Predictive and Prognostic Utility of the Serum Level of Resistin-Like Molecule Beta for Risk Stratification in Patients with Community-Acquired Pneumonia
Abstract
1. Introduction
2. Results
2.1. Population Characteristics of Enrolled Patients
2.2. Level of RELM-β in Each Group and Etiology
2.3. Correlation between Level of RELM-β and CAP Severity
2.4. Value of RELM-β in Predicting Severity in CAP Patients
2.5. Prognostic Ability of RELM-β in CAP Patients of 30-Day Mortality
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Measuring RELM-β and proADM Levels in the Serum
4.3. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Prina, E.; Ranzani, O.T.; Torres, A. Community-acquired pneumonia. Lancet 2015, 386, 1097–1108. [Google Scholar] [CrossRef]
- Pepper, D.J. Treatment for hospitalized patients with severe community-acquired pneumonia. JAMA 2015, 313, 2184. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Martin-Loeches, I.; Menendez, R. Research in community-acquired pneumonia: The next steps. Intensive Care Med. 2017, 43, 1395–1397. [Google Scholar] [CrossRef] [PubMed]
- Fine, M.J.; Auble, T.E.; Yealy, D.M.; Hanusa, B.H.; Weissfeld, L.A.; Singer, D.E.; Coley, C.M.; Marrie, T.J.; Kapoor, W.N. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 1997, 336, 243–250. [Google Scholar] [CrossRef]
- Olson, G.; Davis, A.M. Diagnosis and treatment of adults with community-acquired pneumonia. JAMA 2020, 323, 885. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Fowler, R.; Hayden, F.G. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020, 46, 315–328. [Google Scholar] [CrossRef]
- Cerda-Mancillas, M.C.; Santiago-German, D.; Andrade-Bravo, B.; Pedraza-Olivares, F.; Valenzo-Hernandez, F.; Leanos-Miranda, A.; Isordia-Salas, I. D-dimer as a biomarker of severity and adverse outcomes in patients with community acquired pneumonia. Arch. Med. Res. 2020, 51, 429–435. [Google Scholar] [CrossRef]
- Esposito, S.; Di Gangi, M.; Cardinale, F.; Baraldi, E.; Corsini, I.; Da Dalt, L.; Tovo, P.A.; Correra, A.; Villani, A.; Sacco, O.; et al. Sensitivity and specificity of soluble triggering receptor expressed on myeloid cells-1, midregional proatrial natriuretic peptide and midregional proadrenomedullin for distinguishing etiology and to assess severity in community-acquired pneumonia. PLoS ONE 2016, 11, e0163262. [Google Scholar] [CrossRef]
