Seroprevalence and Risk Factors of Crimean-Congo Hemorrhagic Fever in Cattle of Smallholder Farmers in Central Malawi
Abstract
:1. Introduction
2. Results
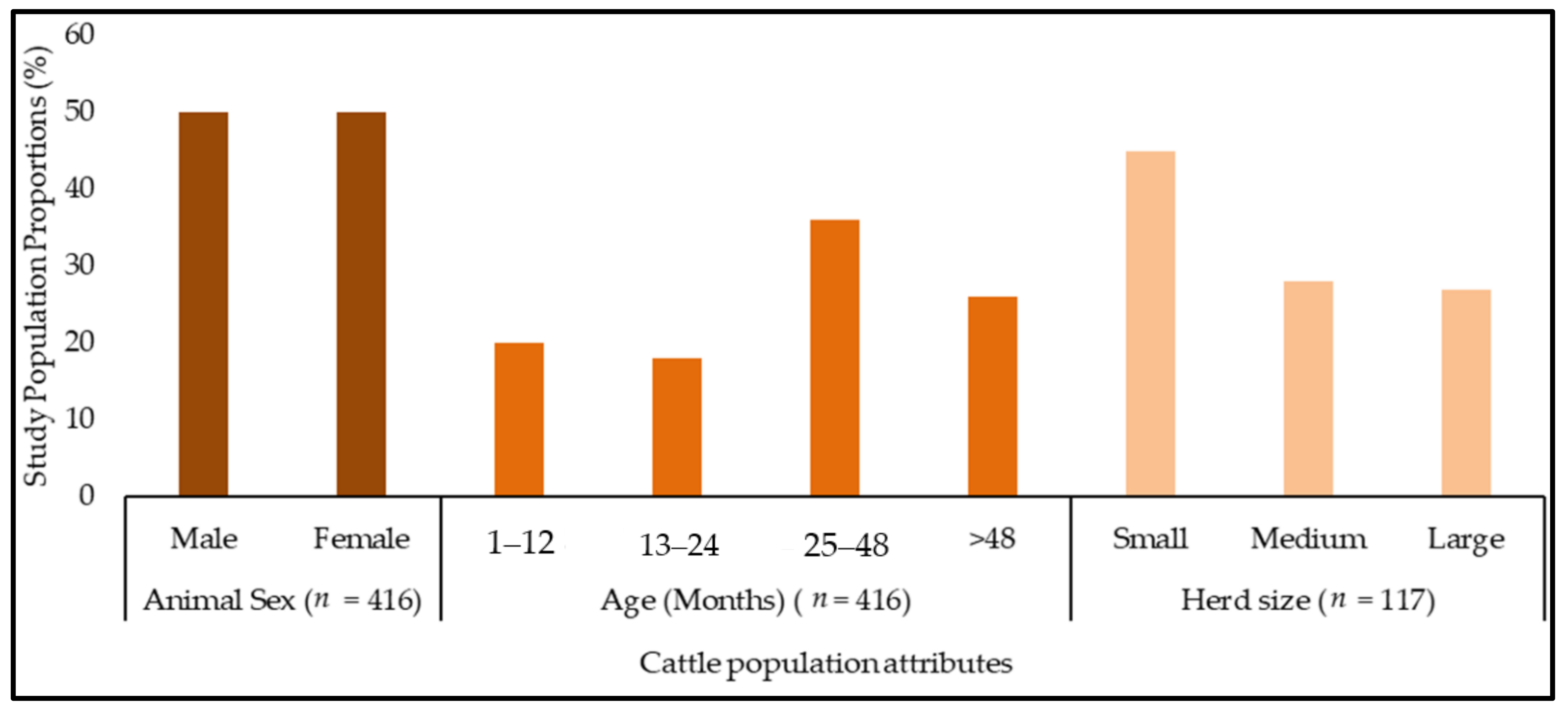
2.1. Description of the Study Population
2.2. Tick Species Identified on Cattle
2.3. Seroprevalence of CCHFV Infection in Cattle
2.4. Risk Factors Associated with Detection of CCHFV-Specific Antibodies in Cattle
3. Discussion
4. Materials and Methods
4.1. Study Sites
4.2. Study and Sampling Design
4.3. Questionnaire Administration
4.4. Cattle Attributes
4.5. Sample (Sera and Tick) Collection from Cattle, Storage, and Transportation
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoogstraal, H. Changing Patterns of Tick-borne Diseases in Modern Society. Annu. Rev. Entomol. 1981, 26, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Maes, P.; Alkhovsky, S.V.; Bào, Y.; Beer, M.; Birkhead, M.; Briese, T.; Buchmeier, M.J.; Calisher, C.H.; Charrel, R.N.; Choi, I.R.; et al. Taxonomy of the order Bunyavirales: Second update 2018. Arch. Virol. 2019, 164, 927–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuehnert, P.A.; Stefan, C.P.; Badger, C.V.; Ricks, K.M. Crimean-Congo Hemorrhagic Fever Virus (CCHFV): A Silent but Widespread Threat. Curr. Trop. Med. Rep. 2021, 8, 141–147. [Google Scholar] [CrossRef]
- Spengler, J.R.; Bergeron, E.; Rollin, P.E. Seroepidemiological Studies of Crimean-Congo Hemorrhagic Fever Virus in Domestic and Wild Animals. PLoS Negl. Trop. Dis. 2016, 10, e0004210. [Google Scholar] [CrossRef] [Green Version]
- Mourya, D.T.; Yadav, P.D.; Shete, A.M.; Gurav, Y.K.; Raut, C.G.; Jadi, R.S.; Pawar, S.D.; Nichol, S.T.; Mishra, A.C. Detection, Isolation and Confirmation of Crimean-Congo Hemorrhagic Fever Virus in Human, Ticks and Animals in Ahmadabad, India, 2010–2011. PLoS Negl. Trop. Dis. 2012, 6, e1653. [Google Scholar] [CrossRef]
- Müller, M.A.; Devignot, S.; Lattwein, E.; Corman, M.V.; Maganga, G.D.; Gloza-Rausch, F.; Binger, T.; Vallo, P.; Emmerich, P.; Cottontail, V.M.; et al. Evidence for Widespread Infection of African Bats with Crimean-Congo Hemorrhagic Fever-like Viruses. Nat. Publ. Group 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeller, H.G.; Cornet, J.P.; Camicas, J.L. Crimean-Congo Haemorrhagic Fever Virus Infection in Birds: Field Investigations in Senegal. Res. Virol. 1994, 145, 105–109. [Google Scholar] [CrossRef]
