Diagnostic Findings in a Confirmed Outbreak of Brucella ovis Infection in a Traditional Sheep Farm in Sicily (South-Italy)
Abstract
:1. Introduction
2. Results
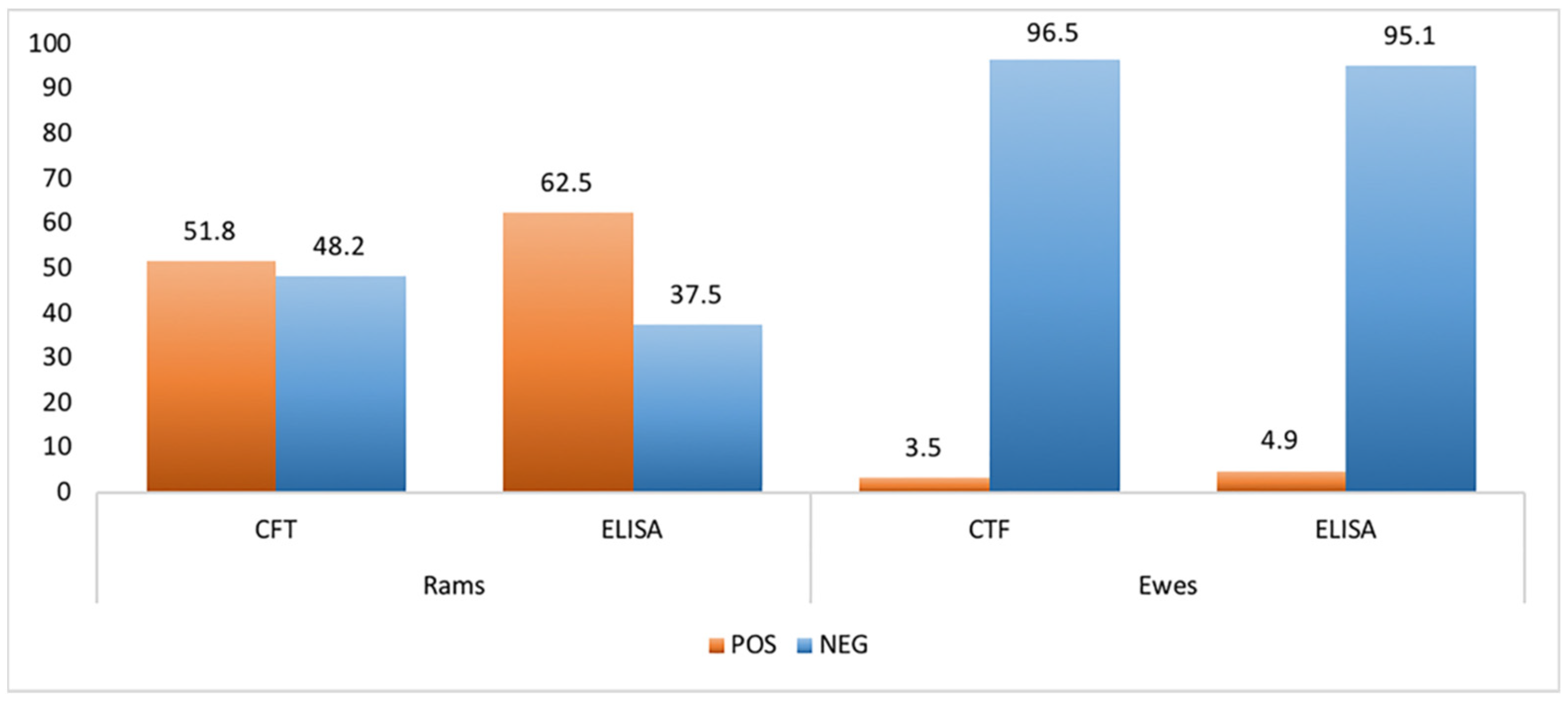
2.1. Serological Analysis
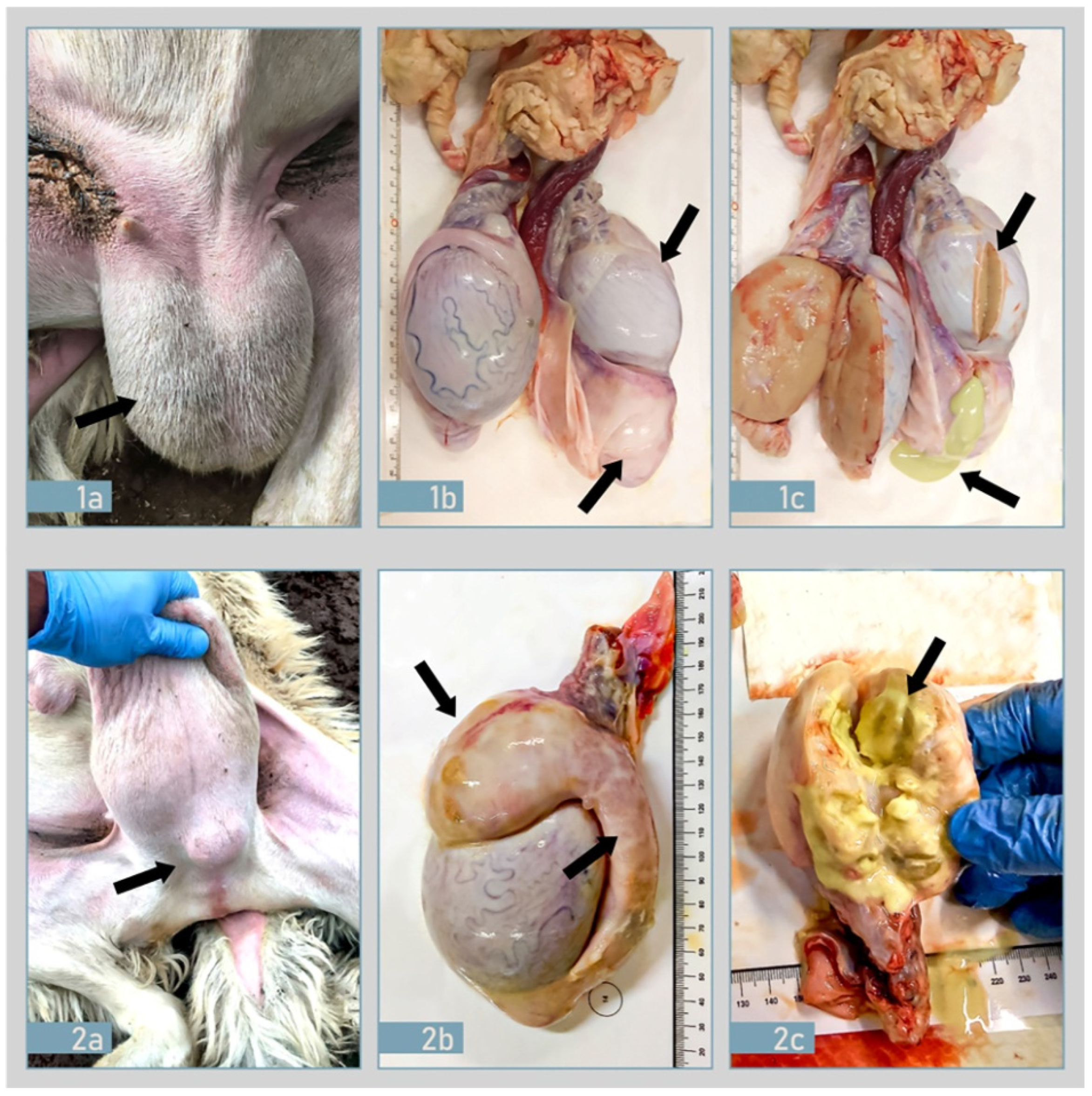
2.2. Ante-Mortem and Post-Mortem Examination
2.3. Histopathological and Immunohistochemical Findings
2.4. Molecular Findings
2.5. Microbiological Findings
3. Discussion
4. Materials and Methods
4.1. Anamnestic Data
4.2. Serological Analysis
4.3. Molecular Analysis
4.4. Clinical Examination and Sampling
4.5. Histology-Hematoxylin & Eosin Staining
4.6. Immunohistochemistry (IHC)
4.7. Bacteriological Analysis and B. ovis Identification
4.8. Brucella spp. Typing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olsen, S.C.; Palmer, M.V. Advancement of knowledge of Brucella over the past 50 years. Vet. Pathol. 2014, 51, 1076–1089. [Google Scholar] [CrossRef]
- Blasco, J.M. Brucella ovis. In Animal Brucellosis; Nielsen, K., Duncan, J.R., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 351–378. [Google Scholar]
- Blasco, J.M. Brucella ovis infection. In Infectious and Parasitic Diseases of Livestock; Lefeòvre, P.C., Blancou, J., Chermette, R., Uilenberg, G., Eds.; Lavoisier: Paris, France, 2010; Volume 2, pp. 1047–1063. [Google Scholar]
- Marco, J.; Gonzalez, L.; Cuervo, L.; de Heredia, F.B.; Barberan, M.; Marin, C.; Blasco, J. Brucella ovis infection in two flocks of sheep. Vet. Rec. 1994, 135, 254–256. [Google Scholar] [CrossRef]
- Sergeant, E.S. Seroprevalence of Brucella ovis infection in commercial ram flocks in the Tamworth area. N. Z. Vet. J. 1994, 42, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Praud, A.; Champion, J.L.; Corde, Y.; Drapeau, A.; Meyer, L.; Garin-Bastuji, B. Assessment of the diagnostic sensitivity and specificity of an indirect ELISA kit for the diagnosis of Brucella ovis infection in rams. BMC Vet. Res. 2012, 8, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commission Delegated Regulation (EU). 