Early Lesion of Post-Primary Tuberculosis: Subclinical Driver of Disease and Target for Vaccines and Host-Directed Therapies
Abstract
:1. Introduction
2. Results
3. Discussion
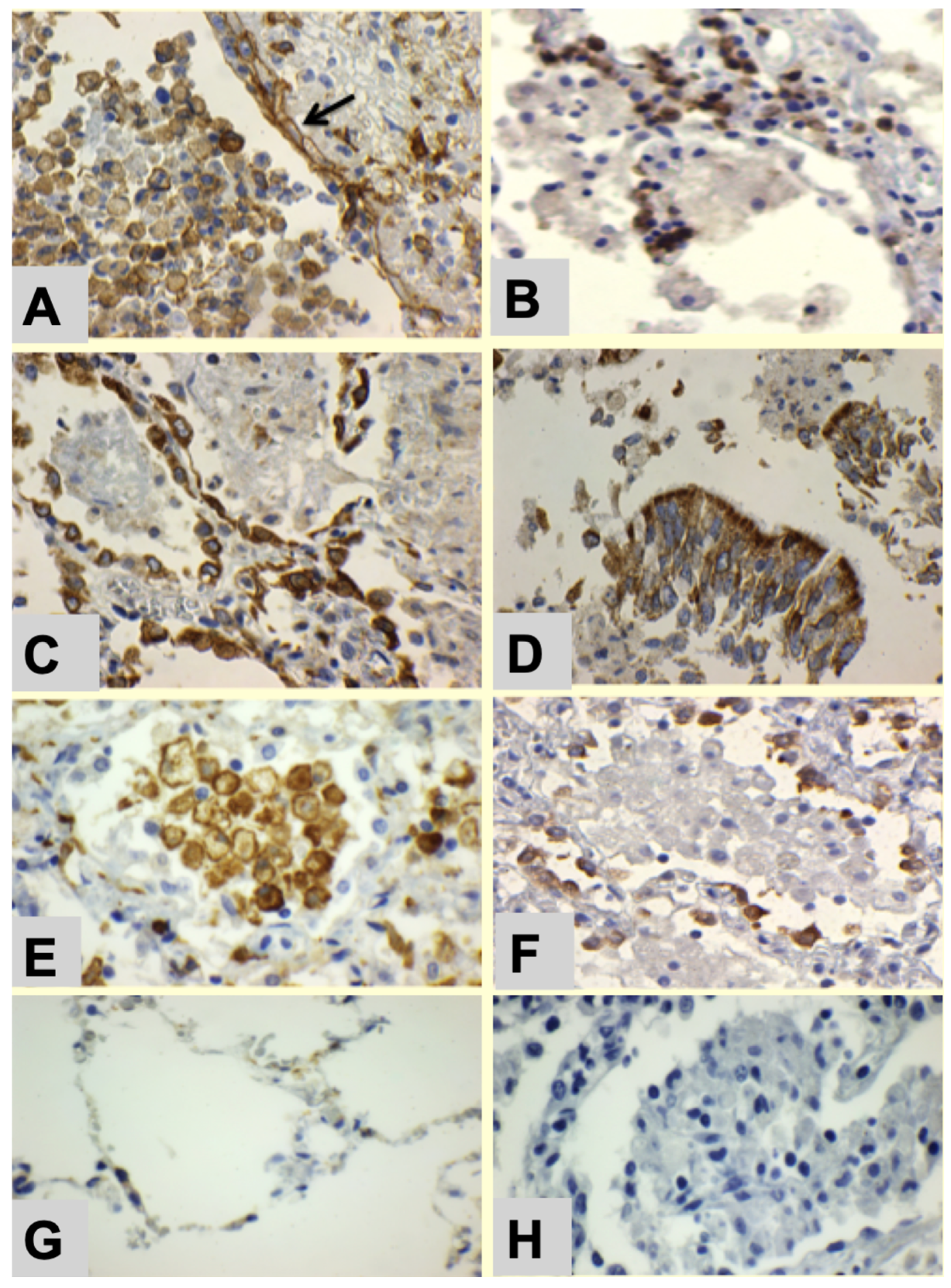
- Bronchial obstruction traps alveolar macrophages to produce post-obstructive lipid pneumonia [14].
- Alveolar lining cells use FAS to produce lipids.
- Alveolar macrophages with M2 phenotype (CD163 Staining) become foamy by accumulating host lipids and secreted mycobacterial antigens.
- Sensitized tissue resident T cells (TRM) accumulate in alveolar walls.
- PD-L1 expression on alveolar macrophages and alveolar pneumocytes suppress PD-1+ T cell activity.
