The Enhancement of the Photodynamic Therapy and Ciprofloxacin Activity against Uropathogenic Escherichia coli Strains by Polypodium vulgare Rhizome Aqueous Extract
Abstract
:1. Introduction
2. Results
2.1. P. vulgare Extract Quantitative Analysis
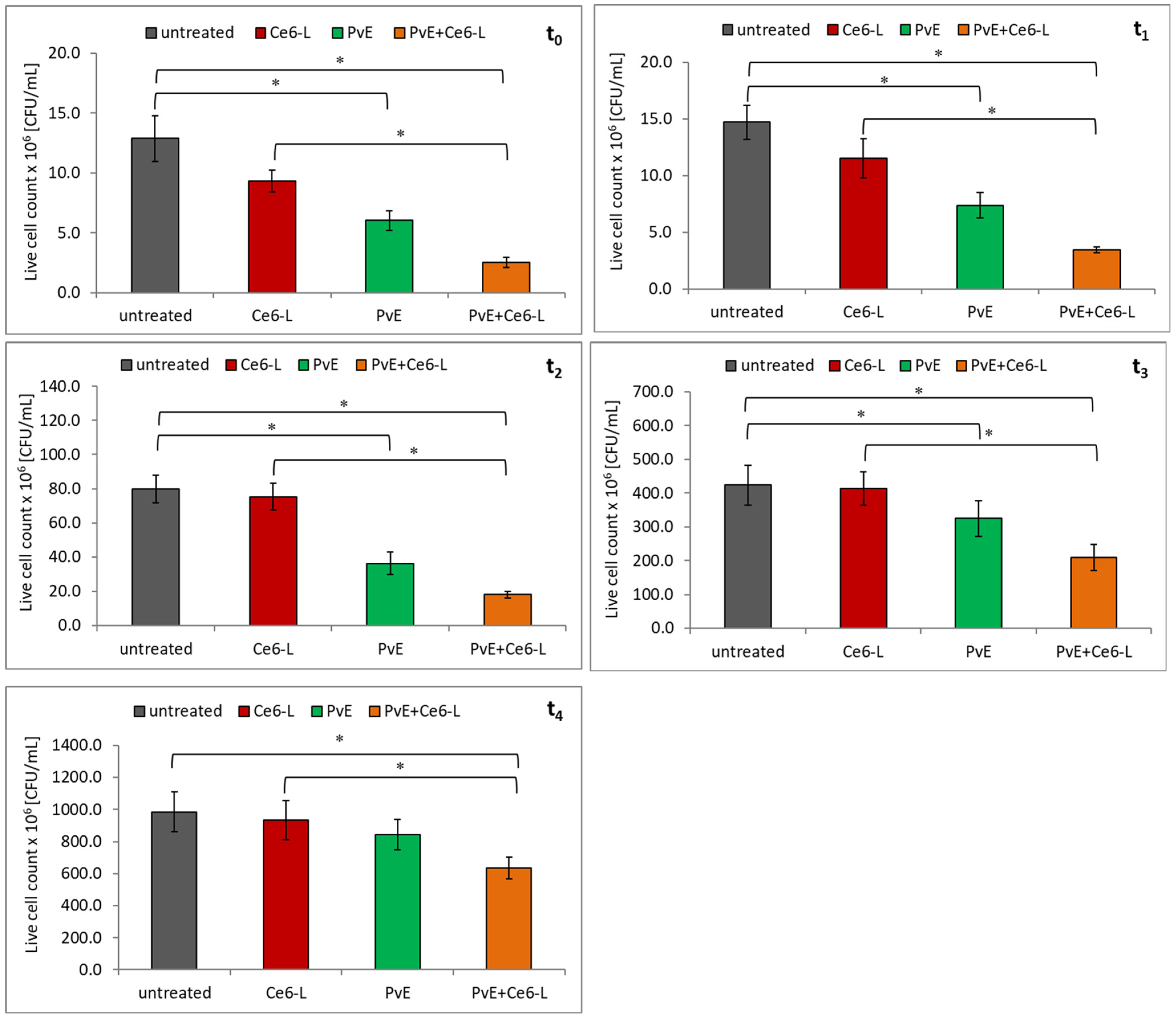
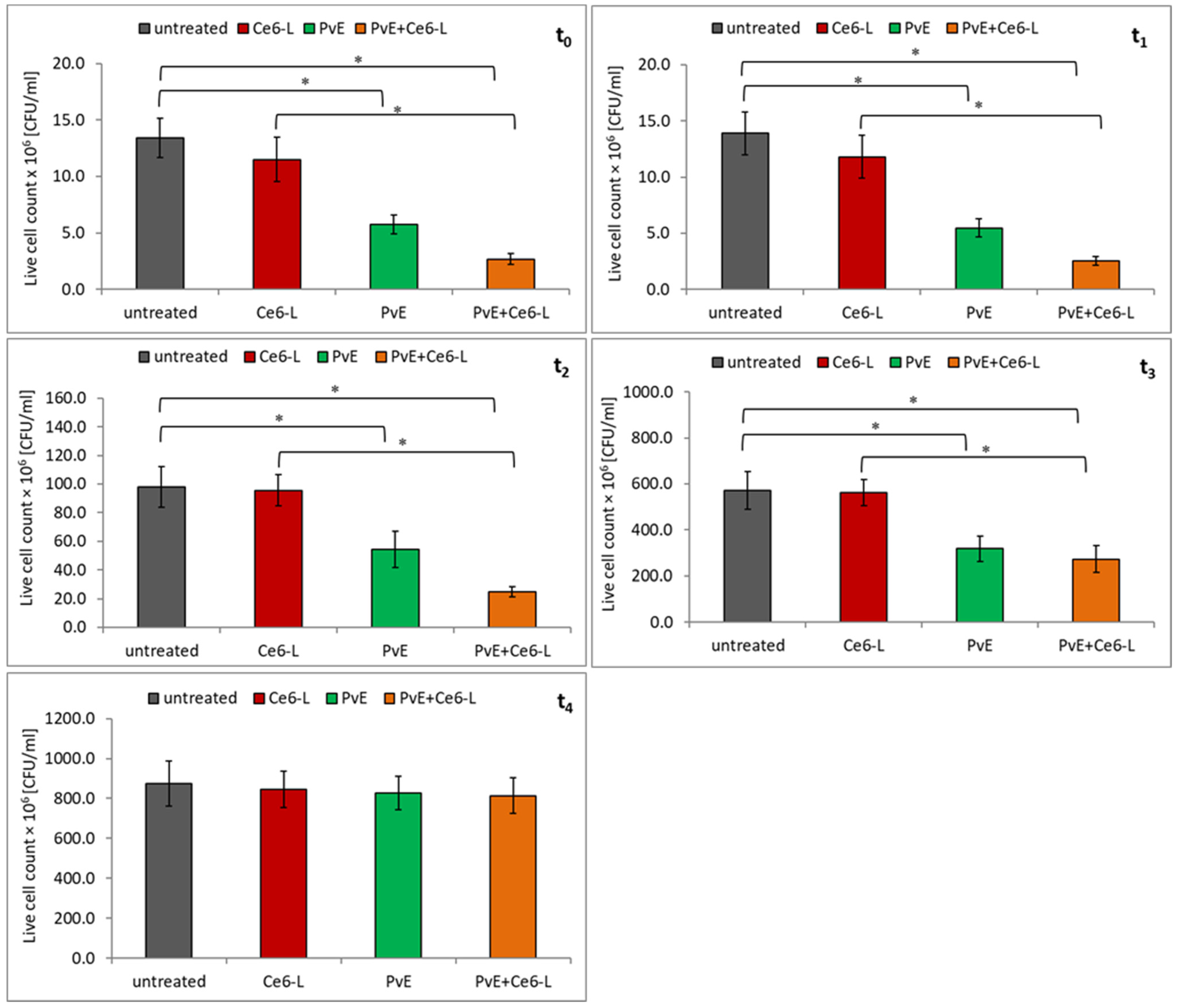
2.2. Antibacterial Effect of PDT and P. vulgare Extract
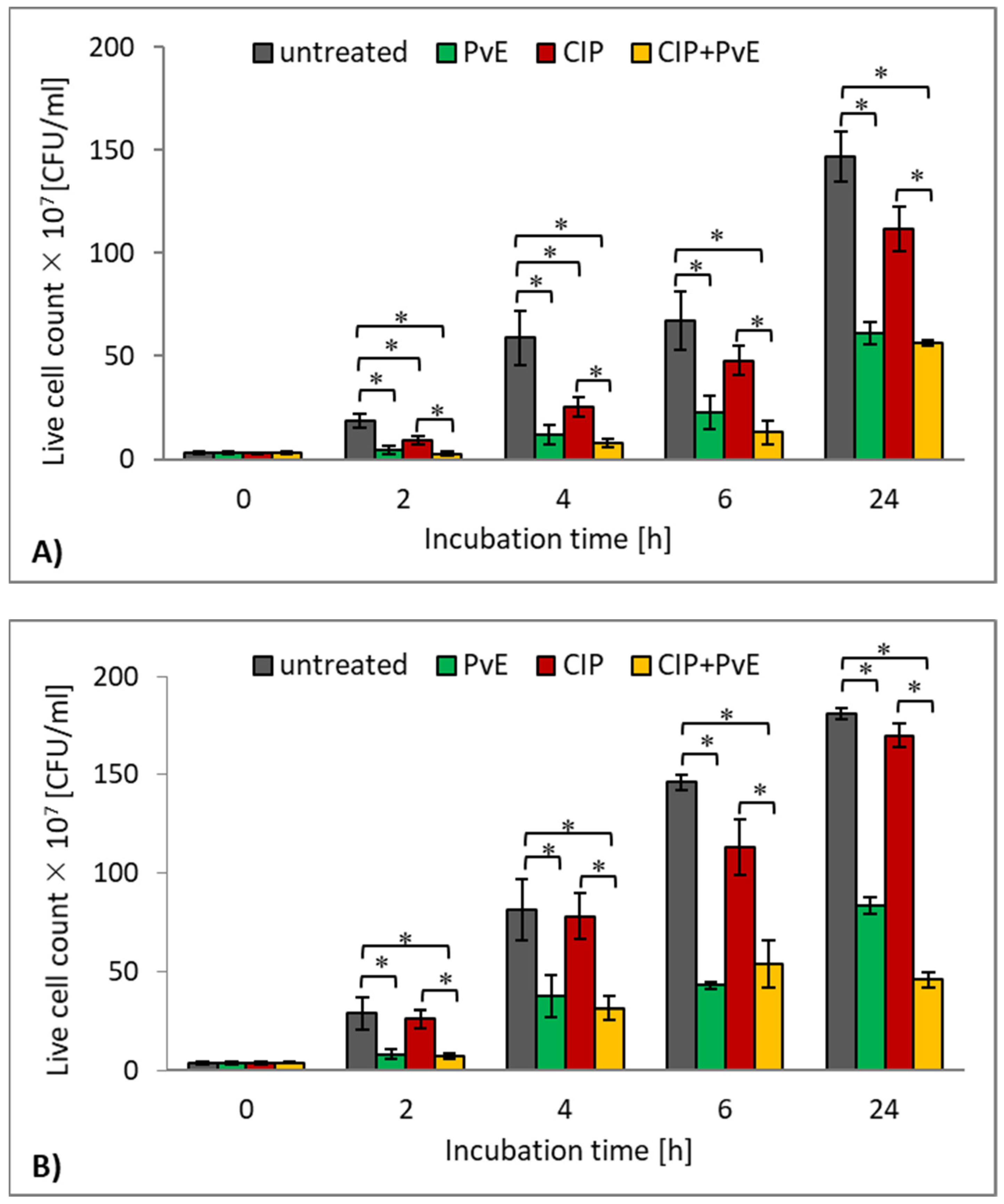
2.3. Antibacterial Effect of CIP and P. vulgare Extract on Planktonic Cultures
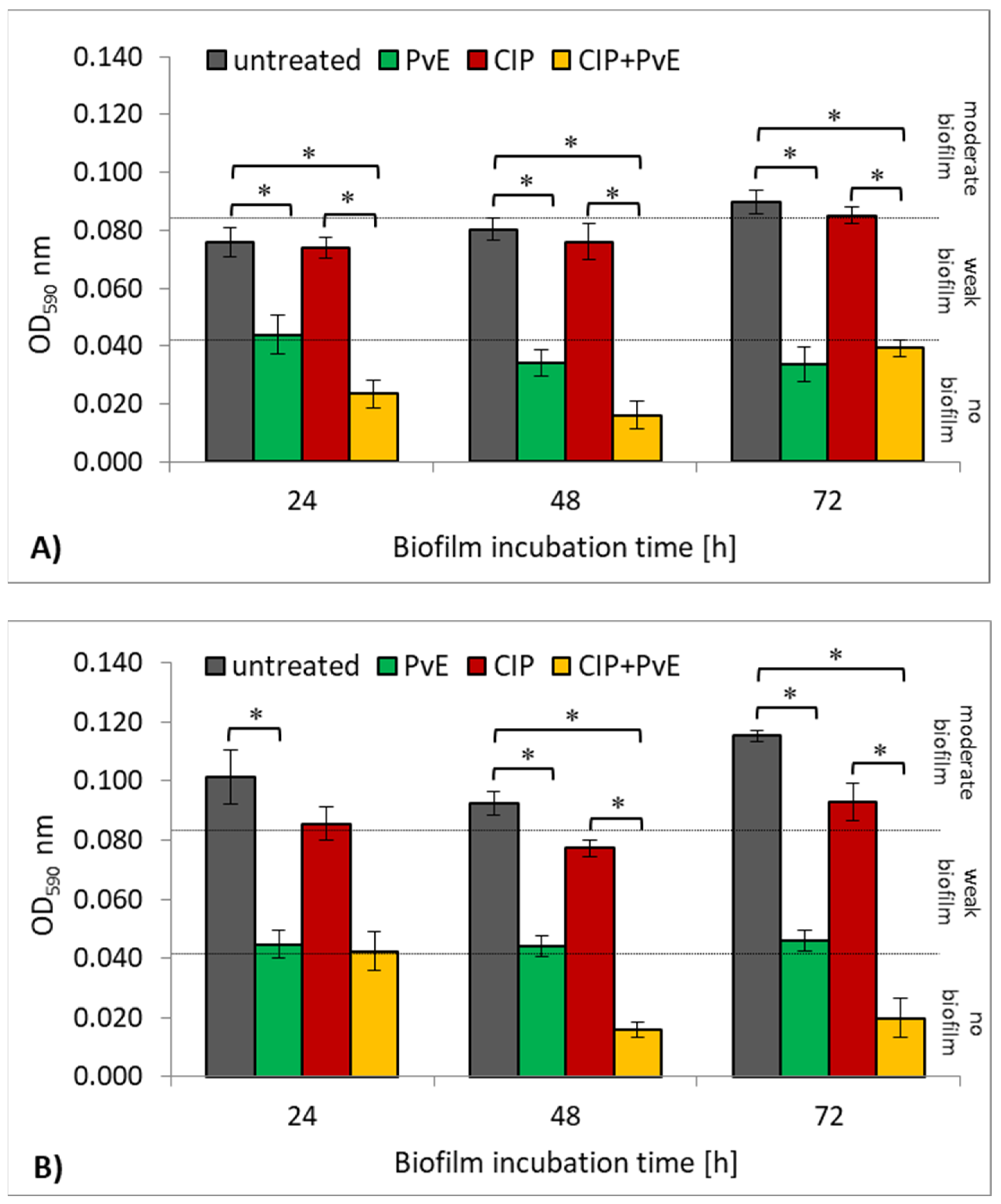
2.4. Antibacterial Effect of CIP and P. vulgare Extract on Biofilm Cultures
3. Discussion
4. Materials and Methods
4.1. Microorganisms
4.2. Antimicrobial Agents
4.2.1. Antibiotic
4.2.2. Plant Material
4.2.3. Photosensitizer and Light Source
4.3. P. vulgare Rhizome Aqueous Extract Preparation
4.4. P. vulgare Rhizome Aqueous Extract Quantification
4.5. MIC Determination
