The Role of TLR2 and TLR4 in Recognition and Uptake of the Apicomplexan Parasite Eimeria bovis and Their Effects on NET Formation
Abstract
:1. Introduction
2. Results
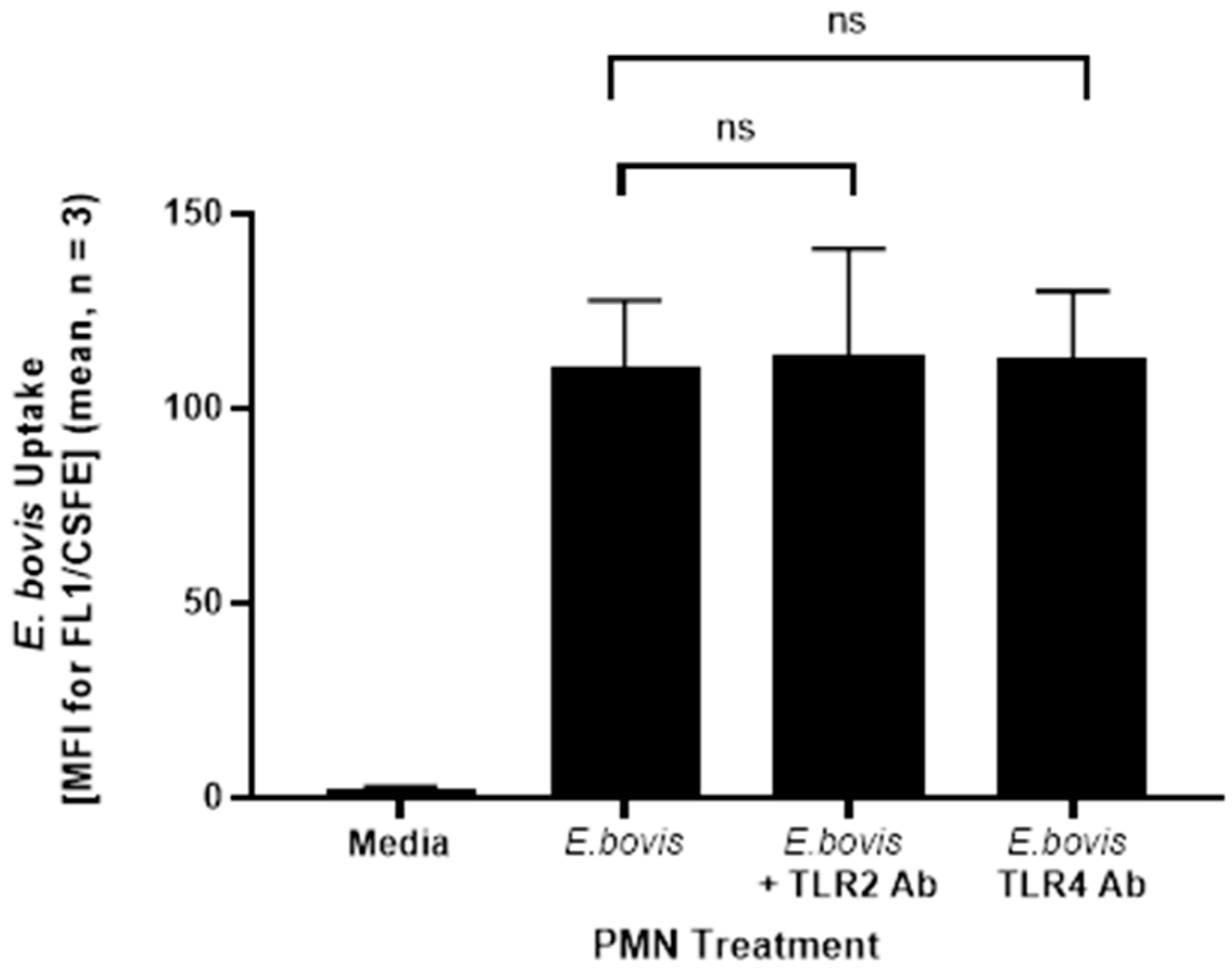
2.1. Addition of TLR2/4 Antibodies Does Not Seem to Inhibit Phagocytosis of E. bovis by PMN, but Seems to Stabilize Their Surface Expression
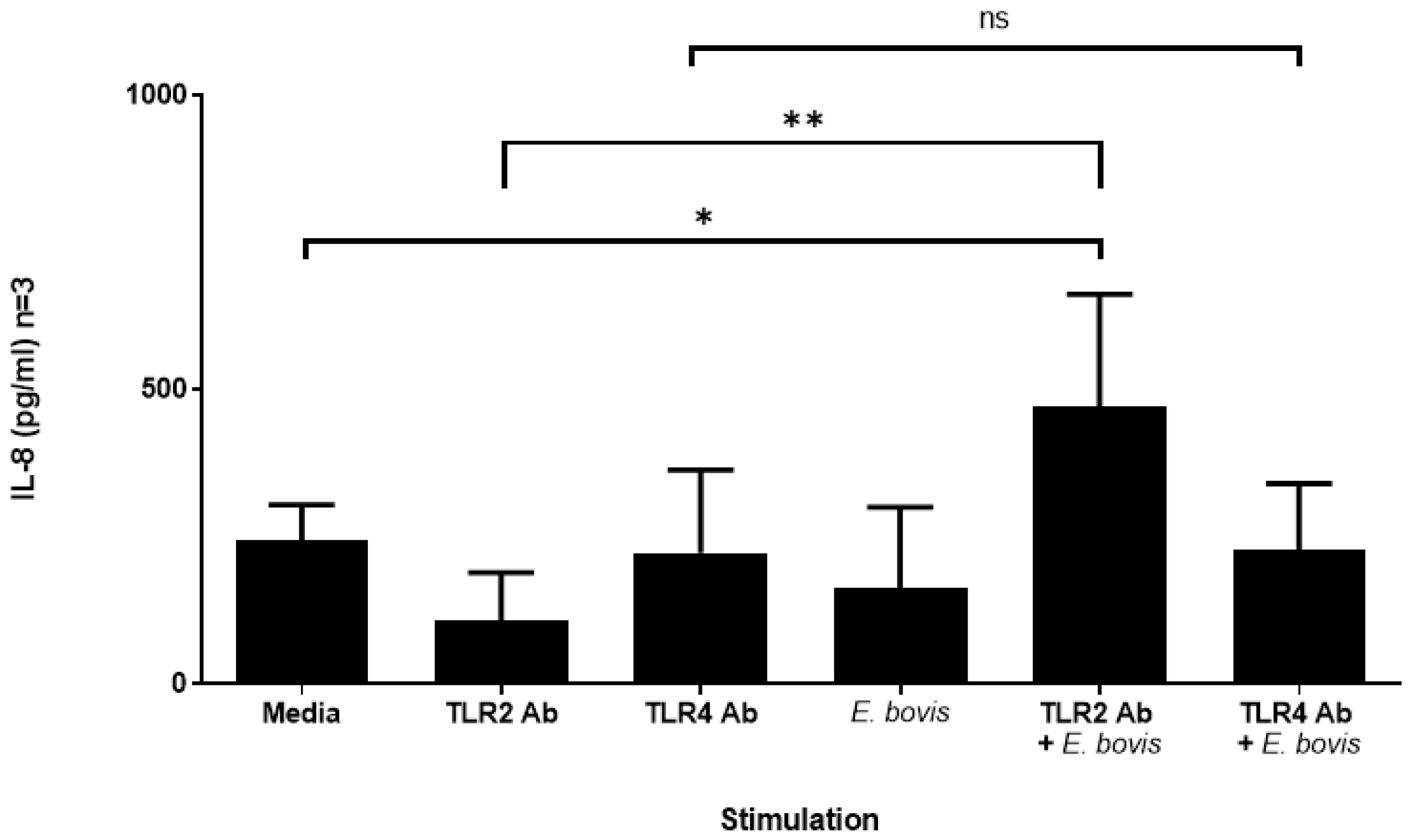
2.2. Exposure of Bovine PMN to E. bovis Increases IL-8 Secretion in the Presence of TLR2 Antibodies
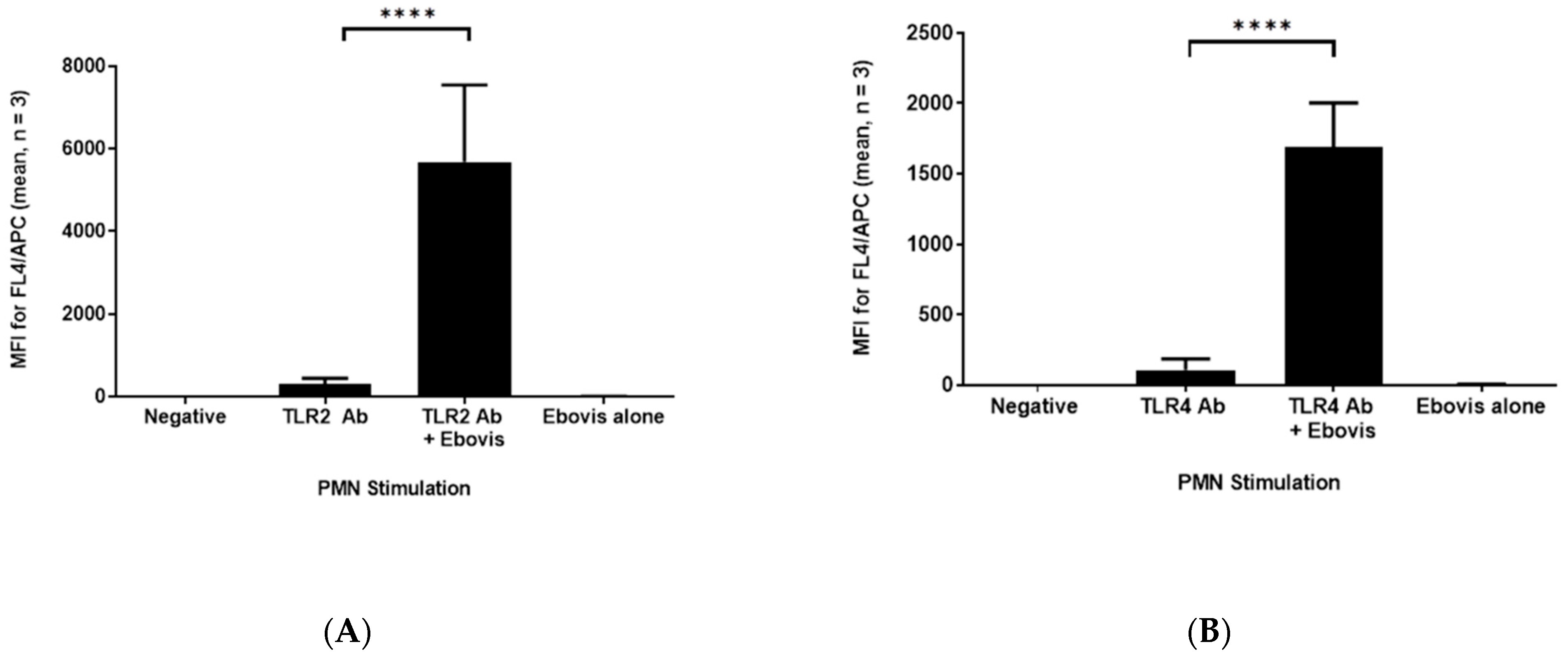
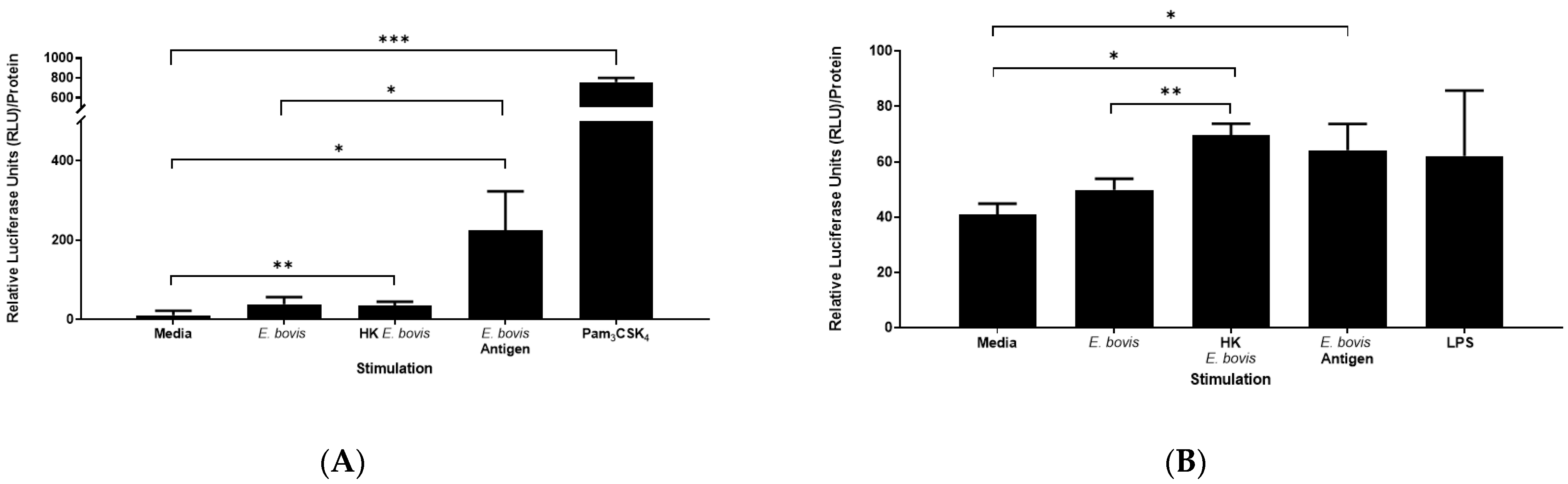
2.3. Induction of TLR2 and TLR4 Activation by E. bovis Sporozoites
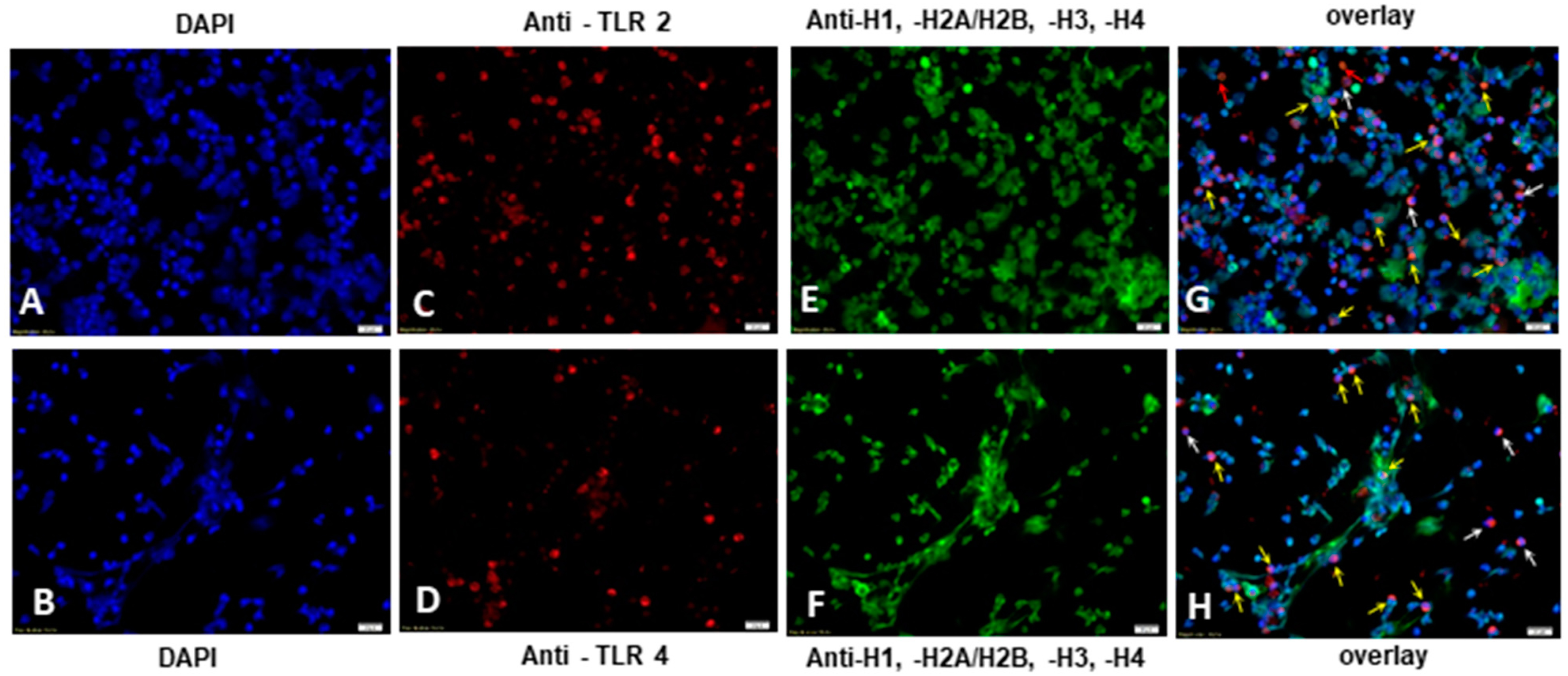
2.4. E. bovis-Induced TLR2 and TLR4 Activation Resulted in NETosis of Exposed Bovine PMN
3. Discussion
4. Materials and Methods
4.1. Parasites
4.2. Isolation of Bovine PMN
4.3. Blocking of TLRs and Phagocytosis Assay
4.4. IL-8 ELISA
4.5. TLR Stimulation Assay
4.6. Induction of NETosis and TLR2 Expression Via Fluorescence Microscopy Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Antibody | Supplier | Details | Isotype |
|---|---|---|---|
| Alexa Fluor 647 conjugated anti-human TLR4 | Novus | NBP2-24773 (clone 76B357.1) | IgG2a |
| Alexa Fluor 647 conjugated anti-bovine TLR2 | Bio-Rad | HCA152A647 (clone AbD12538) | HuCal Fab |
| Mouse anti-sheep IL-8 | Bio-Rad | MCA 1660 (clone 8M6) | IgG2a |
| Rabbit anti-sheep IL-8 | Bio-Rad | AHP425 (polyclonal) | Polyclonal IgG |
| Goat anti-rabbit HRP | DAKO | P0448 | - |
| Mouse anti-histone | Merck | MAB3422 (clone H11-4) | IgG1 |
| Alexa Fluor 488 goat anti-mouse IgG | Life Technologies | Recombinant polyclonal | IgG |
References
- Daugschies, A.; Najdrowski, M. Eimeriosis in Cattle: Current Understanding. J. Veter-Med. Ser. B 2005, 52, 417–427. [Google Scholar] [CrossRef] [PubMed]
- López-Osorio, S.; Silva, L.; Taubert, A.; Chaparro-Gutiérrez, J.J.; Hermosilla, C.R. Concomitant in vitro development of Eimeria zuernii- and Eimeria bovis-macromeronts in primary host endothelial cells. Parasitol. Int. 2018, 67, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla, C.; Bürger, H.-J.; Zahner, H. T cell responses in calves to a primary Eimeria bovis infection: Phenotypical and functional changes. Vet. Parasitol. 1999, 84, 1–2. [Google Scholar] [CrossRef]