- Verma, R.; Khanna, P.; Prinja, S.; Rajput, M. Crimean-Congo hemorrhagic fever: An outbreak in India. Aust. Med. J. 2011, 4, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Balinandi, S.; Patel, K.; Ojwang, J.; Kyondo, J.; Mulei, S.; Tumusiime, A.; Lubwama, B.; Nyakarahuka, L.; Klena, J.D.; Lutwama, J.; et al. Investigation of an isolated case of human Crimean-Congo hemorrhagic fever in Central Uganda. Int. J. Infect. Dis. 2015, 68, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Abbas, T.; Xu, Z.; Younus, M.; Qayyum, A.; Riaz, M.T. Seasonality in hospital admissions of Crimean-Congo hemorrhagic fever and its dependence on ambient temperature-empirical evidence from Pakistan. Int. J. Biometeorol. 2017, 61, 1893–1897. [Google Scholar] [CrossRef]
- Hoogstraal, H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J. Med. Entomol. 1979, 15, 307–417. [Google Scholar] [CrossRef] [PubMed]
- Hawman, D.W.; Feldmann, H. Recent advances in understanding Crimean-Congo hemorrhagic fever virus. F1000 Fac. Rev. 2018, 7, 1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Abri, S.S.; Abaidani, I.A.; Fazlalipour, M.; Mostafavi, E.; Leblebicioglu, H.; Pshenichnaya, N.; Memish, Z.A.; Hewson, R.; Petersen, E.; Mala, P.; et al. Current status of Crimean-Congo hemorrhagic fever in the World Health Organization Eastern Mediterranean Region: Issues, challenges, and future directions. Int. J. Infect Dis. 2017, 58, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Goswami, T.K.; Singh, D.K.; Saminathan, M.; Verma, A.K.; Dhama, K. An Emerging Threat of Crimean Congo Hemorrhagic Fever: Call for Preparedness. Adv. Anim. Vet. Sci. 2014, 2, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Mertens, M.; Vatansever, Z.; Mrenoshki, S.; Krstevski, K.; Stefanovska, J.; Djadjovski, I.; Cvetkovikj, I.; Farkas, R.; Schuster, I.; Donnet, F.; et al. Circulation of Crimean-Congo Hemorrhagic Fever Virus in the Former Yugoslav Republic of Macedonia Revealed by Screening of Cattle Sera Using a Novel Enzyme-linked Immunosorbent Assay. PLoS Negl. Trop. Dis. 2015, 9, e0003519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fajs, L.; Jakupi, X.; Ahmeti, S.; Humolli, I.; Dedushaj, I.; Avsic-Zupanc, T. Molecular Epidemiology of Crimean-Congo Hemorrhagic Fever Virus in Kosovo. PLoS Negl. Trop. Dis. 2014, 8, e2647. [Google Scholar] [CrossRef] [PubMed]
- WHO. Introduction to Crimean-Congo Hemorrhagic Fever. 2018. Available online: https://www.who.int/publications/i/item/introduction-to-crimean-congo-haemorrhaigc-feveron (accessed on 23 November 2020).
