Short- and Long-Term Immunological Responses in Chronic HCV/HIV Co-Infected Compared to HCV Mono-Infected Patients after DAA Therapy
Abstract
:1. Introduction
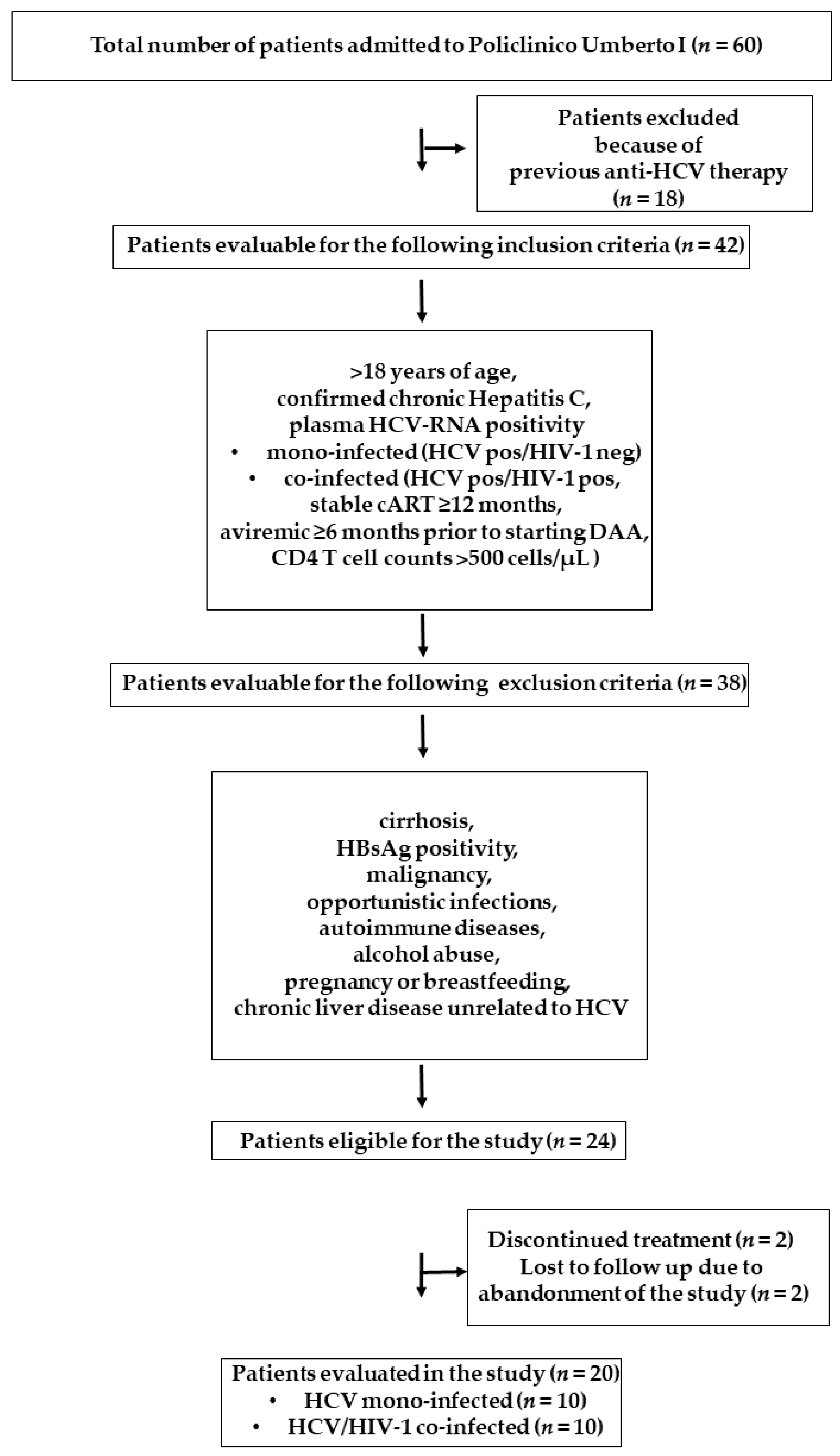
2. Materials and Methods
2.1. Ethics
2.2. Study Design and Sample Collection