- Suhwold, A.; Hermosilla, C.; Seeger, T.; Zahner, H.; Taubert, A. T cell reactions of Eimeria bovis primary and challenge-infected calves. Parasitol. Res. 2010, 106, 595–605. [Google Scholar] [CrossRef]
- Taubert, A.; Wimmers, K.; Ponsuksili, S.; Jimenez, C.A.; Zahner, H.; Hermosilla, C. Microarray-based transcriptional profiling of Eimeria bovis-infected bovine endothelial host cells. Vet. Res. 2010, 41, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrendt, J.H.; Ruiz, A.; Zahner, H.; Taubert, A.; Hermosilla, C. Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria bovis. Vet. Immunol. Immunopathol. 2010, 133, 1–8. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; Huertas, S.J.M.; Conejeros, I.; Alarcón, P.; Hidalgo, M.A.; Burgos, R.A.; Hermosilla, C.; Taubert, A. Eimeria bovis-triggered neutrophil extracellular trap formation is CD11b-, ERK 1/2-, p38 MAP kinase- and SOCE-dependent. Vet. Res. 2015, 46, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, L.M.; Muñoz-Caro, T.; Burgos, R.A.; Hidalgo, M.A.; Taubert, A.; Hermosilla, C. Far beyond Phagocytosis: Phagocyte—Derived Ex tracellular Traps Act Efficiently against Protozoan Parasites In Vitro and In Vivo. Mediat. Inflamm. 2016, 5898074. [Google Scholar] [CrossRef] [Green Version]
- Hermosilla, C.; Stamm, I.; Taubert, A.; Lutz, K.; Zahner, H.; Menge, C. Fluorescent Eimeria bovis sporozoites and meront stages in vitro: A helpful tool to study parasite-host cell interactions. Parasitol. Res. 2008, 102, 777–786. [Google Scholar] [CrossRef]
- Munoz-Caro, T.; Machado Ribeiro da Silva, L.; Renteria-Solis, Z.; Taubert, A.; Hermosilla, C. Neutrophil extracellular traps in the intestinal mucosa of Eimeria-infected animals. Asian Pac. J. Trop Biomed. 2016, 6, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003, 5, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005, 23, 197–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Chavakis, T.; Bierhaus, A.; Al-Fakhri, N.; Schneider, D.; Witte, S.; Linn, T.; Nagashima, M.; Morser, J.; Arnold, B.; Preissner, K.T.; et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: A novel pathway for inflammatory cell recruitment. J. Exp. Med. 2003, 198, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Deppisch, R.; Denefleh, B.; Hug, F.; Andrassy, K.; Hansch, G.M. Expression patterns of the lipopolysaccharide receptor CD14, and the FCgamma receptors CD16 and CD64 on polymorphonuclear neutrophils: Data from patients with severe bacterial infections and lipopolysaccharide-exposed cells. Shock 2003, 19, 5–12. [Google Scholar] [CrossRef]
- Conejeros, I.; Gibson, A.J.; Werling, D.; Muñoz-Caro, T.; Hermosilla, C.; Taubert, A.; Burgos, R.A. Effect of the synthetic Toll-like receptor ligands LPS, Pam3CSK4, HKLM and FSL-1 in the function of bovine polymorphonuclear neutrophils. Dev. Comp. Immunol. 2015, 52, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Kubelkova, K.; Macela, A. Innate Immune Recognition: An Issue More Complex Than Expected. Front. Cell Infect. Microbiol. 2019, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef]
- Brinkmann, V.; Zychlinsky, A. Beneficial suicide: Why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007, 5, 577–582. [Google Scholar] [CrossRef]
- Hermosilla, C.; Caro, T.M.; Silva, L.M.; Ruiz, A.; Taubert, A. The intriguing host innate immune response: Novel anti-parasitic defence by neutrophil extracellular traps. Parasitology 2014, 141, 1489–1498. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; R, M.C.R.; Silva, L.; Magdowski, G.; Gärtner, U.; McNeilly, T.N.; Taubert, A.; Hermosilla, C. Leucocyte-derived extracellular trap formation significantly contributes to Haemonchus contortus larval entrapment. Parasit Vectors 2015, 26, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Caro, T.; Conejeros, I.; Zhou, E.; Pikhovych, A.; Gärtner, U.; Hermosilla, C.; Kulke, D.; Taubert, A. Dirofilaria immitis Microfilariae and Third-Stage Larvae Induce Canine NETosis Resulting in Different Types of Neutrophil Extracellular Traps. Front. Immunol. 2018, 8, 968. [Google Scholar] [CrossRef] [PubMed]
- Bainton, D.F.; Ullyot, J.L.; Farquhar, M.G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J. Exp. Med. 1971, 134, 907–934. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N.; Cowland, J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997, 89, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef]
- Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J. Cell Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef] [Green Version]
- Von Köckritz-Blickwede, M.; Nizet, V. Innate immunity turned inside-out: Antimicrobial defense by phagocyte extracellular traps. J. Mol. Med. 2009, 87, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Paape, M.J.; Bannerman, D.D.; Zhao, X.; Lee, J.W. The bovine neutrophil: Structure and function in blood and milk. Vet. Res. 2003, 34, 597–627. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.; Caro, T.M.; Gerstberger, R.; Vila-Viçosa, M.J.M.; Cortes, H.C.E.; Hermosilla, C.; Taubert, A. The apicomplexan parasite Eimeria arloingi induces caprine neutrophil extracellular traps. Parasitol. Res. 2014, 113, 2797–2807. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Hammond, M.E.; Lapointe, G.R.; Feucht, P.H.; Hilt, S.; Gallegos, C.A.; Gordon, C.A.; Giedlin, M.A.; Mullenbach, G.; Tekamp-Olson, P. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J. Immunol. 1995, 1, 1428–1433. [Google Scholar]
- Peixoto, R.; Silva, L.M.; López-Osório, S.; Zhou, E.; Gärtner, U.; Conejeros, I.; Taubert, A.; Hermosilla, C. Fasciola hepatica induces weak NETosis and low production of intra- and extracellular ROS in exposed bovine polymorphonuclear neutrophils. Dev. Comp. Immunol. 2021, 114. [Google Scholar] [CrossRef]
- Pichyangkul, S.; Yongvanitchit, K.; Kum-arb, U.; Hemmi, H.; Akira, S.; Krieg, A.M.; Heppner, D.G.; Stewart, V.A.; Hasegawa, H.; Looareesuwan, S.G.; et al. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J. Immunol. 2004, 172, 4926–4933. [Google Scholar] [CrossRef] [PubMed]
- Krishnegowda, G.; Hajjar, A.M.; Zhu, J.; Douglass, E.J.; Uematsu, S.; Akira, S.; Woods, A.S.; Gowda, D.C. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: Cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J. Biol. Chem. 2005, 280, 8606–8616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazzinelli, R.T.; Denkers, E.Y. Protozoan encounters with Toll-like receptor signalling pathways: Implications for host parasitism. Nat. Rev. Immunol. 2006, 6, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Reizis, B.; DeFranco, A.L. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity 2008, 15, 272–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct Target. Ther 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Parroche, P.; Lauw, F.N.; Goutagny, N.; Latz, E.; Monks, B.G.; Visintin, A.; Halmen, K.A.; Lamphier, M.; Olivier, M.; Bartholomeu, D.C.; et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc. Natl. Acad. Sci. USA 2007, 104, 1919–1924. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Krishnegowda, G.; Li, G.; Gowda, D.C. Proinflammatory responses by glycosylphosphatidylinositols (GPIs) of Plasmodium falciparum are mainly mediated through the recognition of TLR2/TLR1. Exp. Parasitol. 2011, 128, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debierre-Grockiego, F.; Campos, M.A.; Azzouz, N.; Schmidt, J.; Bieker, U.; Resende, M.G.; Mansur, D.S.; Weingart, R.; Schmidt, R.R.; Golenbock, D.T.; et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 2007, 179, 1129–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, W.A.; Souza Mdo, C.; Ramos-Martinez, E.; Nagpal, K.; Dutra, M.S.; Melo, M.B.; Bartholomeu, D.C.; Ghosh, S.; Golenbock, D.T.; Gazzinelli, R.T. Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe 2013, 13, 42–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koblansky, A.A.; Jankovic, D.; Oh, H.; Hieny, S.; Sungnak, W.; Mathur, R.; Hayden, M.S.; Akira, S.; Sher, A.; Ghosh, S. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity 2013, 38, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raetz, M.; Kibardin, A.; Sturge, C.R.; Pifer, R.; Li, H.; Burstein, E.; Ozato, K.; Larin, S.; Yarovinsky, F. Cooperation of TLR12 and TLR11 in the IRF8-Dependent IL-12 Response to Toxoplasma gondii Profilin. J. Immunol. 2013, 191, 4818–4827. [Google Scholar] [CrossRef] [Green Version]
- Sousa-Rocha, D.; Thomaz-Tobias, M.; Diniz, L.F.A.; Souza, P.S.S.; Pinge-Filho, P.; Toledo, K.A. Trypanosoma cruzi and Its Soluble Antigens Induce NET Release by Stimulating Toll-Like Receptors. PLoS ONE 2015, 10, e0139569. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Pandey, S.P.; Jha, M.K.; Chandel, H.S.; Saha, B. Leishmania expressed lipophosphoglycan interacts with Toll-like receptor (TLR)-2 to decrease TLR-9 expression and reduce anti-leishmanial responses. Clin. Exp. Immunol. 2013, 172, 403–409. [Google Scholar] [CrossRef]