4.6. Preparation of Bacterial Suspensions
4.7. Planktonic Bacterial Cultures Assay
4.8. Biofilm Assay
4.9. Determination of Bacterial Survival
4.10. PDT Experimental Conditions
4.11. Biofilm Visualization by DAPI Staining
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.-A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Garcez, A.S.; Kaplan, M.; Jensen, G.J.; Scheidt, F.R.; Oliveira, E.M.; Suzuki, S.S. Effects of antimicrobial photodynamic therapy on antibiotic-resistant Escherichia coli. Photodiagn. Photodyn. Ther. 2020, 32, 102029. [Google Scholar] [CrossRef]
- Von Tappeiner, H.; Jesionek, A. Therapeutische versuche mit fluoreszierenden stiffen. Münch. Med. Wochenschr. 1903, 47, 2042–2044. [Google Scholar]
- Fritsch, C.; Lang, K.; Neuse, W.; Ruzicka, T.; Lehmann, P. Photodynamic diagnosis and therapy in dermatology. Skin Pharmacol. Appl. Skin Physiol. 1998, 11, 358–753. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Zhang, X.; Guo, W.; Li, F.; Yu, M.; Kong, X.; Wu, W.; Hong, Z. Highly water-soluble and tumor-targeted photosensitizers for photodynamic therapy. Org. Biomol. Chem. 2015, 13, 7681–7694. [Google Scholar] [CrossRef] [PubMed]
- Carrera, E.T.; Dias, H.B.; Corbi, T.S.C.; Marcantonio, R.A.C.; Bernardi, A.C.A.; Bagnato, V.S.; Hamblin, M.R.; Rastelli, A.N.S. The application of antimicrobial photodynamic therapy (aPDT) in dentistry: A critical review. Laser Phys. 2016, 26, 123001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremer, N.; Sherman, S.; Lapidoth, M.; Enk, C.D.; Leshem, Y.A.; Mimouni, T.; Dudkiewicz, D.; Hodak, E.; Levi, A. Self-administered daylight-activated photodynamic therapy for the treatment of hand eczema: A prospective proof-of-concept study. Dermatol. Ther. 2020, 33, e14329. [Google Scholar] [CrossRef] [PubMed]