2.3. Viral Loads, Clinical Parameters, and Fibroscan Assessment
2.4. Immunophenotypic Analysis
2.5. Quantitative Real-Time Reverse Transcription-PCR and IP-10 Detection
2.6. Measurement of Kynurenine: Tryptophan Ratios
2.7. Statistical Analysis
3. Results
3.1. Study Population Characteristics
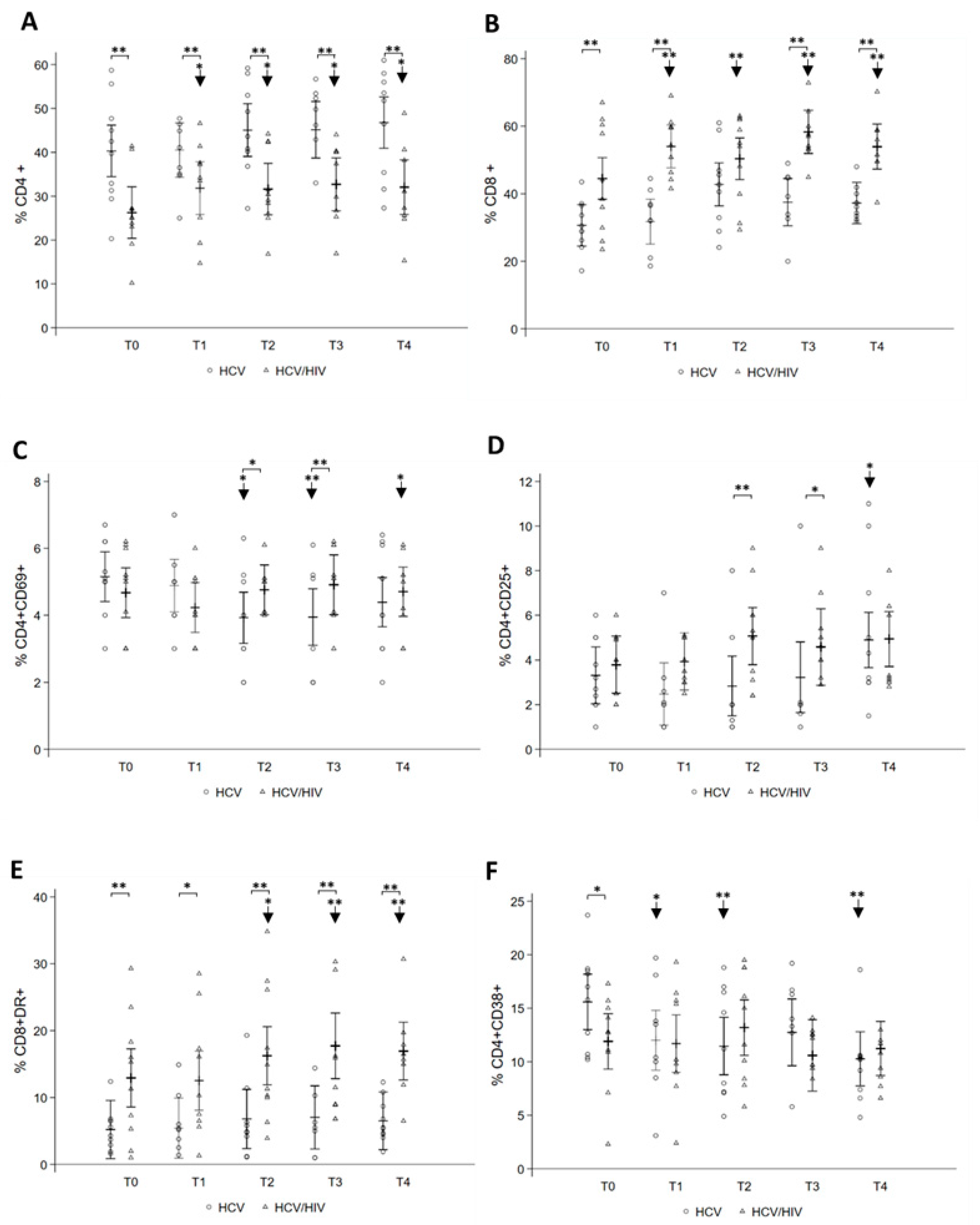
3.2. Longitudinal Changes in Peripheral Immune Phenotype after DAA Treatment
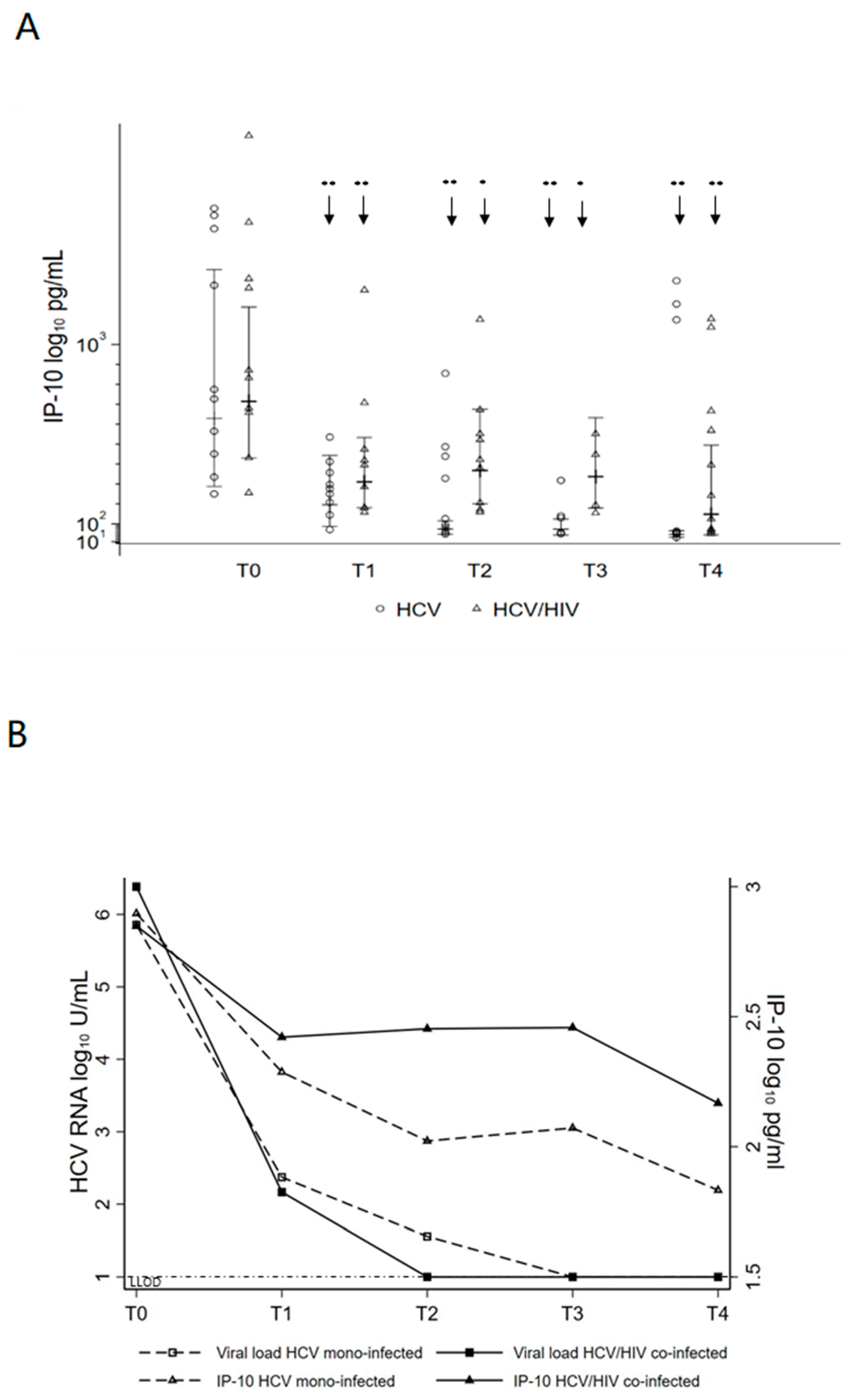
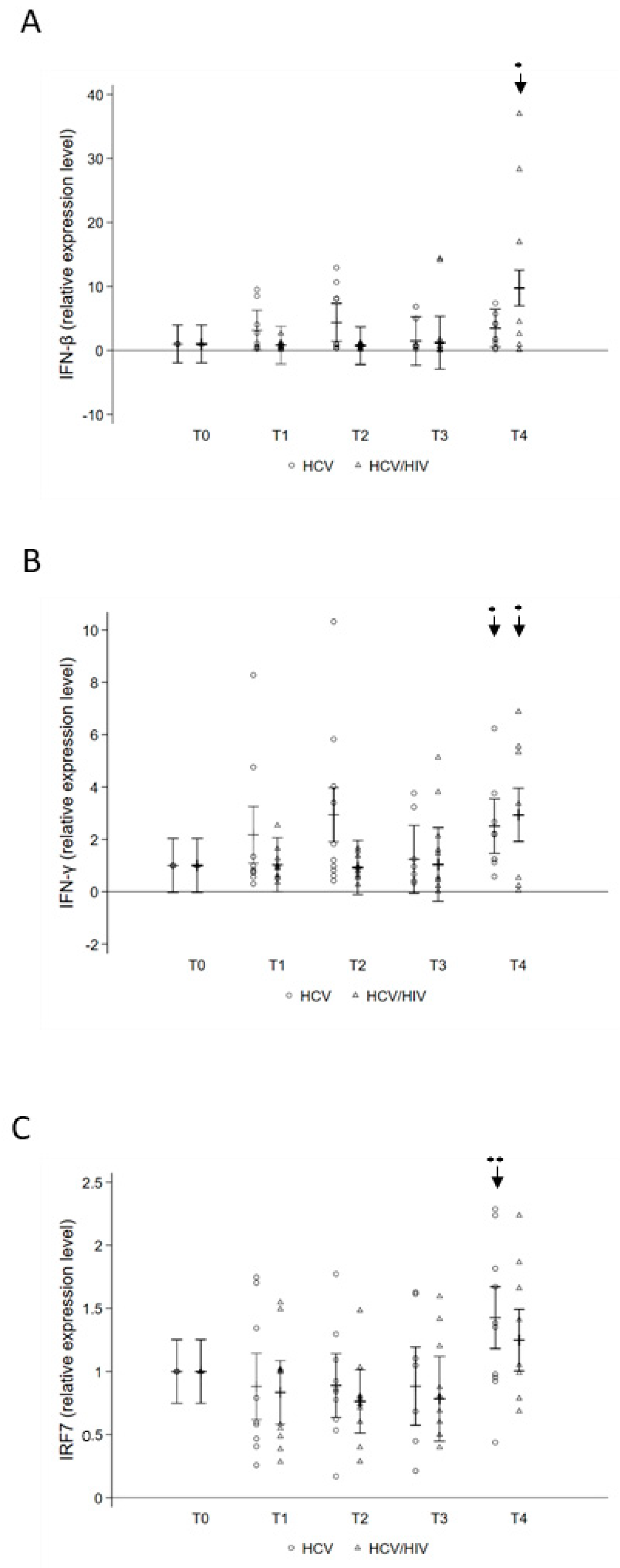
3.3. Changes in Systemic Inflammation after DAA Treatment
3.4. Kynurenine-to-Tryptophan Ratio in HCV Mono- and HCV/HIV Co-Infected Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Hepatitis Report 2017; World Health Organization: Geneva, Swizerland, 2017; Available online: https://apps.who.int/iris/handle/10665/255016 (accessed on 5 November 2021).