- Hammond, D.; Bowman, G.; Davis, L.; Simms, B. The Endogenous Phase of the Life Cycle of Eimeria bovis. J. Parasitol. 1946, 32, 409–427. [Google Scholar] [CrossRef]
- Hermosilla, C.; Zahner, H.; Taubert, A. Eimeria bovis modulates adhesion molecule gene transcription in and PMN adhesion to infected bovine endothelial cells. Int. J. Parasitol. 2006, 36, 423–431. [Google Scholar] [CrossRef]
- Hermosilla, C.; Ruiz, A.; Taubert, A. Eimeria bovis: An update on parasite-host cell interactions. Int. J. Med. Microbiol. 2012, 302, 210–215. [Google Scholar] [CrossRef]
- Behrendt, J.H.; Hermosilla, C.; Hardt, M.; Failing, K.; Zahner, H.; Taubert, A. PMN-mediated immune reactions against Eimeria bovis. Vet. Parasitol. 2008, 151, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.; Piercy, J.; Coffey, T.J. Expression of TOLL-like receptors (TLR) by bovine antigen-presenting cells—Potential role in pathogen discrimination? Veter-Immunol. Immunopathol. 2006, 112, 2–11. [Google Scholar] [CrossRef]
- Hodgson, P.D.; Aich, P.; Manuja, A.; Hokamp, K.; Roche, F.M.; Brinkman, F.S.L.; Potter, A.; Babiuk, L.A.; Griebel, P. Effect of Stress on Viral–Bacterial Synergy in Bovine Respiratory Disease: Novel Mechanisms to Regulate Inflammation. Comp. Funct. Genom. 2005, 6, 244–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Schepper, S.; De Ketelaere, A.; Bannerman, D.D.; Paape, M.J.; Peelman, L.; Burvenich, C. The toll-like receptor-4 (TLR-4) pathway and its possible role in the pathogenesis of Escherichia coli mastitis in dairy cattle. Vet. Res. 2008, 39, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conejeros, I.; Patterson, R.; Burgos, R.; Hermosilla, C.; Werling, D. Induction of reactive oxygen species in bovine neutrophils is CD11b, but not dectin-1-dependent. Veter-Immunol. Immunopathol. 2011, 139, 308–312. [Google Scholar] [CrossRef]
- Hermosilla, C.; Barbisch, B.; Heise, A.; Kowalik, S.; Zahner, H. Development of Eimeria bovis in vitro: Suitability of several bovine, human and porcine endothelial cell lines, bovine fetal gastrointestinal, Madin-Darby bovine kidney (MDBK) and African green monkey kidney (VERO) cells. Parasitol. Res. 2001, 88, 301–307. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cronin, J.; Hodges, R.; Pedersen, S.; Sheldon, I.M. Enzyme Linked Immunosorbent Assay for Quantification of Bovine Interleukin-8 to Study Infection and Immunity in the Female Genital Tract. Am. J. Reprod. Immunol. 2015, 73, 372–382. [Google Scholar] [CrossRef]