- Sorvillo, T.E.; Rodriguez, S.E.; Hudson, P.; Carey, M.; Rodriguez, L.L.; Spiropoulou, C.F.; Bird, B.H.; Spengler, J.R.; Bente, D.A. Towards a Sustainable One Health Approach to Crimean-Congo Hemorrhagic Fever Prevention: Focus Areas and Gaps in Knowledge. Trop. Med. Infect. Dis. 2020, 5, 113. [Google Scholar] [CrossRef]
- Bartolini, B.; Gruber, C.E.M.; Koopmans, M.; Avšič, T.; Bino, S.; Christova, I.; Grunow, R.; Hewson, R.; Korukluoglu, G.; Lemos, C.M.; et al. Laboratory management of Crimean-Congo haemorrhagic fever virus infections: Perspectives from two European networks. Eurosurveillance. 2019, 24, 1800093. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, E.; Zivcec, M.; Chakrabarti, A.K.; Nichol, S.T.; Albariño, C.G.; Spiropoulou, C.F. Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin. PLoS Pathog. 2015, 11, e1004879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escadafal, C.; Ölschläger, S.; Avšič-Županc, T.; Papa, A.; Vanhomwegen, J.; Wolf, R.; Mirazimi, A.; Teichmann, A.; Donoso-Mantke, O.; Niedrig, M. First International External Quality Assessment of Molecular Detection of Crimean-Congo Hemorrhagic Fever Virus. PLoS Negl. Trop. Dis. 2012, 6, e1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munibullah; Yousaf, A.; Shah, M.A.; Habibullah; Sadia, H.; Sohoo., M.R. Crimean—Congo Hemorrhagic Fever a Threat to Public Health. J. Bacteriol. Infec. Dis. 2018, 2, 1–7. Available online: https://www.alliedacademies.org/articles/crimeancongo-hemorrhagic-fever-a-threat-to-public-health-10191.html (accessed on 23 June 2020).
- Nasirian, H. Crimean-Congo hemorrhagic fever (CCHF) seroprevalence: A systematic review and meta-analysis. Acta. Trop. 2019, 196, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Balinandi, S.; von Brömssen, C.; Tumusiime, A.; Kyondo, J.; Kwon, H.; Monteil, V.M.; Ali Mirazimi, A.; Lutwama, J.; Mugisha, L.; Malmberg, M. Serological and molecular study of Crimean-Congo Hemorrhagic Fever Virus in cattle from selected districts in Uganda. J. Virol. Methods. 2021, 290, 114075. [Google Scholar] [CrossRef] [PubMed]
- Vescio, F.M.; Busani, L.; Mughini-Gras, L.; Khoury, C.; Avellis, L.; Taseva, E.; Rezza, G.; Christova, I. Environmental correlates of Crimean-Congo hemorrhagic fever incidence in Bulgaria. BMC Public Health 2012, 12, 1116. [Google Scholar] [CrossRef] [Green Version]
- Palomar, A.M.; Portillo, A.; Santibáñez, P.; Mazuelas, D.; Arizaga, J.; Crespo, A.; Gutiérrez, O.; Cuadrado, J.F.; Oteo, J.A. Crimean-Congo Hemorrhagic Fever Virus in Ticks from Migratory Birds, Morocco. Emerg. Infect. Dis. 2013, 19, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Wahid, B.; Altaf, S.; Naeem, N.; Ilyas, N.; Idrees, M. Scoping Review of Crimean-Congo Hemorrhagic Fever (CCHF) Literature and Implications of Future Research. J. Coll. Physicians Surg. Pak. 2019, 29, 563–573. [Google Scholar] [CrossRef]