- Held, L.; Eigentler, T.K.; Leiter, U.; Garbe, C.; Berneburg, M.J. Effective combination of photodynamic therapy and imiquimod 5% cream in the treatment of actinic keratoses: Three cases. Biomed. Res. Int. 2013, 2013, 102698. [Google Scholar] [CrossRef] [Green Version]
- Mansoori, B.; Mohammadi, A.; Doustvandi, M.A.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic therapy for cancer: Role of natural products. Photodiagn. Photodyn. 2019, 26, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.O.; Tsubone, T.M.; Pavani, C.; Baptista, M.S. Photodynamic efficiency: From molecular photochemistry to cell death. Int. J. Mol. Sci. 2015, 16, 20523–20559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tichaczek-Goska, D.; Wojnicz, D.; Symonowicz, K.; Ziółkowski, P.; Hendrich, A.B. Photodynamic enhancement of the activity of antibiotics used in urinary tract infections. Lasers Med. Sci. 2019, 34, 1547–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fekrazad, R.; Zare, H.; Vand, S.M. Photodynamic therapy effect on cell growth inhibition induced by Radachlorin and toluidine blue O on Staphylococcus aureus and Escherichia coli: An in vitro study. Photodiagn. Photodyn. Ther. 2016, 15, 213–217. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Wintner, A.; Seed, P.C.; Brauns, T.; Gelfand, J.A.; Hamblin, M.R. Antimicrobial photodynamic therapy mediated by methylene blue and potassium iodide to treat urinary tract infection in a female rat model. Sci. Rep. 2018, 8, 7257. [Google Scholar] [CrossRef]
- Firoozeh, F.; Saffari, M.; Neamati, F.; Zibaei, M. Detection of virulence genes in Escherichia coli isolated from patients with cystitis and pyelonephritis. Int. J. Infect. Dis. 2014, 29, 219–222. [Google Scholar] [CrossRef] [Green Version]
- EAU Guidelines. Edn. Presented at the EAU Annual Congress Milan Italy. 2021. Available online: https://uroweb.org/guideline/urological-infections/#3 (accessed on 2 November 2021).