- Alan, M.; Grolimund, E.; Kutz, A.; Christ-Crain, M.; Thomann, R.; Falconnier, C.; Hoess, C.; Henzen, C.; Zimmerli, W.; Mueller, B.; et al. Clinical risk scores and blood biomarkers as predictors of long-term outcome in patients with community-acquired pneumonia: A 6-year prospective follow-up study. J. Intern. Med. 2015, 278, 174–184. [Google Scholar] [CrossRef]
- Luo, Q.; He, X.; Ning, P.; Zheng, Y.; Yang, D.; Xu, Y.; Shang, Y.; Gao, Z. Admission pentraxin-3 level predicts severity of community-acquired pneumonia independently of etiology. Proteom. Clin. Appl. 2019, 13, e1800117. [Google Scholar] [CrossRef]
- Bello, S.; Lasierra, A.B.; Minchole, E.; Fandos, S.; Ruiz, M.A.; Vera, E.; de Pablo, F.; Ferrer, M.; Menendez, R.; Torres, A. Prognostic power of proadrenomedullin in community-acquired pneumonia is independent of aetiology. Eur. Respir. J. 2012, 39, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Kruger, S.; Ewig, S.; Giersdorf, S.; Hartmann, O.; Suttorp, N.; Welte, T. German Competence Network for the Study of Community Acquired Pneumonia Study, G. Cardiovascular and inflammatory biomarkers to predict short-and long-term survival in community-acquired pneumonia: Results from the German Competence Network, CAPNETZ. Am. J. Respir. Crit. Care Med. 2010, 182, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Brown, E.J.; Wright, C.M.; Bhat, S.; Banerjee, R.R.; Dai, C.Y.; Enders, G.H.; Silberg, D.G.; Wen, X.; Wu, G.D.; et al. A family of tissue-specific resistin-like molecules. Proc. Natl. Acad. Sci. USA 2001, 98, 502–506. [Google Scholar] [CrossRef]
- Shojima, N.; Ogihara, T.; Inukai, K.; Fujishiro, M.; Sakoda, H.; Kushiyama, A.; Katagiri, H.; Anai, M.; Ono, H.; Fukushima, Y.; et al. Serum concentrations of resistin-like molecules beta and gamma are elevated in high-fat-fed and obese db/db mice, with increased production in the intestinal tract and bone marrow. Diabetologia 2005, 48, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Grainge, C.; Dulay, V.; Ward, J.; Sammut, D.; Davies, E.; Green, B.; Lau, L.; Cottey, L.; Haitchi, H.M.; Davies, D.E.; et al. Resistin-like molecule-beta is induced following bronchoconstriction of asthmatic airways. Respirology 2012, 17, 1094–1100. [Google Scholar] [CrossRef]