2018/1629 of 25 July 2018 Amending the List of Diseases Set Out in Annex II to Regulation (EU) 2016/429 of the European Parliament and of the Council on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (Animal Health Law); Commission Delegated Regulation (EU): Brussels, Belgium, 2018. [Google Scholar]
- OIE (World Organization for Animal Health). Infection with Brucella ovis. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE (World Organization for Animal Health): Paris, France, 2016. [Google Scholar]
- OIE (World Organisation for Animal Health). Ovine epididymitis (Brucella ovis) in Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2018. [Google Scholar]
- Costa, L.F.; Pessoa, M.S.; Guimarães, L.B.; Faria, A.K.; Morão, R.P.; Mol, J.P.; Garcia, L.N.; Almeida, A.C.; Gouveia, A.M.; Silva, M.X.; et al. Serologic and molecular evidence of Brucella ovis infection in ovine and caprine flocks in the State of Minas Gerais, Brazil. BMC Res. Notes. 2016, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Hinić, V.; Brodard, I.; Thomann, A.; Cvetnić, Z.; Makaya, P.V.; Frey, J.; Abril, C. Novel identification and differentiation of Brucella melitensis, B. abortus, B. suis, B. ovis, B. canis, and B. neotomae suitable for both conventional and real-time PCR systems. J. Microbiol. Methods 2008, 75, 375–378. [Google Scholar] [CrossRef]
- De Massis, F.; Zilli, K.; Di Donato, G.; Nuvoloni, R.; Pelini, S.; Sacchini, L.; D’Alterio, N.; Di Giannatale, E. Distribution of Brucella field strains isolated from livestock, wildlife populations, and humans in Italy from 2007 to 2015. PLoS ONE. 2019, 14, e0213689. [Google Scholar] [CrossRef] [PubMed]
- Buckrell, B.C.; McEwen, S.A.; Johnson, W.H.; Savage, N.C. Epididymitis Caused by Brucella ovis in a Southern Ontario Sheep Flock. Can. Vet. J. 1985, 26, 293–296. [Google Scholar] [PubMed]