- Lizundia, R.; Sauter, K.-S.; Taylor, G.; Werling, D. Host species-specific usage of the TLR4-LPS receptor complex. Innate Immun. 2008, 14, 223–231. [Google Scholar] [CrossRef]
- Patterson, N.J.; Gã¼Nther, J.; Gibson, A.J.; Eofford, V.; Coffey, T.J.; Esplitter, G.; Emonk, I.; Eseyfert, H.-M.; Werling, D. Two TIR-like domain containing proteins in a newly emerging zoonotic Staphylococcus aureus strain sequence type 398 are potential virulence factors by impacting on the host innate immune response. Front. Microbiol. 2014, 5, 662. [Google Scholar] [CrossRef]
- Willcocks, S.; Offord, V.; Seyfert, H.-M.; Coffey, T.J.; Werling, D. Species-specific PAMP recognition by TLR2 and evidence for species-restricted interaction with Dectin-1. J. Leukoc. Biol. 2013, 94, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shi, X.; Bauer, I.; Günther, J.; Seyfert, H.-M. Lingual antimicrobial peptide and IL-8 expression are oppositely regulated by the antagonistic effects of NF-κB p65 and C/EBPβ in mammary epithelial cells. Mol. Immunol. 2011, 48, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Gantner, B. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect. 2004, 6, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.R.; Ma, A.C.; A Tavener, S.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villagra-Blanco, R.; Silva, L.; Conejeros, I.; Taubert, A.; Hermosilla, C. Pinniped- and Cetacean-Derived ETosis Contributes to Combating Emerging Apicomplexan Parasites (Toxoplasma gondii, Neospora caninum) Circulating in Marine Environments. Biology 2019, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Godínez, C.; Carrero, J.C. The state of art of neutrophil extracellular traps in protozoan and helminthic infections. Biosci. Rep. 2019, 39, BSR20180916. [Google Scholar] [CrossRef] [Green Version]