- Khatun, M.; Ray, R.B. Mechanisms underlying Hepatitis C Virus-associated hepatic fibrosis. Cells 2019, 8, 1249. [Google Scholar] [CrossRef] [Green Version]
- Peters, L.; Klein, M.B. Epidemiology of hepatitis C virus in HIV-infected patients. Curr. Opin. HIV AIDS 2015, 10, 297–302. [Google Scholar] [CrossRef]
- Debes, J.D.; Bohjanen, P.R.; Boonstra, A. Mechanisms of accelerated liver fibrosis progression during HIV Infection. J. Clin. Transl. Hepatol. 2016, 4, 328–335. [Google Scholar]
- Ganesan, M.; Poluektova, L.Y.; Kharbanda, K.K.; Osna, N.A. Human immunodeficiency virus and hepatotropic virus co-morbidities as the inducers of liver injury progression. World J. Gastroenterol. 2019, 25, 398–410. [Google Scholar] [CrossRef]
- Zeremski, M.; Petrovic, L.M.; Talal, A.H. The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. J. Viral Hepat. 2007, 14, 675–687. [Google Scholar] [CrossRef]
- Chigbu, D.I.; Loonawat, R.; Sehgal, M.; Patel, D.; Jain, P. Hepatitis C Virus Infection: Host-virus interaction and mechanisms of viral persistence. Cells 2019, 8, 376. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, V.D.; Landay, A.L.; Sandberg, J.K. Innate immunity and chronic immune activation in HCV/HIV-1 co-infection. Clin. Immunol. 2010, 135, 12–25. [Google Scholar] [CrossRef]
- Carlton-Smith, C.; Holmes, J.A.; Naggie, S.; Lidofsky, A.; Lauer, G.M.; Kim, A.Y.; Chung, R.T.; of the ACTG A5327 Study Group. IFN-free therapy is associated with restoration of type I IFN response in HIV-1 patients with acute HCV infection who achieve SVR. J. Viral Hepat. 2018, 25, 465–472. [Google Scholar] [CrossRef]
- Coppola, N.; Martini, S.; Pisaturo, M.; Sagnelli, C.; Filippini, P.; Sagnelli, E. Treatment of chronic hepatitis C in patients with HIV/HCV coinfection. World J. Virol. 2015, 4, 1–12. [Google Scholar] [CrossRef]
- Lau, G.; Benhamou, Y.; Chen, G.; Li, J.; Shao, Q.; Ji, D.; Li, F.; Li, B.; Liu, J.; Hou, J.; et al. Efficacy and safety of 3-week response-guided triple direct-acting antiviral therapy for chronic hepatitis C infection: A phase 2, open-label, proof-of-concept study. Lancet Gastroenterol. Hepatol. 2016, 1, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Sikavi, C.; Chen, P.H.; Lee, A.D.; Saab, E.G.; Choi, G.; Saab, S. Hepatitis C and human immunodeficiency virus coinfection in the era of direct-acting antiviral agents: No longer a difficult-to-treat population. Hepatology 2018, 67, 847–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, L.F.; Chan, A.; Zheng, J.; Chow, S.C.; Wilder, J.M.; Muir, A.J.; Naggie, S. Direct-Acting Antivirals improve access to care and cure for patients with HIV and chronic HCV infection. Open Forum Infect. Dis. 2017, 5, ofx264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Cortés, L.F.; Trujillo-Rodríguez, M.; Báez-Palomo, A.; Benmarzouk-Hidalgo, O.J.; Dominguez-Molina, B.; Milanés-Guisado, Y.; Espinosa, N.; Viciana, P.; Gutiérrez-Valencia, A. Eradication of Hepatitis C Virus (HCV) reduces immune activation, microbial translocation, and the HIV DNA level in HIV/HCV-coinfected patients. J. Infect. Dis. 2018, 218, 624–632. [Google Scholar] [CrossRef] [Green Version]