- Homer, R.J. Airway remodeling and RELM-beta. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L303-4. [Google Scholar] [CrossRef][Green Version]
- Mishra, A.; Wang, M.; Schlotman, J.; Nikolaidis, N.M.; DeBrosse, C.W.; Karow, M.L.; Rothenberg, M.E. Resistin-like molecule-beta is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L305-13. [Google Scholar] [CrossRef]
- Lin, Q.; Johns, R.A. Resistin family proteins in pulmonary diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L422–L434. [Google Scholar] [CrossRef]
- Florin, T.A.; Ambroggio, L.; Brokamp, C.; Zhang, Y.; Nylen, E.S.; Rattan, M.; Crotty, E.; Belsky, M.A.; Krueger, S.; Epperson, T.N.; et al. Proadrenomedullin predicts severe disease in children with suspected community-acquired pneumonia. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Angelini, D.J.; Su, Q.; Yamaji-Kegan, K.; Fan, C.; Teng, X.; Hassoun, P.M.; Yang, S.C.; Champion, H.C.; Tuder, R.M.; Johns, R.A. Resistin-like molecule-beta in scleroderma-associated pulmonary hypertension. Am. J. Respir. Cell. Mol. Biol. 2009, 41, 553–561. [Google Scholar] [CrossRef]
- Boucherat, O.; Paulin, R.; Provencher, S.; Bonnet, S. New insights into HIMF (Hypoxia-Induced Mitogenic Factor)-mediated signaling pathways in pulmonary hypertension. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2451–2453. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Fan, C.; Gomez-Arroyo, J.; Van Raemdonck, K.; Meuchel, L.W.; Skinner, J.T.; Everett, A.D.; Fang, X.; Macdonald, A.A.; Yamaji-Kegan, K.; et al. HIMF (Hypoxia-Induced Mitogenic Factor) signaling mediates the HMGB1 (High Mobility Group Box 1)-dependent endothelial and smooth muscle cell crosstalk in pulmonary hypertension. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2505–2519. [Google Scholar] [CrossRef]
- Knipper, J.A.; Willenborg, S.; Brinckmann, J.; Bloch, W.; Maass, T.; Wagener, R.; Krieg, T.; Sutherland, T.; Munitz, A.; Rothenberg, M.E.; et al. Interleukin-4 receptor alpha signaling in myeloid cells controls collagen fibril assembly in skin repair. Immunity 2015, 43, 803–816. [Google Scholar] [CrossRef]