- Rehman, K.; Bettani, M.A.K.; Veletzky, L.; Afridi, S.; Ramharter, M. Outbreak of Crimean-Congo hemorrhagic fever with atypical clinical presentation in the Karak District of Khyber Pakhtunkhwa, Pakistan. Infect. Dis. Poverty 2018, 7, 116. [Google Scholar] [CrossRef] [Green Version]
- Richards, G.A.; Weyer, J.; Blumberg, L. Viral Haemorrhagic Fevers in South Africa. S. Afr. Med. J. 2015, 105, 748–751. [Google Scholar] [CrossRef] [Green Version]
- Jauréguiberry, S.; Tattevin, P.; Tarantola, A.; Legay, F.; Tall, A.; Nabeth, P.; Zeller, H.; Michelet, C. Imported Crimean-Congo hemorrhagic Fever. J. Clin. Microbiol. 2005, 43, 4905–4907. [Google Scholar] [CrossRef] [Green Version]
- Leblebicioglu, H.; Ozaras, R.; Fletcher, T.E.; Beeching, N.J.; ESCMID Study Group for Infections in Travelers and Migrants (ESGITM). Crimean-Congo hemorrhagic fever in travelers: A systematic review. Travel Med. Infect. Dis. 2016, 14, 73–80. [Google Scholar] [CrossRef]
- Temur, A.I.; Jens, H.K.; David, B.P.; Dmitry, A.; Maryam, K. Epidemiology of Crimean-Congo Hemorrhagic Fever (CCHF) in Africa—Underestimated for Decades. Am. J. Trop. Med. Hyg. 2021, 104, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Muianga, A.F.; Watson, R.; Varghese, A.; Chongo, I.S.; Ali, S.; Monteiro, V.; Inalda, F.; Chelene, I.; António, V.; Hewson, R.; et al. First serological evidence of Crimean-Congo haemorrhagic fever in febrile patients in Mozambique. Int. J. Infect. Dis. 2017, 62, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, M.; Simuunza, M.; Saasa, N.; Dautu, G.; Mori-Kajihara, A.; Qiu, Y.; Nakao, R.; Eto, Y.; Furumoto, H.; Bernard, M.; et al. Serologic and molecular evidence for circulation of Crimean-Congo hemorrhagic fever virus in ticks and cattle in Zambia. PLoS Negl. Trop. Dis. 2021, 15, e0009452. [Google Scholar] [CrossRef]
- Mares, R.G. Animal health and production in Malawi past, present and future. Trop. Anim. Health Prod. 1973, 5, 272–277. [Google Scholar] [CrossRef] [PubMed]
- National Statistical Office of Malawi. Malawi Population and Housing Main Report 2018 (May). Available online: https://malawi.unfpa.org/sites/default/files/resourcepdf/2018 (accessed on 2 January 2021).
- Ministry of Agriculture, Department of Animal Health and Livestock Development. Policy Document on Livestock in Malawi. Ministry of Agriculture, Department of Animal Health and Livestock Development: Lilongwe, Malawi, 2006. Available online: http://extwprlegs1.fao.org/docs/pdf/mlw169552.pdf (accessed on 2 January 2021).
