [CrossRef]
- Akgül, H.; Tez, M.; Ünal, A.E.; Keskek, M.; Sayek, I.; Özçelik, T. Echinococcus against cancer: Why not? Cancer 2003, 98, 1999–2000. [Google Scholar] [CrossRef]
- Daneshpour, S.; Bahadoran, M.; Hejazi, S.H.; Eskandarian, A.A.; Mahmoudzadeh, M.; Darani, H.Y. Common antigens between hydatid cyst and cancers. Adv. Biomed. Res. 2016, 5. [Google Scholar] [CrossRef]
- Darani, H.Y.; Daneshpour, S.; Kefayat, A.H.; Mofid, M.R.; Rad, S.R. Effect of Hydatid Cyst Fluid Antigens on Induction of Apoptosis on Breast Cancer Cells. Adv. Biomed. Res. 2019, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, S.L.; McManus, D.P. Echinococcus granulosus: Cure for Cancer Revisited. Front. Med. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Sava, M.; Huynh, T.; Frugoli, A.; Kong, L.; Salehpour, M.; Barrows, B. Colorectal Cancer Related to Chronic Strongyloides stercoralis Infection. Case Rep. Gastrointest. Med. 2020, 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Catalano, C.; Aron, J.; Bansal, R.; Leytin, A. Colorectal Cancer Associated with Strongyloides stercoralis Colitis. ACG Case Rep. J. 2017, 4, e104. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Kishimoto, K.; Kinjo, N.; Hokama, A.; Kinjo, F.; Fujita, J. Association between Strongyloides stercoralis infection and biliary tract cancer. Parasitol. Res. 2007, 101, 1345–1348. [Google Scholar] [CrossRef]
- Tanaka, T.; Hirata, T.; Parrott, G.; Higashiarakawa, M.; Kinjo, T.; Kinjo, T.; Hokama, A.; Fujita, J. Relationship Among Strongyloides stercoralis Infection, Human T-Cell Lymphotropic Virus Type 1 Infection, and Cancer: A 24-Year Cohort Inpatient Study in Okinawa, Japan. Am. J. Trop. Med. Hyg. 2016, 94, 365–370. [Google Scholar] [CrossRef]
- Menezes, R.C.; Tortelly, R.; Gomes, D.C.; Pinto, R.M. Nodular typhlitis associated with the nematodes Heterakis gallinarum and Heterakis isolonche in pheasants: Frequency and pathology with evidence of neoplasia. Mem. Inst. Oswaldo Cruz 2003, 98, 1011–1016. [Google Scholar] [CrossRef]