- Najafi Fard, S.; Schietroma, I.; Corano Scheri, G.; Giustini, N.; Serafino, S.; Cavallari, E.N.; Pinacchio, C.; De Girolamo, G.; Ceccarelli, G.; Scagnolari, C.; et al. Direct-acting antiviral therapy enhances total CD4+ and CD8+ T-cells responses, but does not alter T-cells activation among HCV mono-infected, and HCV/HIV-1 co-infected patients. Clin. Res. Hepatol. Gastroenterol. 2018, 4, 319–329. [Google Scholar] [CrossRef]
- Emmanuel, B.; El-Kamary, S.S.; Magder, L.S.; Stafford, K.A.; Charurat, M.E.; Poonia, B.; Chairez, C.; McLaughlin, M.; Hadigan, C.; Masur, H.; et al. Immunological recovery in T-cell activation after sustained virologic response among HIV positive and HIV negative chronic Hepatitis C patients. Hepatol. Int. 2019, 13, 270–276. [Google Scholar] [CrossRef]
- Brochado-Kith, Ó.; Martínez, I.; Berenguer, J.; González-García, J.; Salgüero, S.; Sepúlveda-Crespo, D.; Díez, C.; Hontañón, V.; Ibañez-Samaniego, L.; Pérez-Latorre, L.; et al. HCV Cure with Direct-Acting Antivirals Improves Liver and Immunological Markers in HIV/HCV-Coinfected Patients. Front. Immunol. 2021, 12, 723196. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, Y. Viral and host factors associated with outcomes of hepatitis C virus infection. Mol. Med. Rep. 2017, 5, 2909–2924. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef]
- Mehraj, V.; Routy, J.P. Tryptophan catabolism in chronic viral infections: Handling uninvited guests. Int. J. Tryptophan Res. 2015, 8, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Jenabian, M.A.; Mehraj, V.; Costiniuk, C.T.; Vyboh, K.; Kema, I.; Rollet, K.; Ramirez, P.R.; Klein, M.B.; Routy, J.P. Influence of Hepatitis C Virus sustained virological response on immunosuppressive tryptophan catabolism in ART-treated HIV/HCV coinfected patients. J. Acquir. Immune Defic. Syndr. 2016, 71, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Lepiller, Q.; Soulier, E.; Li, Q.; Lambotin, M.; Barths, J.; Fuchs, D.; Stoll-Keller, F.; Liang, T.J.; Barth, H. Antiviral and immunoregulatory effects of indoleamine-2,3-Dioxygenase in hepatitis C virus infection. J. Innate Immun. 2015, 7, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Kardashian, A.; Ma, Y.; Yin, M.T.; Scherzer, R.; Nolan, O.; Aweeka, F.; Tien, P.C.; Price, J.C. High Kynurenine:Tryptophan ratio is associated with liver fibrosis in HIV-monoinfected and HIV/Hepatitis C virus-coinfected women. Open Forum Infect. Dis. 2019, 6, ofz281. [Google Scholar] [CrossRef]
- Capone, A.; Lo Presti, A.; Sernicola, L.; Farcomeni, S.; Ferrantelli, F.; Maggiorella, M.T.; Mee, E.T.; Rose, N.J.; Cella, E.; Ciccozzi, M.; et al. Genetic diversity in the env V1-V2 region of proviral quasispecies from long-term controller MHC-typed cynomolgus macaques infected with SHIVSF162P4cy. J. Gen. Virol. 2018, 99, 1717–1728. [Google Scholar] [CrossRef]
- Sgarbanti, M.; Marsili, G.; Remoli, A.L.; Stellacci, E.; Mai, A.; Rotili, D.; Perrotti, E.; Acchioni, C.; Orsatti, R.; Iraci, N.; et al. IκB kinase ε targets interferon regulatory factor 1 in activated T lymphocytes. Mol. Cell. Biol. 2014, 34, 1054–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neesgaard, B.; Ruhwald, M.; Weis, N. Inducible protein-10 as a predictive marker of antiviral hepatitis C treatment: A systematic review. World J. Hepatol. 2017, 9, 677–688. [Google Scholar] [CrossRef]