- Li, G.; Messina, J.P.; Peter, B.G.; Snapp, S.S. Mapping Land Suitability for Agriculture in Malawi. Land Degrad. Dev. 2017, 28, 2001–2016. [Google Scholar] [CrossRef]
- Pigott, D.M.; Deshpande, A.; Letourneau, I.; Morozoff, C.; Reiner Jr, R.C.; Kraemer, M.U.G.; Brent, S.E.; Bogoch, I.I.; Khan, K.; Biehl, M.H.; et al. Local, National, and Regional Viral Haemorrhagic Fever Pandemic Potential in Africa: A Multistage Analysis. Lancet 2017, 390, 2662–2672. [Google Scholar] [CrossRef] [Green Version]
- Berggren, S.A. Cattle ticks in Malawi. Vet. Parasitol. 1978, 4, 289–297. [Google Scholar] [CrossRef]
- Schwartz, J.; Yen, M.Y. Toward a Collaborative Model of Pandemic Preparedness and Response: Taiwan’s Changing Approach to Pandemics. J. Microbiol. Immunol. Infect. 2017, 50, 125–132. [Google Scholar] [CrossRef]
- Nasirian, H. New Aspects about Crimean-Congo Hemorrhagic Fever (CCHF) Cases and Associated Fatality Trends: A Global Systematic Review and Meta-Analysis. Comp. Immunol. Microbiol. Infect. Dis. 2020, 69, e101429. [Google Scholar] [CrossRef]
- Swanepoel, R.; Shepherd, A.J.; Leman, P.A.; Shepherd, S.P.; McGillivray, G.M.; Erasmus, M.J.; . Searle, L.A.; Gill, D.E. Epidemiologic and clinical features of Crimean-Congo hemorrhagic fever in southern Africa. Am. J. Trop. Med. Hyg. 1987, 36, 120–132. [Google Scholar] [CrossRef]
- Chauhan, R.P.; Zelalem, G.D.; Noreddin, A.; El Zowalaty, M.E. Systematic Review of Important Viral Diseases in Africa in Light of the ‘One Health’ Concept. Pathogens 2020, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Messina, J.P.; Pigott, D.M.; Golding, N.; Duda, K.A.; Brownstein, J.S.; Weissa, D.J.; Gibsona, H.; Robinsond, T.P.; Gilberte, M.; Winta, G.R.W.; et al. The global distribution of Crimean-Congo hemorrhagic fever. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Joseph Wu, T.S.; Kagoli, M.; Kaasbøll, J.J.; Bjune, G.A. Integrated Disease Surveillance and Response (IDSR) in Malawi: Implementation gaps and challenges for timely alert. PLoS ONE 2018, 13, e0200858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himeidan, Y.E.; Kweka, E.J.; Mahgoub, M.M.; El Amin, A.; El Rayah, A.; Ouma, J.O. Recent Outbreaks of Rift Valley Fever in East Africa and the Middle East. Front. Public Health 2014, 2, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Rojas, J.L.G.; Schmidt, C.G.; Michel, V.; et al. Rift Valley Fever—Epidemiological Update and Risk of Introduction into Europe. EFSA J. 2020, 18, e06041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, A.; Lutwama, J.; Sserugga, J.; Gély, M.; Pittiglio, C.; Pinto, J.; Chevalier, V. Development and Assessment of a Geographic Knowledge-Based Model for Mapping Suitable Areas for Rift Valley Fever Transmission in Eastern Africa. PLoS Negl. Trop. Dis. 2016, 10, e0004999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakizimana, J.N.; Yona, C.; Kamana, O.; Nauwynck, H.; Misinzo, G. African Swine fever Virus Circulation between Tanzania and Neighboring Countries: A Systematic Review and Meta-Analysis. Viruses 2021, 13, 306. [Google Scholar] [CrossRef]
- Simulundu, E.; Chambaro., H.M.; Sinkala, Y.; Kajihara, M.; Ogawa, H.; Mori, A.; Ndebe, J.; Dautu, G.; Mataa, L.; Lubaba, C.H.; et al. Co-circulation of multiple genotypes of African swine fever viruses among domestic pigs in Zambia (2013–2015). Transbound. Emerg. Dis. 2018, 65, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Sas, M.A.; Mertens, M.; Kadiat, J.G.; Schuster, I.; Pongombo, C.P.S.; Maloba, A.G.K.; Groschup, M.H. Serosurvey for Crimean-Congo Hemorrhagic Fever Virus Infections in Ruminants in Katanga Province, the Democratic Republic of the Congo. Ticks Tick Borne Dis. 2017, 8, 858–861. [Google Scholar] [CrossRef]
- Maiga, O.; Sas, M.A.; Rosenke, K.; Kamissoko, B.; Mertens, M.; Sogoba, N.; Traore, A.; Sangare, M.; Niang, M.; Schwan, T.G.; et al. Serosurvey of Crimean-Congo Hemorrhagic Fever Virus in Cattle, Mali, West Africa. Am. J. Trop. Med. Hyg. 2017, 96, 1341–1345. [Google Scholar] [CrossRef]