- Machicado, C.; Marcos, L.A. Carcinogenesis associated with parasites other than Schistosoma, Opisthorchis and Clonorchis: A systematic review. Int. J. Cancer 2016, 138, 2915–2921. [Google Scholar] [CrossRef]
- Hurst, R.J.M.; Else, K.J. Trichuris murisresearch revisited: A journey through time. Parasitology 2013, 140, 1325–1339. [Google Scholar] [CrossRef]
- Hayes, K.S.; Cliffe, L.J.; Bancroft, A.J.; Forman, S.P.; Thompson, S.; Booth, C.; Grencis, R.K. Chronic Trichuris muris infection causes neoplastic change in the intestine and exacerbates tumour formation in APC min/+ mice. PLoS Negl. Trop. Dis. 2017, 11, e0005708. [Google Scholar] [CrossRef]
- Herrera, L.A.; Ostrosky-Wegman, P. Do helminths play a role in carcinogenesis? Trends Parasitol. 2001, 17, 172–175. [Google Scholar] [CrossRef]
- Fabbi, M.; Carbotti, G.; Ferrini, S. Context-dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP. J. Leukoc. Biol. 2015, 97, 665–675. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Dominguez, C.; Hamilton, D.H.; Palena, C. The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Compagno, D.; Tiraboschi, C.; Garcia, J.D.; Rondón, Y.; Corapi, E.; Velazquez, C.; Laderach, D.J. Galectins as Checkpoints of the Immune System in Cancers, Their Clinical Relevance, and Implication in Clinical Trials. Biomolecules 2020, 10, 750. [Google Scholar] [CrossRef]
- Meléndez, R.D.; Suárez-Pellín, C.; Suárez-Pellın, C. Spirocerca lupi and dogs: The role of nematodes in carcinogenesis. Trends Parasitol. 2001, 17, 516. [Google Scholar] [CrossRef]
- Qi, H.; Liu, S.; Guo, C.; Wang, J.; Greenaway, F.T.; Sun, M.-Z. Role of annexin A6 in cancer. Oncol. Lett. 2015, 10, 1947–1952. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Gong, J.; Emurshid, A. Extracellular HSPs: The Complicated Roles of Extracellular HSPs in Immunity. Front. Immunol. 2016, 7, 159. [Google Scholar] [CrossRef]