- Fang, C.L.; Yin, L.J.; Sharma, S.; Kierstein, S.; Wu, H.F.; Eid, G.; Haczku, A.; Corrigan, C.J.; Ying, S. Resistin-like molecule-beta (RELM-beta) targets airways fibroblasts to effect remodelling in asthma: From mouse to man. Clin. Exp. Allergy 2015, 45, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Song, N.; Hu, X.; Zhu, A.; Wei, X.; Liu, J.; Yuan, S.; Mao, W.; Chen, X. Inhibition of RELM-beta prevents hypoxia-induced overproliferation of human pulmonary artery smooth muscle cells by reversing PLC-mediated KCNK3 decline. Life Sci. 2020, 246, 117419. [Google Scholar] [CrossRef] [PubMed]
- LeMessurier, K.S.; Palipane, M.; Tiwary, M.; Gavin, B.; Samarasinghe, A.E. Chronic features of allergic asthma are enhanced in the absence of resistin-like molecule-beta. Sci. Rep. 2018, 8, 7061. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Baek, H.A.; Yu, H.; Lee, H.J.; Park, B.H.; Ullenbruch, M.; Liu, J.; Nakashima, T.; Choi, Y.Y.; Wu, G.D.; et al. FIZZ2/RELM-beta induction and role in pulmonary fibrosis. J. Immunol. 2011, 187, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Dietschmann, A.; Schruefer, S.; Krappmann, S.; Voehringer, D. Th2 cells promote eosinophil-independent pathology in a murine model of allergic bronchopulmonary aspergillosis. Eur. J. Immunol. 2020, 50, 1044–1056. [Google Scholar] [CrossRef]
- Meyer Sauteur, P.M. Challenges and progress toward determining pneumonia etiology. Clin. Infect. Dis. 2020, 71, 514–516. [Google Scholar] [CrossRef]
- Meyer Sauteur, P.M.; Truck, J.; van Rossum, A.M.C.; Berger, C. Circulating antibody-secreting cell response during mycoplasma pneumoniae childhood pneumonia. J. Infect. Dis. 2020, 222, 136–147. [Google Scholar] [CrossRef]
- Morampudi, V.; Dalwadi, U.; Bhinder, G.; Sham, H.P.; Gill, S.K.; Chan, J.; Bergstrom, K.S.; Huang, T.; Ma, C.; Jacobson, K.; et al. The goblet cell-derived mediator RELM-beta drives spontaneous colitis in Muc2-deficient mice by promoting commensal microbial dysbiosis. Mucosal Immunol. 2016, 9, 1218–1233. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Itoh, K.; Park, S.H.; Kaku, M.; Ishii, K.; Sasano, H.; Naitoh, T.; Unno, M.; Fukushima, K. Resistin-like molecule beta, a colonic epithelial protein, exhibits antimicrobial activity against Staphylococcus aureus including methicillin-resistant strains. Surg. Today 2020, 50, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Meyer Sauteur, P.M.; Berger, C. Proadrenomedullin in Mycoplasma pneumoniae community-acquired pneumonia in children. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, H.P.; Xu, F.; Wu, B.Q.; Lin, H.C. Early diagnosis of necrotizing enterocolitis by plasma RELMbeta and thrombocytopenia in preterm infants: A pilot study. Pediatr. Neonatol. 2019, 60, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Loftus, C.M. Carotid endarterectomy: Current indications for elective and emergency surgery. Postgrad. Med. 1987, 82, 241–244, 246–248. [Google Scholar] [CrossRef]
- Cataudella, E.; Giraffa, C.M.; Di Marca, S.; Pulvirenti, A.; Alaimo, S.; Pisano, M.; Terranova, V.; Corriere, T.; Ronsisvalle, M.L.; Di Quattro, R.; et al. Neutrophil-To-Lymphocyte ratio: An emerging marker predicting prognosis in elderly adults with community-acquired pneumonia. J. Am. Geriatr. Soc. 2017, 65, 1796–1801. [Google Scholar] [CrossRef]
- Qiu, Y.; Su, Y.; Tu, G.W.; Ju, M.J.; He, H.Y.; Gu, Z.Y.; Yang, C.; Luo, Z. Neutrophil-to-Lymphocyte ratio predicts mortality in adult renal transplant recipients with severe community-acquired pneumonia. Pathogens 2020, 9, 913. [Google Scholar] [CrossRef]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007, 44, S27–S72. [Google Scholar] [CrossRef]
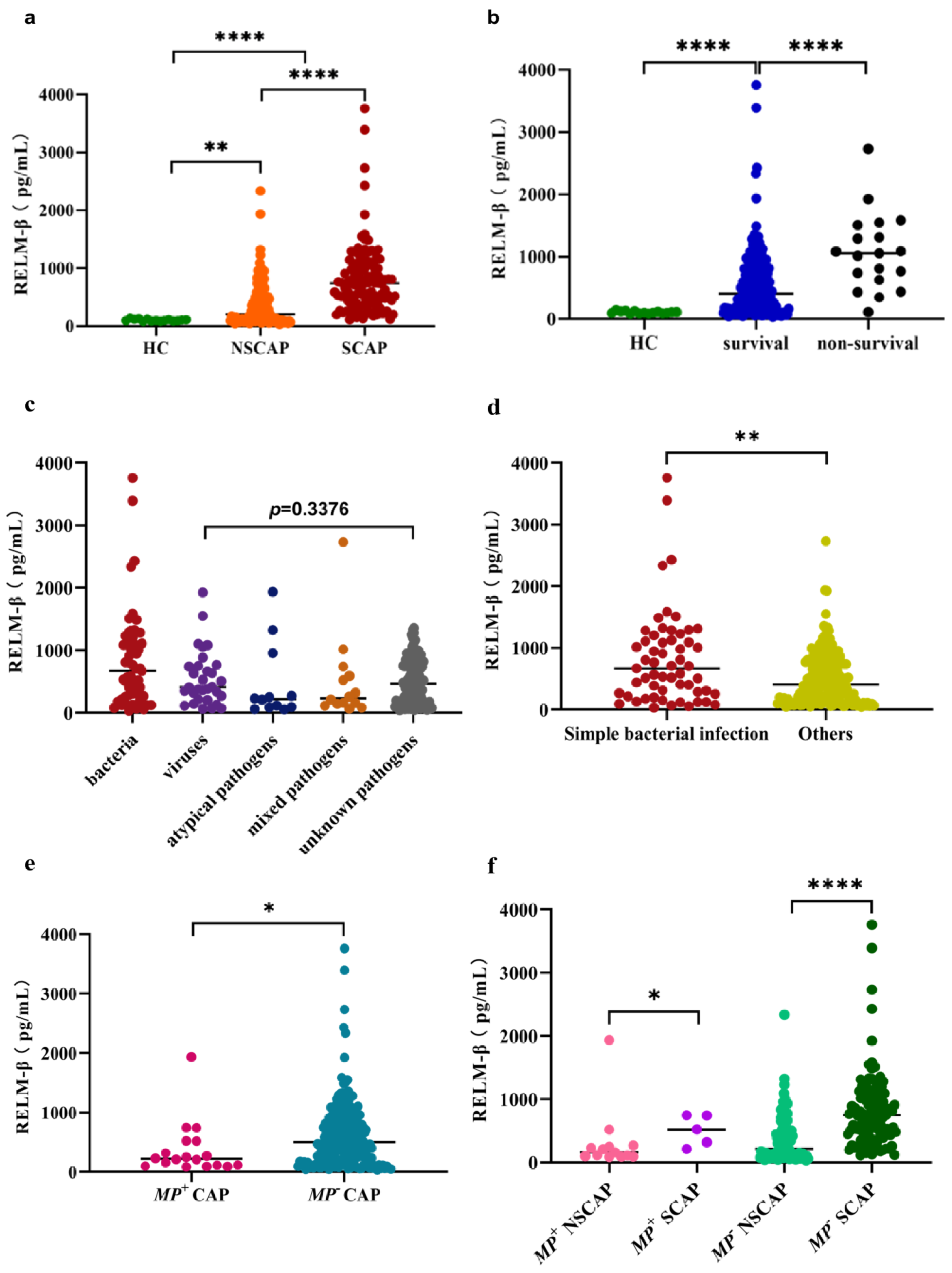
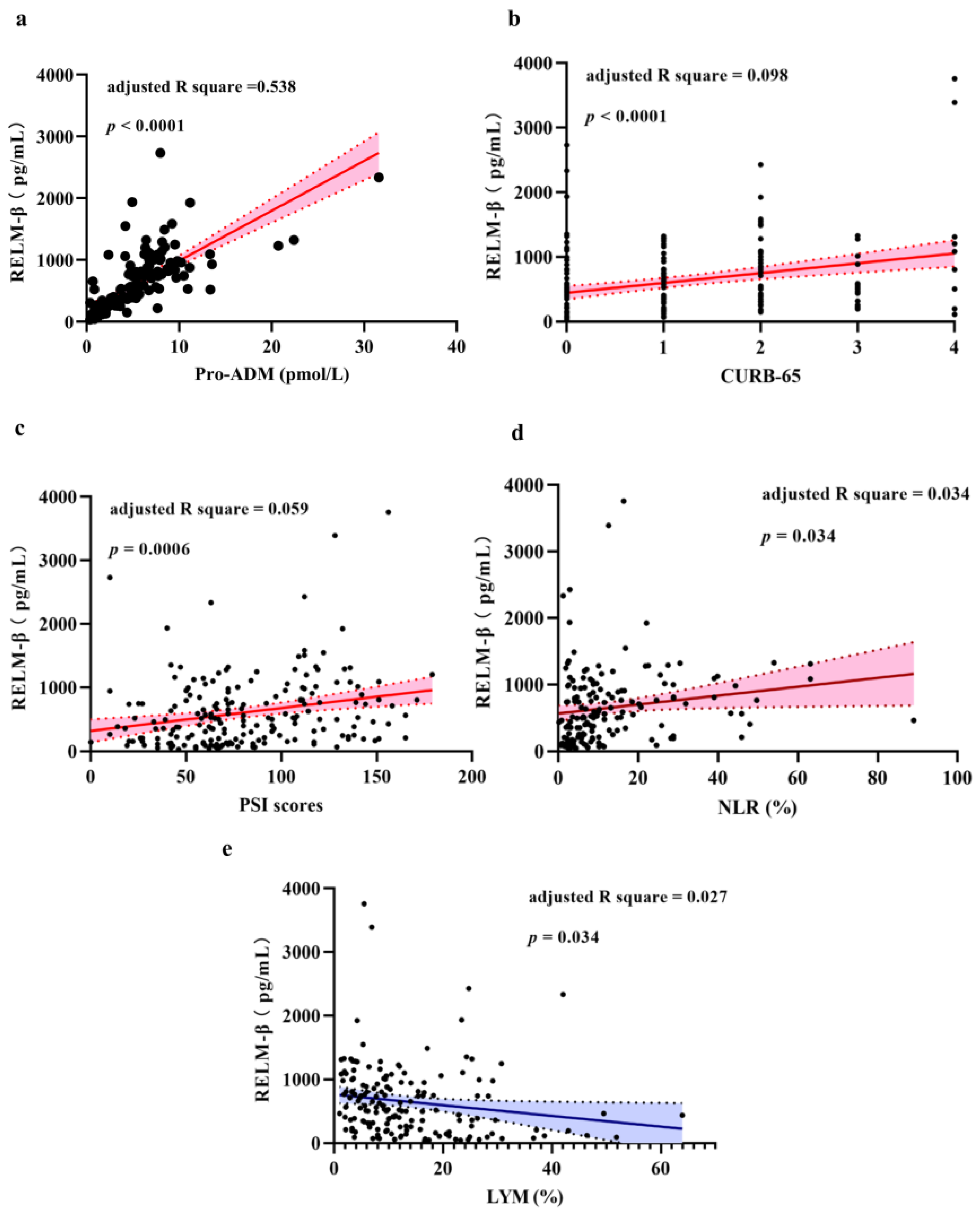

| Characteristic | NSCAP (N = 112) | SCAP (N = 114) | p-Value |
|---|---|---|---|
| Male sex—no. (%) | 80 (71.43) | 82 (71.93) | 0.525 † |
| Age—years | 55 (46.50–62.00) | 57.72 ± 17.82 | 0.213 ††† |
| Smoking history—no. (%) | 18 (16.07) | 19 (16.67) | 0.524 † |
| Underlying diseases— no. (%) | |||
| Chronic heart failure | 5 (4.46) | 10 (8.77) | |
| Diabetes mellitus | 18 (16.07) | 18 (15.79) | |
| Cerebrovascular disease | 9 (8.04) | 24 (21.05) | |
| Chronic liver disease | 5 (4.46) | 6 (5.26) | |
| Chronic renal disease | 1 (0.86) | 3 (2.63) | |
| Bronchiectasis | 1 (0.86) | 2 (1.75) | |