- Sas, M.A.; Mertens, M.; Isselmou, E.; Reimer, N.; Mamy, B.; Doumbia, B.; Groschup, M.H. Crimean-Congo Hemorrhagic Fever Virus-Specific Antibody Detection in Cattle in Mauritania. Vector Borne Zoonotic Dis. 2017, 17, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Mangombi, J.B.; Roqueplo, C.; Sambou, M.; Dahmani, M.; Mediannikov, O.; Comtet, L.; Davoust, B. Seroprevalence of Crimean-Congo Hemorrhagic Fever in Domesticated Animals in Northwestern Senegal. Vector Borne Zoonotic Dis. 2020, 20, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Sas, M.A.; Loic Comtet, L.; Donnet, F.; Mertens, M.; Tordo, N.; Pourquier, P.; Groschup, M.H. A Novel Double-Antigen Sandwich ELISA for the Species-Independent Detection of Crimean-Congo Hemorrhagic Fever Virus-Specific Antibodies. Antivir. Res. 2018, 151, 24–26. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Adam, I.A.; Osman, B.T.; Aradaib, I.E. Epidemiological survey of Crimean Congo hemorrhagic fever virus in cattle in East Darfur State, Sudan. Ticks Tick Borne Dis. 2015, 6, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Msimang, V.; Weyer, j.; Le Roux, C.; Kemp, A.; Burt, F.J.; Tempia, S.; Grobbelaar, A.; Moolla, N.; Rostal, M.K.; Bagge, W.; et al. Risk Factors Associated with Exposure to Crimean-Congo Haemorrhagic Fever Virus in Animal Workers and Cattle, and Molecular Detection in Ticks, South Africa. PLoS Negl. Trop. Dis. 2021, 15, e0009384. [Google Scholar] [CrossRef]
- Schulz, A.; Barry, Y.; Stoek, F.; Ba, A.; Schulz, J.; Haki, M.L.; Sas, M.A.; Doumbia, B.A.; Kirkland, P.; Bah, M.Y.; et al. Crimean-Congo Hemorrhagic Fever Virus Antibody Prevalence in Mauritanian Livestock (Cattle, Goats, Sheep and Camels) Is Stratified by the Animal’s Age. PLoS Negl. Trop. Dis. 2021, 15, e0009228. [Google Scholar] [CrossRef] [PubMed]
- Adam, I.A.; Mahmoud, M.A.M.; Aradaib, I.E. A seroepidemiological survey of Crimean Congo hemorrhagic fever among Cattle in North Kordufan State, Sudan. Virol. J. 2013, 10, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suliman, H.M.; Adam, I.A.; Saeed, S.I.; Abdelaziz, S.A.; Haroun, E.M.; Aradaib, I.E. Crimean Congo Hemorrhagic Fever among the One-Humped Camel (Camelus Dromedaries) in Central Sudan. Virol. J. 2017, 14, 147. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.R.; Bouattor, A.; Camicas, J.L.; Estrada-Pena, A.; Horak, I.G.; Latif, A.A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003; pp. 1–221. ISBN 0-9545173-0-X. [Google Scholar]
- Akuffo, R.; Brandful, J.A.M.; Zayed, A.; Adjei, A.; Watany, N.; Fahmy, N.T.; Hughes, R.; Doman, B.; Voegborlo, S.V.; Aziati, D.; et al. Crimean-Congo Hemorrhagic Fever Virus in Livestock Ticks and Animal Handler Seroprevalence at an Abattoir in Ghana. BMC Infect. Dis. 2016, 16, 324. [Google Scholar] [CrossRef] [Green Version]
- Ozdarendeli, A.; Aydin, K.; Tonbak, S.; Aktas, M.; Altay, K.; Koksal, I.Y.; Bolat, Y.; Dumanli, N.; Kalkan, A. Genetic Analysis of the M RNA Segment of Crimean-Congo Hemorrhagic Fever Virus Strains in Turkey. Arch. Virol. 2008, 153, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Telmadarraiy, Z.; Ghiasi, S.M.; Moradi, M.; Vatandoost, H.; Eshraghian, M.R.; Faghihi, F.; Zarei, Z.; Haeri, A.; Chinikar, S. A Survey of Crimean-Congo Haemorrhagic Fever in Livestock and Ticks in Ardabil Province, Iran during 2004-2005. Scand. J. Infect. Dis. 2010, 42, 137–141. [Google Scholar] [CrossRef]
- Simuunza, M.; Weir, W.; Courcier, E.; Tait, A.; Shiels, B. Epidemiological Analysis of Tick-Borne Diseases in Zambia. Vet. Parasitol. 2011, 175, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Munyeme, M.; Muma, J.B.; Munang’andu, H.M.; Kankya, C.; Skjerve, E.; Tryland, M. Cattle Owners’ Awareness of Bovine Tuberculosis in High and Low Prevalence Settings of the Wildlife-Livestock Interface Areas in Zambia. BMC Vet. Res. 2010, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohoo, I.; Martin, W.; Stryhn, H. Veterinary Epidemiologic Research. AVC Inc.: Charlottetown, PE, Canada, 2004; 88, pp. 38–49. Available online: https://epdf.pub/veterinary-epidemiologic-research.html (accessed on 6 March 2021).