- Schito, M.L.; Barta, J.R. Nonspecific immune responses and mechanisms of resistance to Eimeria papillata infections in mice. Infect. Immun. 1997, 65, 3165–3170. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, M.J.; Radic, M. Neutrophil Extracellular Traps: Double-Edged Swords of Innate Immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [Green Version]
- Zambrano, F.; Schulz, M.; Pilatz, A.; Wagenlehner, F.; Schuppe, H.-C.; Conejeros, I.; Uribe, P.; Taubert, A.; Sánchez, R.; Hermosilla, C. Increase of leucocyte-derived extracellular traps (ETs) in semen samples from human acute epididymitis patients—A pilot study. J. Assist. Reprod. Genet. 2020, 37, 2223–2231. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Keshari, R.S.; Jyoti, A.; Dubey, M.; Kothari, N.; Kohli, M.; Bogra, J.; Barthwal, M.K.; Dikshit, M. Cytokines Induced Neutrophil Extracellular Traps Formation: Implication for the Inflammatory Disease Condition. PLoS ONE 2012, 7, e48111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regli, I.B.; Passelli, K.; Martínez-Salazar, B.; Amore, J.; Hurrell, B.P.; Müller, A.J.; Tacchini-Cottier, F. TLR7 Sensing by Neutrophils Is Critical for the Control of Cutaneous Leishmaniasis. Cell Rep. 2020, 31, 107746. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.S.A.; Lin, C.; Ball, C.J.; King, M.R.; Duhamel, G.E.; Denkers, E.Y. Toxoplasma gondii Triggers Release of Human and Mouse Neutrophil Extracellular Traps. Infect. Immun. 2011, 80, 768–777. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Caro, T.; Gibson, A.J.; Conejeros, I.; Werling, D.; Taubert, A.; Hermosilla, C. The Role of TLR2 and TLR4 in Recognition and Uptake of the Apicomplexan Parasite Eimeria bovis and Their Effects on NET Formation. Pathogens 2021, 10, 118. https://doi.org/10.3390/pathogens10020118
Muñoz-Caro T, Gibson AJ, Conejeros I, Werling D, Taubert A, Hermosilla C. The Role of TLR2 and TLR4 in Recognition and Uptake of the Apicomplexan Parasite Eimeria bovis and Their Effects on NET Formation. Pathogens. 2021; 10(2):118. https://doi.org/10.3390/pathogens10020118
Chicago/Turabian StyleMuñoz-Caro, Tamara, Amanda J. Gibson, Iván Conejeros, Dirk Werling, Anja Taubert, and Carlos Hermosilla. 2021. "The Role of TLR2 and TLR4 in Recognition and Uptake of the Apicomplexan Parasite Eimeria bovis and Their Effects on NET Formation" Pathogens 10, no. 2: 118. https://doi.org/10.3390/pathogens10020118