| Chronic obstructive pulmonary disease | 1 (1.86) | 9 (7.89) | |
| Antibiotic pre-treatment—no. (%) | 36 (32.14) | 37 (32.46) | 0.776 † |
| Physical examination | |||
| T max (℃) | 38.50 (37.88–39.20) | 39.00 (38.00–39.83) | 0.082 ††† |
| Respiratory frequency (times/min) | 20 (18–22) | 28 (22–32) | <0.0001 ††† |
| Heart rate | 90 ± 14 | 102 ± 18 | <0.0001 †† |
| Laboratory results | |||
| WBC (×109/L) | 7.53 (5.45–11.56) | 11.20 (7.30–16.40) | <0.0001 ††† |
| NEU (%) | 73.95 (65.93–82.33) | 84.70 (78.40–90.70) | <0.0001 ††† |
| LYM (%) | 16.55 (10.13–25.13) | 8.00 (3.90–12.60) | <0.0001 ††† |
| NLR | 4.18 (2.62–8.33) | 10.54 (6.24–23.21) | <0.0001 ††† |
| CRP | 51.49 (6.72–139.50) | 105.00 (28.45–172.60) | 0.018 ††† |
| PCT | 0.11 (0.04–0.34) | 0.61 (0.24–5.00) | <0.0001 ††† |
| ESR | 33.00 (19.50–54.00) | 55.00 (38.00–81.00) | 0.0004 ††† |
| Chest X-ray | |||
| Bilateral lung infection—no. (%) | 29 (25.89) | 96 (84.21) | <0.0001 † |
| Pleural effusion—no. (%) | 4 (3.57) | 37 (32.46) | <0.0001 † |
| Detected pathogen—no. (%) | |||
| Bacteria a | 21 (18.75) | 36 (31.58) | |
| Virus b | 10 (8.93) | 21 (18.75) | |
| Atypical pathogen c | 11 (9.82) | 1 (0.88) | |
| Mixed pathogen | 7 (6.25) | 7 (6.14) | |
| Unknown | 63 (56.25) | 49 (42.98) | |
| CURB-65 | |||
| Score points | 0 (0–1) | 2 (1–2) | <0.0001 ††† |
| PSI | |||
| Score points | 62.00 (42.00–72.00) | 98.03 ± 37.61 | <0.0001 †† |
| 30-day mortality-no. (%) | 0 (0.00) | 19 (16.67) | <0.0001 † |
| AUC | 95% CI | Sensitivity | Specificity | Threshold | p-Value | |
|---|---|---|---|---|---|---|
| RELM-β | 0.794 | 0.736–0.845 | 75.44 | 70.54 | >416.04 | <0.0001 |
| WBC | 0.658 | 0.587–0.723 | 36.94 | 89.77 | >13.53 | <0.0001 |
| NEU % | 0.735 | 0.661–0.801 | 70.75 | 66.67 | >79.60 | <0.0001 |
| LYM % | 0.756 | 0.683–0.819 | 67.62 | 75.00 | ≤10.3 | <0.0001 |
| NLR | 0.761 | 0.688–0.824 | 59.62 | 83.33 | >8.78 | <0.0001 |
| CRP | 0.622 | 0.534–0.705 | 65.43 | 60.78 | >66.72 | 0.0180 |
| PCT | 0.750 | 0.663–0.824 | 69.88 | 76.32 | >0.31 | <0.0001 |
| CURB-65 | 0.771 | 0.706–0.828 | 77.27 | 65.88 | >0 | <0.0001 |
| PSI | 0.791 | 0.727–0.845 | 63.06 | 90.80 | >86 | <0.0001 |
| proADM | 0.720 | 0.641–0.791 | 91.30 | 58.23 | >1.84 | <0.0001 |