- Torell, R.; Bruce, B.; Kvasnicka, B. Methods of Determining Age of Cattle. Cattle Producer’s Library: CL712. University of Nevada: Reno, NV, USA, 2003; pp. 1–3. Available online: www.avc-beef.org/AgingCattle-Griffin/AgingCattle-CL712.pdf (accessed on 6 February 2021).
- Mushonga, B.; Shinexuugi, I.; Mbiri, P.; Samkange, A.; Madzingira, O.; Kandiwa, E. Applicability of teeth examination as a tool for age estimation in a semi-arid cattle production environment in Namibia. Trop. Anim. Health Prod. 2020, 52, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- OIE. Collection and Shipment of Diagnostic Specimens. 2018. Available online: https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/1.1.01_COLLECTION.pdf (accessed on 22 January 2020).



| Factor (n) | Category | Number of Herds per Category | Percentage (95% CI) |
|---|---|---|---|
| Grazing land type (n =117) | Dambo | 94 | 80.3 (72.0–87.1) |
| Both (dambo and upland) | 12 | 10.3 (05.1–17.2) | |
| Upland | 11 | 9.4 (4.79–16.20) | |
| Ticks on herd (n = 117) | Present | 106 | 90.6 (83.8–95.2) |
| Absent | 11 | 9.4 (4.8–16.2) | |
| Tick control (n = 108) | Done | 67 | 62.0 (52.2–71.2) |
| Not done | 41 | 38.0 (28.8–47.8) | |
| Method of tick control (n = 108) | No tick control | 41 | 38.0 (28.8–47.8) |
| Spraying | 55 | 50.9 (41.1–60.7) | |
| Dipping | 1 | 0.9 (0.0–5.1) | |
| Mixed methods | 11 | 10.2 (5.2–17.5) | |
| Tick control frequency (n = 108) | None | 41 | 38.0 (28.8–47.1) |
| Whenever necessary | 33 | 30.6 (22.2–40.2) | |
| Monthly | 19 | 17.6 (10.9–26.1) | |
| Fortnightly | 11 | 10.2 (5.2–17.5) | |
| Weekly | 4 | 3.7 (1.0–9.2) | |
| Farmer keeping other stock species (n = 108) | Yes | 107 | 99.1 (95.0–100.0) |
| No | 1 | 0.9 (0.0–5.1) |
| Risk Factor | Category | n | Seroprevalence (%) | 95% CI | p-Value |
|---|---|---|---|---|---|
| District | Dedza | 56 | 57.1 | 43.2–70.3 | 0.025 * |
| Dowa | 67 | 47.8 | 35.4–60.3 | ||
| Kasungu | 56 | 32.1 | 20.3–46.0 | ||
| Lilongwe East | 57 | 35.1 | 22.9–48.9 | ||
| Lilongwe West | 48 | 60.4 | 45.3–74.2 | ||
| Mchinji | 98 | 46.9 | 36.9–57.3 | ||
| Ntchisi | 34 | 59.9 | 35.1–70.2 | ||
| Sex | Male | 208 | 36.5 | 30.0–43.5 | <0.001 * |
| Female | 208 | 57.2 | 50.2–64.0 | ||
| Age (Months) | 1–12 | 83 | 25.3 | 16.4–36.0 | <0.001 * |
| 13–24 | 80 | 31.3 | 21.4–42.6 | ||
| 25–48 | 151 | 58.3 | 50.0–66.2 | ||
| >48 | 102 | 59.8 | 49.6–69.4 | ||
| Ticks on herd | Present | 384 | 48.7 | 43.6–53.8 | 0.016 * |
| Absent | 32 | 25.0 | 11.5–43.4 | ||
| Grazing land type | Dambo | 326 | 44.8 | 39.3–50.4 | 0.013 * |
| Both (Dambo and upland) | 40 | 40.0 | 24.9–56.7 | ||
| Upland | 50 | 33.0 | 51.2–78.8 | ||
| Tick control | Done | 254 | 46.9 | 40.6–53.2 | 0.854 |