- Casey, J.L.; Feld, J.J.; MacParland, S.A. Restoration of HCV-specific immune responses with antiviral therapy: A case for DAA treatment in acute HCV infection. Cells 2019, 8, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdomo-Celis, F.; Taborda, N.A.; Rugeles, M.T. CD8 + T-Cell response to HIV infection in the era of antiretroviral therapy. Front. Immunol. 2019, 10, 1896. [Google Scholar] [CrossRef]
- Semmo, N.; Klenerman, P. CD4+ T cell responses in hepatitis C virus infection. World J. Gastroenterol. 2007, 13, 4831–4838. [Google Scholar] [CrossRef]
- Vranjkovic, A.; Deonarine, F.; Kaka, S.; Angel, J.B.; Cooper, C.; Crawley, A.M. Direct-Acting Antiviral treatment of HCV infection does not resolve the dysfunction of circulating CD8 + T-cells in advanced liver disease. Front. Immunol. 2019, 10, 1926. [Google Scholar] [CrossRef] [Green Version]
- Hengst, J.; Falk, C.S.; Schlaphoff, V.; Deterding, K.; Manns, M.P.; Cornberg, M.; Wedemeyer, H. Direct-Acting Antiviral-induced hepatitis C virus clearance does not completely restore the altered cytokine and chemokine milieu in patients with chronic hepatitis C. J. Infect. Dis. 2016, 214, 1965–1974. [Google Scholar] [CrossRef] [Green Version]
- Rial-Crestelo, D.; Sepúlveda, M.A.; González-Gasca, F.J.; Geijo-Martínez, P.; Martínez-Alfaro, E.; Barberá, J.R.; Yzusqui, M.; Casallo, S.; García, M.; Hornero, C.M.; et al. Does fibrosis really regress in HIV/hepatitis C virus co-infected patients after treatment with direct antiviral agents? AIDS 2020, 34, 427–432. [Google Scholar] [CrossRef]
- Nagaoki, Y.; Imamura, M.; Nishida, Y.; Daijo, K.; Teraoka, Y.; Honda, F. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: Comparison with interferon-based therapy. J. Med. Virol. 2019, 91, 650–658. [Google Scholar] [CrossRef]
- Carrat, F.; Fontaine, H.; Dorival, C.; Simony, M.; Diallo, A.; Hezode, C.; for the French ANRS CO22 Hepather Cohort. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: A prospective cohort study. Lancet 2019, 393, 1453–1464. [Google Scholar] [CrossRef]
- Meissner, E.G.; Kohli, A.; Higgins, J.; Lee, Y.J.; Prokunina, O.; Wu, D.; Orr, C.; Masur, H.; Kottilil, S. Rapid changes in peripheral lymphocyte concentrations during interferon- free treatment of chronic hepatitis C virus infection. Hepatol. Commun. 2017, 1, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Smits, M.; Zoldan, K.; Ishaque, N.; Gu, Z.; Jechow, K.; Wieland, D.; Conrad, C.; Eils, R.; Fauvelle, C.; Baumert, T.F.; et al. Follicular T helper cells shape the HCV-specific CD4+ T cell repertoire after virus elimination. J. Clin. Investig. 2020, 130, 998–1009. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, S.; Bhatta, M.; Ward, H.; Romani, S.; Lee, R.; Rosenthal, E.; Osinusi, A.; Kohli, A.; Masur, H.; Kottilil, S.; et al. Multitarget Direct-Acting Antiviral therapy is associated with superior immunologic recovery in patients coinfected with Human Immunodeficiency Virus and Hepatitis C Virus. Hepatol. Commun. 2018, 2, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Auma, A.; Shive, C.; Damjanovska, S.; Kowal, C.; Cohen, D.E.; Bhattacharya, D.; Alston-Smith, B.; Osborne, M.; Kalayjian, R.; Balagopal, A.; et al. T-cell activation is correlated with monocyte activation in HCV/HIV coinfection and declines during HCV Direct-Acting Antiviral Therapy. Open Forum Infect. Dis. 2021, 8, ofab079. [Google Scholar] [CrossRef] [PubMed]