- Wagenlehner, F.M.E.; Wullt, B.; Perletti, G. Antimicrobials in urogenital infections. Int. J. Antimicrob. Agents 2011, 38, 3–10. [Google Scholar] [CrossRef]
- Bonkat, G.; Widmer, A.F.; Rieken, M.; van der Merwe, A.; Braissant, O.; Müller, G.; Wyler, S.; Frei, R.; Gasser, T.C.; Bachmann, A. Microbial biofilm formation and catheter-associated bacteriuria in patients with suprapubic catheterization. World J. Urol. 2013, 31, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Dar, P.A.; Sofi, G.; Jafri, M.A. Polypodium vulgare Linn. a versatile herbal medicine: A review. Int. J. Pharm. Sci. Res. 2012, 3, 1616–1620. [Google Scholar]
- Biegański, J.; Jamiołkowski, S. Our Herbs and Treatment with Them; St. Jamiołkowski & T. J. Evert; Drukarnia Polska Spółdzielni Wydawniczej “Zryw”: Łódź, Poland, 1949; pp. 157–158. [Google Scholar]
- Muszyński, J.K. Pharmacognosy; PZWL: Warsaw, Poland, 1957; pp. 60–61. [Google Scholar]
- Gleńsk, M.; Tichaczek-Goska, D.; Środa-Pomianek, K.; Włodarczyk, M.; Wesolowski, C.A.; Wojnicz, D. Differing antibacterial and antibiofilm properties of Polypodium vulgare L. rhizome aqueous extract and one of its purified active ingredients-osladin. J. Herb. Med. 2019, 17–18, 100261. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Mahmoodi Kordi, F.; Ali Ahmadi, A.; Bahadori, S.; Valizadeh, H. Antibacterial evaluation and preliminary phytochemical screening of selected ferns from Iran. Res. J. Pharmacogn. Phytochem. 2015, 2, 53–59. [Google Scholar]
- Grzybek, J. Phytochemical and biological investigations on Polypodium vulgare L. Acta Pol. Pharm. 1983, 40, 259–263. [Google Scholar]
- Sofiane, G.; Wafa, N.; Ouarda, D. Antioxidant, antimicrobial and anti-inflammatory activities of flavonoids and tannins extracted from Polypodium vulgare L. Asian J. Biochem. Pharm. Res. 2015, 5, 114–212. [Google Scholar]
- Khan, A.; Siddiqui, A.; Jafri, M.A.; Asif, M. Ethnopharmacological studies of Polypodium vulgare Linn: A comprehensive review. J. Drug Deliv. Ther. 2018, 8, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Bottoni, M.; Milani, F.; Colombo, L.; Nallio, K.; Colombo, P.S.; Giuliani, C.; Bruschi, P.; Fico, G. Using medicinal plants in Valmalenco (Italian Alps): From tradition to scientific approaches. Molecules 2020, 25, 4144. [Google Scholar] [CrossRef] [PubMed]
- Gleńsk, M.; Dudek, M.K.; Ciach, M.; Włodarczyk, M. Isolation and structural determination of flavan-3-ol derivatives from the Polypodium vulgare L. rhizomes water extract. Nat. Prod. Res. 2021, 35, 1474–1483. [Google Scholar] [CrossRef]
- Simon, A.; Ványolós, A.; Béni, Z.; Dékány, M.; Tóth, G.; Báthori, M. Ecdysteroids from Polypodium vulgare L. Steroids 2011, 76, 1419–1424. [Google Scholar] [CrossRef]
- European Medicines Agency Evaluation of Medicines for Human Use. EMEA Assessment Report on Polypodium vulgare L., Rhizoma; European Medicines Agency: London, UK, 2008. [Google Scholar]
- Farras, A.; Mitjans, M.; Maggi, F.; Caprioli, G.; Vinardell, M.P.; López, V. Polypodium vulgare L. (Polypodiaceae) as a source of bioactive compounds: Polyphenolic profile, cytotoxicity and cytoprotective properties in different cell lines. Front. Pharmacol. 2021, 12, 727528. [Google Scholar] [CrossRef] [PubMed]
- EMA Committee on Herbal Medicinal Products (HMPC). Assessment Report on Polypodium vulgare L., Rhizoma; HMPC: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Rojewska, M.; Smułek, W.; Prochaska, K.; Kaczorek, E. Combined effect of nitrofurantoin and plant surfactant on bacteria phospholipid membrane. Molecules 2020, 25, 2527. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Das, N.; Mishra, S.K.; Bishayee, A.; Ali, E.S.; Bishayee, A. The phytochemical, biological, and medicinal attributes of phytoecdysteroids: An updated review. Acta Pharma. Sin. B 2021, 11, 1740–1766. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Zoetendal, E.; Mackie, R.I. Bacterial mechanisms to overcome inhibitory effects of dietary tannins. Microb. Ecol. 2005, 50, 197–205. [Google Scholar] [CrossRef]