| Not done | 133 | 45.9 | 37.2–54.7 | ||
| Animal source | Within district | 331 | 57.7 | 42.2–53.3 | 0.241 * |
| Outside district | 56 | 39.3 | 26.5–53.3 | ||
| Presence of other stocks in herd | Present | 383 | 46.7 | 41.7–51.9 | 0.336 |
| Absent | 4 | 25.0 | 0.1–80.6 | ||
| Herd size | Small | 127 | 52.0 | 42.9–60.9 | 0.210 * |
| Medium | 113 | 48.7 | 39.2–58.3 | ||
| Large | 176 | 42.1 | 34.7–49.7 |
| Risk Factor | Category | OR | CI | p-Value |
|---|---|---|---|---|
| District | Mchinji | r | ||
| Dedza | 2.2 | 1.0–4.9 | 0.050 * | |
| Dowa | 0.6 | 0.3–1.5 | 0.309 | |
| Kasungu | 0.7 | 0.3–1.6 | 0.408 | |
| Lilongwe East | 1.2 | 0.5–2.6 | 0.669 | |
| Lilongwe West | 2.8 | 1.2–6.5 | 0.016 * | |
| Ntchisi | 5.1 | 1.4–18.6 | 0.013 * | |
| Age (Months) | 1–12 | r | ||
| 13–24 | 1.2 | 0.6–2.6 | 0.626 | |
| 25–48 | 4.4 | 2.2–8.6 | <0.001 * | |
| >48 | 4.3 | 2.1–9.0 | <0.001 * | |
| Animal Sex | Male | r | ||
| Female | 2.5 | 1.6–4.0 | <0.001 * | |
| Ticks on herd | Absent | r | ||
| Present | 3.2 | 1.2–8.5 | 0.02 * | |
| Grazing land type | Dambo | r | ||
| Both (Dambo and Upland) | 0.5 | 0.2–1.5 | 0.244 | |
| Upland | 4.4 | 1.8–10.9 | 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phonera, M.C.; Simuunza, M.C.; Kainga, H.; Ndebe, J.; Chembensofu, M.; Chatanga, E.; Kanyanda, S.; Changula, K.; Muleya, W.; Mubemba, B.; et al. Seroprevalence and Risk Factors of Crimean-Congo Hemorrhagic Fever in Cattle of Smallholder Farmers in Central Malawi. Pathogens 2021, 10, 1613. https://doi.org/10.3390/pathogens10121613
Phonera MC, Simuunza MC, Kainga H, Ndebe J, Chembensofu M, Chatanga E, Kanyanda S, Changula K, Muleya W, Mubemba B, et al. Seroprevalence and Risk Factors of Crimean-Congo Hemorrhagic Fever in Cattle of Smallholder Farmers in Central Malawi. Pathogens. 2021; 10(12):1613. https://doi.org/10.3390/pathogens10121613
Chicago/Turabian StylePhonera, Marvin Collen, Martin Chitolongo Simuunza, Henson Kainga, Joseph Ndebe, Mwelwa Chembensofu, Elisha Chatanga, Setiala Kanyanda, Katendi Changula, Walter Muleya, Benjamin Mubemba, and et al. 2021. "Seroprevalence and Risk Factors of Crimean-Congo Hemorrhagic Fever in Cattle of Smallholder Farmers in Central Malawi" Pathogens 10, no. 12: 1613. https://doi.org/10.3390/pathogens10121613
APA StylePhonera, M. C., Simuunza, M. C., Kainga, H., Ndebe, J., Chembensofu, M., Chatanga, E., Kanyanda, S., Changula, K., Muleya, W., Mubemba, B., Chitanga, S., Kajihara, M., Sawa, H., Njunga, G., Takada, A., & Simulundu, E. (2021). Seroprevalence and Risk Factors of Crimean-Congo Hemorrhagic Fever in Cattle of Smallholder Farmers in Central Malawi. Pathogens, 10(12), 1613. https://doi.org/10.3390/pathogens10121613