- Kardashian, A.; Peters, M.G.; Tien, P.C.; Price, J.C. The pathogenesis of liver disease in people living with human immunodeficiency virus: The emerging role of the microbiome. Clin. Liver Dis. (Hoboken) 2020, 15, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellor, A.L.; Munn, D.H. Tryptophan catabolism and T-cell tolerance: Immunosuppression by starvation? Immunol. Today 1999, 20, 469–473. [Google Scholar] [CrossRef]


| HCV (n = 10) | HCV/HIV-1 (n = 10) | |
|---|---|---|
| Age a | 52.5 (48–66) | 50.5 (48–60) |
| Sex (male/female) % male | 50% | 90% |
| HCV-RNA (copies × 106/mL) (T0) a | 2.39 (0.069–14.1) | 3.3 (0.39–10.5) |
| HIV-RNA (copies × 106/mL) (T0) | ND | <37 |
| ALT level, IU/L a | T0 [65.6 (39–154)] T4 [18.5 (13–23)] (p = 0.0020) † | T0 [83 (25–383)] T4 [20 (8–45)] (p = 0.0059) † |
| AST level, IU/L a | T0 [48 (34–117)] T4 [16 (9–22)] (p = 0.0020) † | T0 [68.5 (16–221)] T4 [20 (15–65)] (p = 0.0195) † |
| GGT level, IU/L a | T0 [67 (16–442)] T4 [19 (11–48)] (p = 0.0039) † | T0 [75 (31–209)] T4 [34.5 (20–119)] (p = 0.1055) ‡ |
| Liver stiffness (kPa) a | T0 [10.1 (4.6–12.5)] T4 [5.4 (2.7–9.9)] (p = 0.0050) † | T0 [10 (4–14)] T4 [7 (4–10)] (p = 0.0130) † |
| APRI score | T0 [0.7 (0.5–1.1)] T4 [0.2 (0.1–0.3)] (p = 0.0020) † | T0 [0.7 (0.5–1.4)] T4 [0.3 (0.2–0.3)] (p = 0.0547) ‡ |
| FIB-4 index | T0 [1.8 (1.7–2.4)] T4 [1.1 (0.7–1.4)] (p = 0.0020) † | T0 [1.9 (1.3–2.3)] T4 [1.3 (1.2–1.5)] (p = 0.1641) † |
| Genotype 1/others (%) | 50% | 60% |
| Genotype 2/others (%) | 20% | 20% |
| Genotype 3/others (%) | 30% | 20% |
| Sofosbuvir + Daclatasvir | 5 | 6 |
| Sofosbuvir + Ledispavir | 5 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farcomeni, S.; Moretti, S.; Fimiani, C.; Sulekova, L.F.; Vescio, F.; Sernicola, L.; Maggiorella, M.T.; Remoli, A.L.; Picconi, O.; Mosca, L.; et al. Short- and Long-Term Immunological Responses in Chronic HCV/HIV Co-Infected Compared to HCV Mono-Infected Patients after DAA Therapy. Pathogens 2021, 10, 1488. https://doi.org/10.3390/pathogens10111488
Farcomeni S, Moretti S, Fimiani C, Sulekova LF, Vescio F, Sernicola L, Maggiorella MT, Remoli AL, Picconi O, Mosca L, et al. Short- and Long-Term Immunological Responses in Chronic HCV/HIV Co-Infected Compared to HCV Mono-Infected Patients after DAA Therapy. Pathogens. 2021; 10(11):1488. https://doi.org/10.3390/pathogens10111488
Chicago/Turabian StyleFarcomeni, Stefania, Sonia Moretti, Caterina Fimiani, Lucia Fontanelli Sulekova, Fenicia Vescio, Leonardo Sernicola, Maria T. Maggiorella, Anna Lisa Remoli, Orietta Picconi, Luciana Mosca, and et al. 2021. "Short- and Long-Term Immunological Responses in Chronic HCV/HIV Co-Infected Compared to HCV Mono-Infected Patients after DAA Therapy" Pathogens 10, no. 11: 1488. https://doi.org/10.3390/pathogens10111488
APA StyleFarcomeni, S., Moretti, S., Fimiani, C., Sulekova, L. F., Vescio, F., Sernicola, L., Maggiorella, M. T., Remoli, A. L., Picconi, O., Mosca, L., Esvan, R., Biliotti, E., Ciccozzi, M., Sgarbanti, M., Taliani, G., & Borsetti, A. (2021). Short- and Long-Term Immunological Responses in Chronic HCV/HIV Co-Infected Compared to HCV Mono-Infected Patients after DAA Therapy. Pathogens, 10(11), 1488. https://doi.org/10.3390/pathogens10111488