4. Material and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, R.L. Pathology of post primary tuberculosis of the lung: An illustrated critical review. Tuberculosis 2011, 91, 497–509. [Google Scholar] [CrossRef] [Green Version]
- Rich, A. The Pathogenesis of Tuberculosis, 2nd ed.; Charles C Thomas: Springfield, IL, USA, 1951; 1028p. [Google Scholar]
- Canetti, G. The Tubercle Bacillus in the Pulmonary Lesion of Man. In Histobacteriology and Its Bearing on the Therapy of Pulmonary Tuberculosis; Springer Publishing Compani Inc.: New York, NY, USA, 1955; 226p. [Google Scholar]
- Medlar, E.M. The pathogenesis of minimal pulmonary tuberculosis; a study of 1225 necropsies in cases of sudden and unexpected death. Am. Rev. Tuberc. 1948, 58, 583–611. [Google Scholar]
- Im, J.G.; Itoh, H. Tree-in-Bud Pattern of Pulmonary Tuberculosis on Thin-Section CT: Pathological Implications. Korean J. Radiol. 2018, 19, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L. The Pathogenesis of Tuberculosis-The Koch Phenomenon Reinstated. Pathogens 2020, 9, 813. [Google Scholar] [CrossRef]
- Hunter, R.L. Tuberculosis as a three-act play: A new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis 2016, 97, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, R.L.; Actor, J.K.; Hwang, S.A.; Karev, V.; Jagannath, C. Pathogenesis of post primary tuberculosis: Immunity and hypersensitivity in the development of cavities. Ann. Clin. Lab Sci. 2014, 44, 365–387. [Google Scholar] [PubMed]
- Mustafa, T.; Leversen, N.A.; Sviland, L.; Wiker, H.G. Differential in vivo expression of mycobacterial antigens in Mycobacterium tuberculosis infected lungs and lymph node tissues. BMC Infect. Dis. 2014, 14, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malen, H.; Berven, F.S.; Fladmark, K.E.; Wiker, H.G. Comprehensive analysis of exported proteins from Mycobacterium tuberculosis H37Rv. Proteomics 2007, 7, 1702–1718. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L. The Pathogenesis of Tuberculosis: The Early Infiltrate of Post-primary (Adult Pulmonary) Tuberculosis: A Distinct Disease Entity. Front. Immunol. 2018, 9, 2108. [Google Scholar] [CrossRef] [Green Version]
- Basaraba, R.J.; Hunter, R.L. Pathology of Tuberculosis: How the Pathology of Human Tuberculosis Informs and Directs Animal Models. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Medlar, E.M. Pathogenic concepts of tuberculosis. Am. J. Med. 1950, 9, 611–622. [Google Scholar] [CrossRef]
- Hunter, R.L. On the pathogenesis of post primary tuberculosis: The role of bronchial obstruction in the pathogenesis of cavities. Tuberculosis 2011, 91, S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Hunter, R.L.; Hwang, S.A. Morphoproteomic-Guided Host-Directed Therapy for Tuberculosis. Front. Immunol. 2017, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Welsh, K.J.; Risin, S.A.; Actor, J.K.; Hunter, R.L. Immunopathology of postprimary tuberculosis: Increased T-regulatory cells and DEC-205-positive foamy macrophages in cavitary lesions. Clin. Dev. Immunol. 2011, 2011, 307631. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.A.; Ali, Y.; Fedotova, E.; Hunter, R.L.; Brown, R.E. Morphoproteomics Identifies the Foamy Alveolar Macrophage as an M2 Phenotype with PD-L1 Expression in the Early Lesion of Post-Primary Tuberculosis: Implications for Host Immune Surveillance and Therapy. Ann. Clin. Lab Sci. 2020, 50, 429–438. [Google Scholar]
- Shim, D.; Kim, H.; Shin, S.J. Mycobacterium tuberculosis Infection-Driven Foamy Macrophages and Their Implications in Tuberculosis Control as Targets for Host-Directed Therapy. Front. Immunol. 2020, 11, 910. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.; Actor, J. The pathogenesis of post-primary tuberculosis. A game changer for vaccine development. Tuberculosis 2019, 116S, S114–S117. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.A.; Welsh, K.J.; Kruzel, M.L.; Actor, J.K. Lactoferrin Augmentation of the BCG Vaccine Leads to Increased Pulmonary Integrity. Tuberc. Res. Treat. 2011, 2011, 835410. [Google Scholar] [CrossRef]
- Majumdar, A.; Wankhade, G.; Kamble, P.D.; Harinath, B.C. Effect of HIV protease inhibitors and Orlistat on mycobacterial ES-31 serine protease, a potential drug target in Mycobacterium tuberculosis. Indian J. Tuberc. 2011, 58, 4–10. [Google Scholar] [PubMed]