- Rafael, L.; Teresinha, N.; Moritz, J.C.; Maria, I.G.; Dalmarco Eduardo, M.; Fröde Tânia, S. Evaluation of antimicrobial and antiplatelet aggregation effects of Solidago chilensis Meyen. Int. J. Green Pharm. 2009, 3, 35–39. [Google Scholar]
- Luke-Marshall, N.R.; Hansen, L.A.; Shafirstein, G.; Campagnari, A.A. Antimicrobial photodynamic therapy with chlorin e6 is bactericidal against biofilms of the primary human otopathogens. mSphere 2020, 5, e00492-20. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.M.; Lee, H.S.; Jeong, D.; Oh, H.K.; Ra, K.H.; Lee, M.Y. Antimicrobial photodynamic therapy using chlorin e6 with halogen light for acne bacteria-induced inflammation. Life Sci. 2015, 124, 56–63. [Google Scholar] [CrossRef]
- Wu, M.F.; Deichelbohrer, M.; Tschernig, T.; Laschke, M.W.; Szentmáry, N.; Hüttenberger, D.; Foth, H.J.; Seitz, B.; Bischoff, M. Chlorin e6 mediated photodynamic inactivation for multidrug resistant Pseudomonas aeruginosa keratitis in mice in vivo. Sci. Rep. 2017, 7, 44537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dascalu Rusu, L.M.; Moldovan, M.; Prodan, D.; Ciotlaus, I.; Popescu, V.; Baldea, I.; Carpa, R.; Sava, S.; Chifor, R.; Badea, M.E. Assessment and characterization of some new photosensitizers for antimicrobial photodynamic therapy (aPDT). Materials 2020, 13, 3012. [Google Scholar] [CrossRef]
- Nie, M.; Silva, R.C.E.; de Oliveira, K.T.; Bagnato, V.S.; de Souza Rastelli, A.N.; Crielaard, W.; Yang, J.; Deng, D.M. Synergetic antimicrobial effect of chlorin e6 and hydrogen peroxide on multi-species biofilms. Biofouling 2021, 37, 656–665. [Google Scholar] [CrossRef]
- Drulis-Kawa, Z.; Bednarkiewicz, A.; Bugla, G.; Stręk, W.; Doroszkiewicz, W. Bactericidal effects of the Fotolon (chlorin e6) on gram-negative and gram-positive strains isolated from wound infections. Adv. Clin. Exp. Med. 2006, 15, 279–283. [Google Scholar]
- de Annunzio, S.R.; de Freitas, L.M.; Blanco, A.L.; da Costa, M.M.; Carmona-Vargas, C.C.; de Oliveira, K.T.; Fontana, C.R. Susceptibility of Enterococcus faecalis and Propionibacterium acnes to antimicrobial photodynamic therapy. J. Photochem. Photobiol. B 2018, 178, 545–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynoso, E.; Ferreyra, D.D.; Durantini, E.N.; Spesia, M.B. Photodynamic inactivation to prevent and disrupt Staphylococcus aureus biofilm under different media conditions. Photodermatol. Photoimmunol. Photomed. 2019, 35, 322–331. [Google Scholar] [CrossRef]
- Ulatowska-Jarża, A.; Zychowicz, J.; Hołowacz, I.; Bauer, J.; Razik, J.; Wieliczko, A.; Podbielska, H.; Müller, G.; Stręk, W.; Bindig, U. Antimicrobial PDT with chlorophyll-derived photosensitizer and semiconductor laser. Med. Laser Appl. 2006, 21, 177–183. [Google Scholar] [CrossRef]
- Embleton, M.L.; Nair, S.P.; Cookson, B.D.; Wilson, M. Selective lethal photosensitization of methicillin-resistant Staphylococcus aureus using an IgG–tin (IV) chlorin e6 conjugate. J. Antimicrob. Chemother. 2002, 50, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Yue, L.; Zheng, M.; Khan, I.M.; Wang, Z. Chlorin e6 conjugated chitosan as an efficient photoantimicrobial agent. Int. J. Biol. Macromol. 2021, 183, 1309–1316. [Google Scholar] [CrossRef]
- Suvorov, N.; Pogorilyy, V.; Diachkova, E.; Vasil’ev, Y.; Mironov, A.; Grin, M. Derivatives of natural chlorophylls as agents for antimicrobial photodynamic therapy. Int. J. Mol. Sci. 2021, 22, 6392. [Google Scholar] [CrossRef]