| RELM-β + CURB-65 | 0.860 | 0.803–0.906 | 84.55 | 71.76 | -- | <0.0001 |
| RELM-β + PSI | 0.861 | 0.805–0.906 | 81.08 | 81.61 | -- | <0.0001 |
| AUC | 95% CI | Sensitivity | Specificity | Threshold | p-Value | |
|---|---|---|---|---|---|---|
| RELM-β | 0.777 | 0.717–0.829 | 57.89 | 87.44 | >1006.14 | <0.0001 |
| WBC | 0.737 | 0.670–0.797 | 57.89 | 85.56 | >15.21 | 0.0009 |
| NEU % | 0.606 | 0.527–0.681 | 86.67 | 49.01 | >80.60 | 0.1552 |
| LYM % | 0.693 | 0.616–0.762 | 60.00 | 79.33 | ≤5.30 | 0.0190 |
| NLR | 0.698 | 0.621–0.767 | 60.00 | 81.21 | >16.75 | 0.0170 |
| CRP | 0.515 | 0.426–0.602 | 41.67 | 80.00 | ≤12.80 | 0.8706 |
| PCT | 0.830 | 0.751–0.892 | 81.82 | 72.73 | >1.33 | <0.0001 |
| CURB-65 | 0.764 | 0.698–0.822 | 73.68 | 71.02 | >1 | <0.0001 |
| PSI | 0.820 | 0.759–0.871 | 89.47 | 75.98 | >106 | <0.0001 |
| proADM | 0.723 | 0.644–0.794 | 92.86 | 52.99 | >4.16 | 0.0001 |
| RELM-β + CURB-65 | 0.844 | 0.786–0.892 | 89.47 | 71.02 | -- | <0.0001 |
| RELM-β + PSI | 0.871 | 0.816–0.915 | 89.47 | 73.18 | -- | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Luo, Q.; Shang, Y.; He, X.; Xu, Y.; Gao, Z. Predictive and Prognostic Utility of the Serum Level of Resistin-Like Molecule Beta for Risk Stratification in Patients with Community-Acquired Pneumonia. Pathogens 2021, 10, 122. https://doi.org/10.3390/pathogens10020122
Chen L, Luo Q, Shang Y, He X, Xu Y, Gao Z. Predictive and Prognostic Utility of the Serum Level of Resistin-Like Molecule Beta for Risk Stratification in Patients with Community-Acquired Pneumonia. Pathogens. 2021; 10(2):122. https://doi.org/10.3390/pathogens10020122
Chicago/Turabian StyleChen, Li, Qiongzhen Luo, Ying Shang, Xinwei He, Yu Xu, and Zhancheng Gao. 2021. "Predictive and Prognostic Utility of the Serum Level of Resistin-Like Molecule Beta for Risk Stratification in Patients with Community-Acquired Pneumonia" Pathogens 10, no. 2: 122. https://doi.org/10.3390/pathogens10020122
APA StyleChen, L., Luo, Q., Shang, Y., He, X., Xu, Y., & Gao, Z. (2021). Predictive and Prognostic Utility of the Serum Level of Resistin-Like Molecule Beta for Risk Stratification in Patients with Community-Acquired Pneumonia. Pathogens, 10(2), 122. https://doi.org/10.3390/pathogens10020122