- Wahdan-Alaswad, R.S.; Cochrane, D.R.; Spoelstra, N.S.; Howe, E.N.; Edgerton, S.M.; Anderson, S.M.; Thor, A.D.; Richer, J.K. Metformin-induced killing of triple-negative breast cancer cells is mediated by reduction in fatty acid synthase via miRNA-193b. Horm. Cancer 2014, 5, 374–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kridel, S.J.; Axelrod, F.; Rozenkrantz, N.; Smith, J.W. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004, 64, 2070–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restrepo, B.I. Metformin: Candidate host-directed therapy for tuberculosis in diabetes and non-diabetes patients. Tuberculosis 2016, 101S, S69–S72. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Jie, L.; Kumar, P.; Hong, G.S.; Leow, M.K.-S.; Paleja, B.; Tsenova, L.; Kurepina, N.; Chen, J.; Zolezzi, F.; et al. Metformin as adjunct antituberculosis therapy. Sci. Transl. Med. 2014, 6, 263ra159. [Google Scholar] [CrossRef]
- Salamon, H.; Bruiners, N.; Lakehal, K.; Shi, L.; Ravi, J.; Yamaguchi, K.D.; Pine, R.; Gennaro, M.L. Cutting edge: Vitamin D regulates lipid metabolism in Mycobacterium tuberculosis infection. J. Immunol. 2014, 193, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Mily, A.; Rekha, R.S.; Kamal, S.M.M.; Arifuzzaman, A.S.M.; Rahim, Z.; Khan, L.; Haq, A.; Zaman, K.; Bergman, P.; Brighenti, S.; et al. Significant Effects of Oral Phenylbutyrate and Vitamin D3 Adjunctive Therapy in Pulmonary Tuberculosis: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0138340. [Google Scholar]
- Hunter, R.L.; Jagannath, C.; Actor, J.K. Pathology of postprimary tuberculosis in humans and mice: Contradiction of long-held beliefs. Tuberculosis 2007, 87, 267–278. [Google Scholar] [CrossRef]
- Liang, Y.; Bai, X.; Zhang, J.; Song, J.; Yang, Y.; Yu, Q.; Li, N.; Wu, X. Ag85A/ESAT-6 chimeric DNA vaccine induces an adverse response in tuberculosis-infected mice. Mol. Med. Rep. 2016, 14, 1146–1152. [Google Scholar] [CrossRef]
- Van Der Meeren, O.; Hatherill, M.; Nduba, V.; Wilkinson, R.; Muyoyeta, M.; Van Brakel, E.; Ayles, H.M.; Henostroza, G.; Thienemann, F.; Scriba, T.; et al. Phase 2b Controlled Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N. Engl. J. Med. 2018, 379, 1621–1634. [Google Scholar] [CrossRef]
- Al-Attiyah, R.; Mustafa, A.S.; Abal, A.T.; El-Shamy, A.S.; Dalemans, W.; Skeiky, Y.A. In vitro cellular immune responses to complex and newly defined recombinant antigens of Mycobacterium tuberculosis. Clin. Exp. Immunol. 2004, 138, 139–144. [Google Scholar] [CrossRef]
- Okada, M.; Kita, Y.; Nakajima, T.; Kanamaru, N.; Hashimoto, S.; Nagasawa, T.; Kaneda, Y.; Yoshida, S.; Nishida, Y.; Nakatani, H.; et al. Novel prophylactic vaccine using a prime-boost method and hemagglutinating virus of Japan-envelope against tuberculosis. Clin. Dev. Immunol. 2011, 2011, 549281. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.K.; Aqdas, M.; Das, D.K.; Singh, S.; Nadeem, S.; Kaur, G.; Agrewala, J.N. A multiple T cell epitope comprising DNA vaccine boosts the protective efficacy of Bacillus Calmette-Guerin (BCG) against Mycobacterium tuberculosis. BMC Infect. Dis. 2020, 20, 677. [Google Scholar] [CrossRef] [PubMed]
- Doimo, N.T.S.; Zárate-Bladés, C.R.; Rodrigues, R.F.; Tefé-Silva, C.; Trotte, M.N.S.; Souza, P.R.M.; Soares, L.S.; Rios, W.M.; Floriano, E.M.; Brandão, I.T.; et al. Immunotherapy of tuberculosis with Mycobacterium leprae Hsp65 as a DNA vaccine triggers cross-reactive antibodies against mammalian Hsp60 but not pathological autoimmunity. Hum. Vaccin Immunother. 2014, 10, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Francisco-Cruz, A.; Wistuba, I.I. State-of-the-Art of Profiling Immune Contexture in the Era of Multiplexed Staining and Digital Analysis to Study Paraffin Tumor Tissues. Cancers 2019, 11, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, R.E.; Hunter, R.L. Early Lesion of Post-Primary Tuberculosis: Subclinical Driver of Disease and Target for Vaccines and Host-Directed Therapies. Pathogens 2021, 10, 1572. https://doi.org/10.3390/pathogens10121572
Brown RE, Hunter RL. Early Lesion of Post-Primary Tuberculosis: Subclinical Driver of Disease and Target for Vaccines and Host-Directed Therapies. Pathogens. 2021; 10(12):1572. https://doi.org/10.3390/pathogens10121572
Chicago/Turabian StyleBrown, Robert E., and Robert L. Hunter. 2021. "Early Lesion of Post-Primary Tuberculosis: Subclinical Driver of Disease and Target for Vaccines and Host-Directed Therapies" Pathogens 10, no. 12: 1572. https://doi.org/10.3390/pathogens10121572
APA StyleBrown, R. E., & Hunter, R. L. (2021). Early Lesion of Post-Primary Tuberculosis: Subclinical Driver of Disease and Target for Vaccines and Host-Directed Therapies. Pathogens, 10(12), 1572. https://doi.org/10.3390/pathogens10121572