- Chou, F.-C.; Chen, H.-Y.; Kuo, C.-C.; Sytwu, H.-K. Role of Galectins in Tumors and in Clinical Immunotherapy. Int. J. Mol. Sci. 2018, 19, 430. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, B.-B.; Hua, H.; Li, B.; Zhang, B.; Yu, Q.; Li, X.-Y.; Liu, Y.; Pan, W.; Liu, X.-Y.; et al. The Dynamics of Treg/Th17 and the Imbalance of Treg/Th17 in Clonorchis sinensis-Infected Mice. PLoS ONE 2015, 10, e0143217. [Google Scholar] [CrossRef]
- Floudas, A.; Cluxton, C.D.; Fahel, J.; Khan, A.R.; Saunders, S.P.; Amu, S.; Alcami, A.; Fallon, P. Composition of the Schistosoma mansoni worm secretome: Identification of immune modulatory Cyclophilin A. PLoS Negl. Trop. Dis. 2017, 11, e0006012. [Google Scholar] [CrossRef]
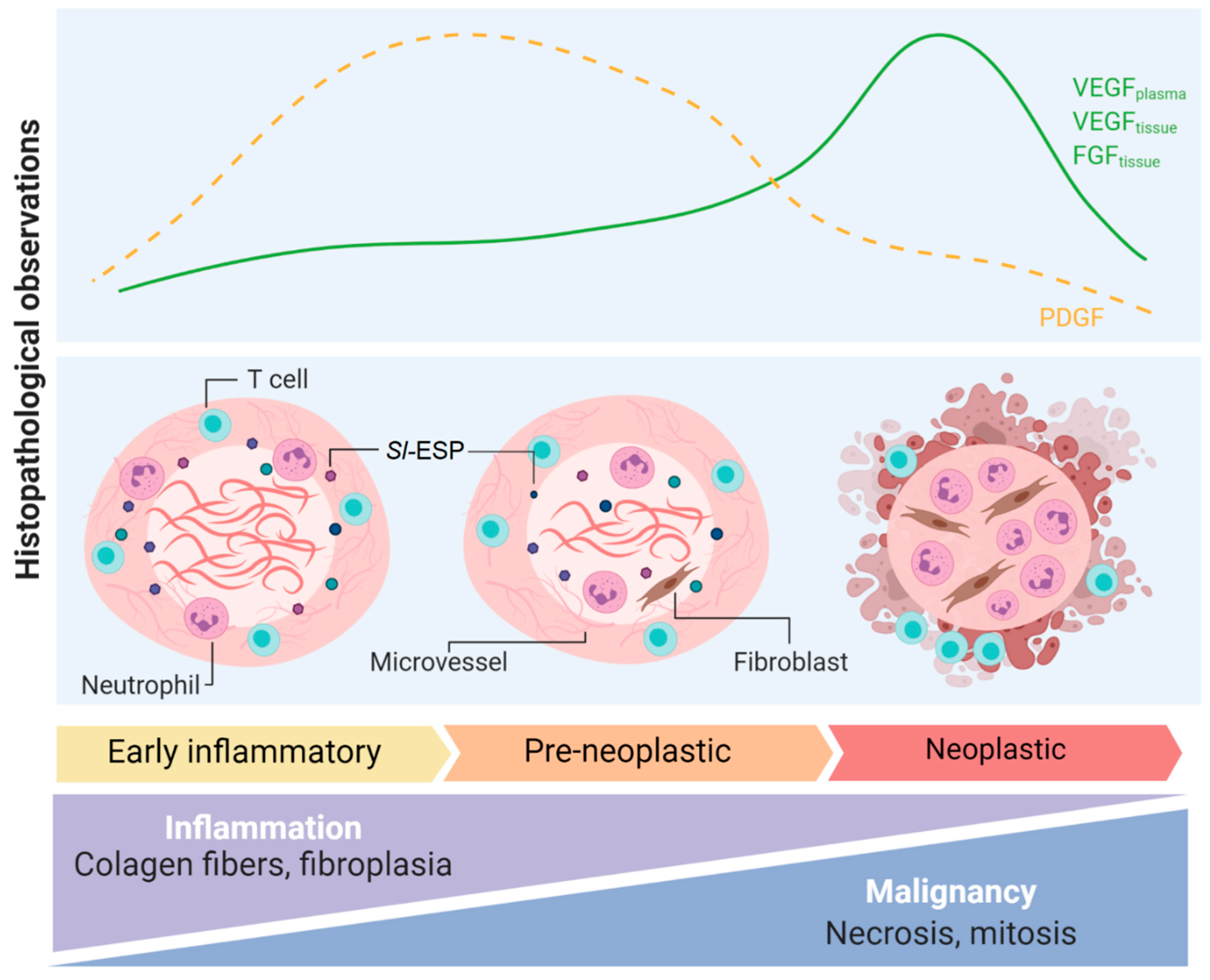
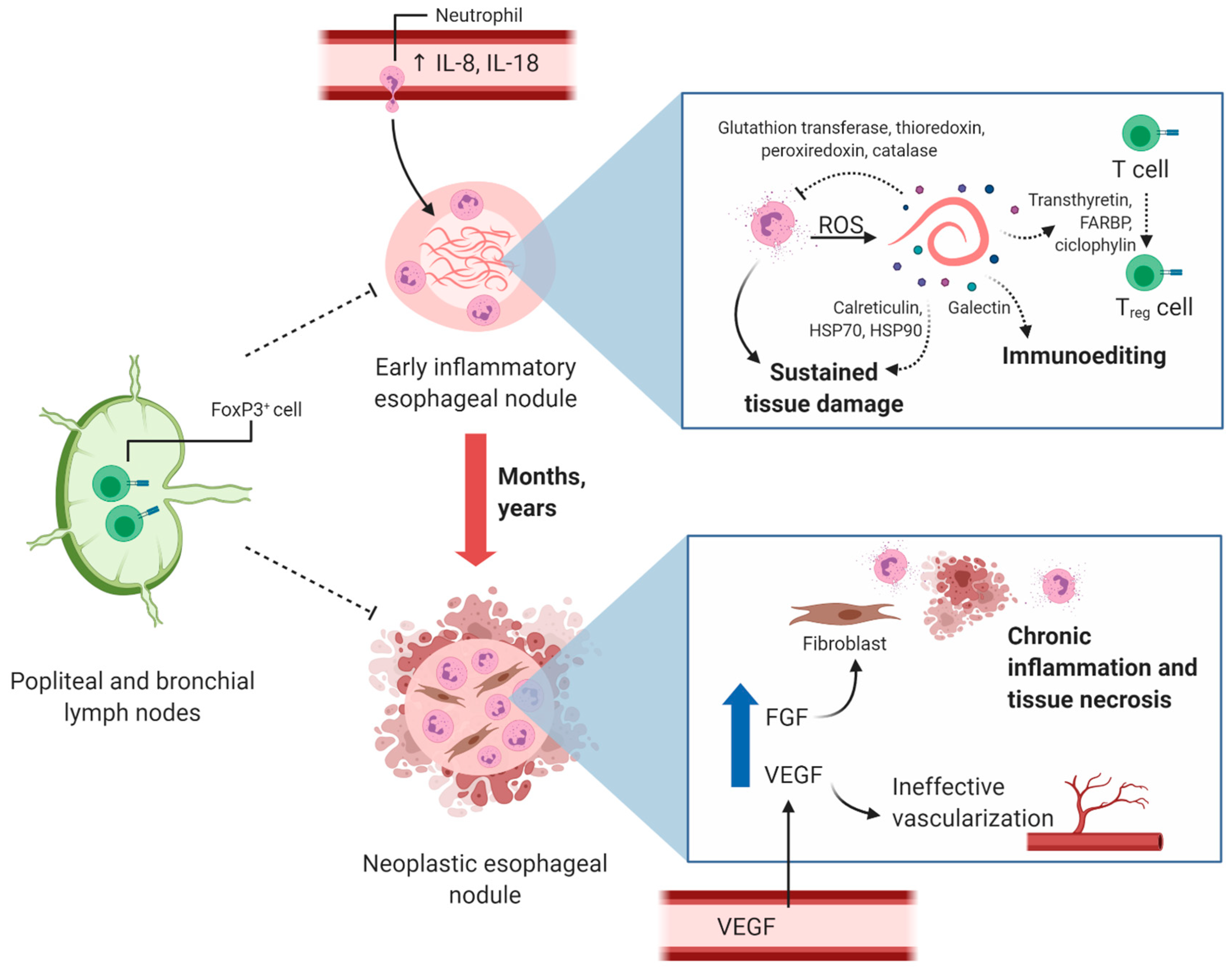
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porras-Silesky, C.; Mejías-Alpízar, M.J.; Mora, J.; Baneth, G.; Rojas, A. Spirocerca lupi Proteomics and Its Role in Cancer Development: An Overview of Spirocercosis-Induced Sarcomas and Revision of Helminth-Induced Carcinomas. Pathogens 2021, 10, 124. https://doi.org/10.3390/pathogens10020124
Porras-Silesky C, Mejías-Alpízar MJ, Mora J, Baneth G, Rojas A. Spirocerca lupi Proteomics and Its Role in Cancer Development: An Overview of Spirocercosis-Induced Sarcomas and Revision of Helminth-Induced Carcinomas. Pathogens. 2021; 10(2):124. https://doi.org/10.3390/pathogens10020124
Chicago/Turabian StylePorras-Silesky, Catalina, María José Mejías-Alpízar, Javier Mora, Gad Baneth, and Alicia Rojas. 2021. "Spirocerca lupi Proteomics and Its Role in Cancer Development: An Overview of Spirocercosis-Induced Sarcomas and Revision of Helminth-Induced Carcinomas" Pathogens 10, no. 2: 124. https://doi.org/10.3390/pathogens10020124
APA StylePorras-Silesky, C., Mejías-Alpízar, M. J., Mora, J., Baneth, G., & Rojas, A. (2021). Spirocerca lupi Proteomics and Its Role in Cancer Development: An Overview of Spirocercosis-Induced Sarcomas and Revision of Helminth-Induced Carcinomas. Pathogens, 10(2), 124. https://doi.org/10.3390/pathogens10020124