- Park, J.H.; Moon, Y.H.; Bang, I.S.; Kim, Y.C.; Kim, S.A.; Ahn, S.G.; Yoon, J.H. Antimicrobial effect of photodynamic therapy using a highly pure chlorin e6. Lasers Med. Sci. 2010, 25, 705–710. [Google Scholar] [CrossRef]
- Yow, C.M.; Tang, H.M.; Chu, E.S.; Huang, Z. Hypericin-mediated photodynamic antimicrobial effect on clinically isolated pathogens. Photochem. Photobiol. 2012, 88, 626–632. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural photosensitizers in antimicrobial photodynamic therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.P.; Lopes, D.P.S.; de Melo Calado, S.P.; Gonçalves, C.V.; Muniz, I.P.R.; Ribeiro, I.S.; Galantini, M.P.L.; da Silva, R.A.A. Efficacy of photoactivated Myrciaria cauliflora extract against Staphylococcus aureus infection—A pilot study. J. Photochem. Photobiol. B. 2019, 191, 107–115. [Google Scholar] [CrossRef]
- Sana, M.; Jameel, H.; Rahman, M. Miracle remedy: Inhibition of bacterial efflux pumps by natural products. J. Infect. Dis. Ther. 2015, 3, 1000213. [Google Scholar] [CrossRef]
- Kurek, A.; Nadkowska, P.; Pliszka, S.; Wolska, K.I. Modulation of antibiotic resistance in bacterial pathogens by oleanolic acid and ursolic acid. Phytomedicine 2012, 19, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.H.; Mohamed, M.S.M.; Khalil, M.S.; Azmy, M.; Mabrouk, M.I. Combination of essential oil and ciprofloxacin to inhibit/eradicate biofilms in multidrug-resistant Klebsiella pneumoniae. J. Appl. Microbiol. 2018, 125, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Malik, T.; Singh, P.; Pant, S.; Chauhan, N.; Lohani, H. Potentiation of antimicrobial activity of ciprofloxacin by Pelargonium graveolens essential oil against selected uropathogens. Phytother. Res. 2011, 25, 1225–1228. [Google Scholar] [CrossRef]
- Nafis, A.; Iriti, M.; Ouchari, L.; El Otmani, F.; Marraiki, N.; Elgorban, A.M.; Syed, A.; Mezrioui, N.; Hassani, L.; Custódio, L. New insight into the chemical composition, antimicrobial and synergistic effects of the Moroccan endemic Thymus atlanticus (Ball) Roussine essential oil in combination with conventional antibiotics. Molecules 2021, 26, 5850. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, M.; Althubiani, A.S.; Abulreesh, H.H.; Qais, F.A.; Khan, M.S.; Ahmad, I. Bioactive extracts of Carum copticum L. enhances efficacy of ciprofloxacin against MDR enteric bacteria. Saudi J. Biol. Sci. 2019, 26, 1848–1855. [Google Scholar] [CrossRef]
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically, 11th ed.; CLSI Standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tichaczek-Goska, D.; Gleńsk, M.; Wojnicz, D. The Enhancement of the Photodynamic Therapy and Ciprofloxacin Activity against Uropathogenic Escherichia coli Strains by Polypodium vulgare Rhizome Aqueous Extract. Pathogens 2021, 10, 1544. https://doi.org/10.3390/pathogens10121544
Tichaczek-Goska D, Gleńsk M, Wojnicz D. The Enhancement of the Photodynamic Therapy and Ciprofloxacin Activity against Uropathogenic Escherichia coli Strains by Polypodium vulgare Rhizome Aqueous Extract. Pathogens. 2021; 10(12):1544. https://doi.org/10.3390/pathogens10121544
Chicago/Turabian StyleTichaczek-Goska, Dorota, Michał Gleńsk, and Dorota Wojnicz. 2021. "The Enhancement of the Photodynamic Therapy and Ciprofloxacin Activity against Uropathogenic Escherichia coli Strains by Polypodium vulgare Rhizome Aqueous Extract" Pathogens 10, no. 12: 1544. https://doi.org/10.3390/pathogens10121544
APA StyleTichaczek-Goska, D., Gleńsk, M., & Wojnicz, D. (2021). The Enhancement of the Photodynamic Therapy and Ciprofloxacin Activity against Uropathogenic Escherichia coli Strains by Polypodium vulgare Rhizome Aqueous Extract. Pathogens, 10(12), 1544. https://doi.org/10.3390/pathogens10121544