- Cvetnić, Ž.; Zdelar-Tuk, M.; Duvnjak, S.; Benić, M.; Mihaljević, Ž.; Habrun, B.; Reil, I.; Cvetnić, M.; Spicic, S. Infectious epididymitis caused by Brucella ovis in Croatian sheep flocks. Turk. J. Vet. Anim. Sci. 2017, 41, 679–685. [Google Scholar] [CrossRef]
- Farina, R.; Cerri, D.; Andreani, A.; Renzoni, G.; Guadagnini, P.F.; Lombardi, G. Epididymitis in rams: First report of the presence of Brucella ovis in Italy. Sel. Vet. 1995, 36, 285–291. [Google Scholar]
- Chiarenza, G.; Villari, S.; Galluzzo, P.; Briganò, S.; Alfano, M.; Tagliarini, A.; Pilato, V.; Guercio, A.; Stancanelli, A. Brucella ovis presence in sicilian farms (Italy). Int. J. Infect. Dis. 2018, 73, 385–386. [Google Scholar] [CrossRef]
- Mancuso, G.; Scornaienchi, D.; Pecora, M.; Lucifora, G. Brucella ovis: Indagine sierologica negli allevamenti della provincia di Cosenza. Dati preliminari. Large Anim. Rev. 2004, 10, 4. [Google Scholar]
- Elderbrook, M.; Schumaker, B.; Cornish, T.; Peck, D.; Sondgeroth, K. Seroprevalence and risk factors of Brucella ovis in domestic sheep in Wyoming, USA. BMC Vet. Res. 2019, 15, 246. [Google Scholar] [CrossRef] [Green Version]
- Van Metre, D.C.; Rao, S.; Kimberling, C.V.; Morley, P.S. Factors associated with failure in breeding soundness examination of Western USA rams. Prev. Vet. Med. 2012, 105, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Bagley, C.V.; Paskett, M.E.; Matthews, N.J.; Stenquist, N.J. Prevalence and causes of ram epididymitis in Utah. J. Am. Vet. Med. Assoc. 1985, 186, 798–801. [Google Scholar]
- Souza, T.S.; Costa, J.N.; Martinez, P.M.; de Lima, C.C.V.; Araújo, B.R.; Costa Nelto, A.O.; Anunciação, A.V.M.; Almeida, M.d.G.A.R.; Pinheiro, R.R. Seroepidemiological survey for Brucella ovis infection in sheep flocks of semi-arid region in Bahia State, Brazil. Vet. Zootec. 2011, 18 (Suppl. 3), 697–700. [Google Scholar]
- Machado, G.; Santos, D.V.; Kohek, I.; Stein, M.C.; Hein, H.E.; Poeta, A.S.; Vidor, A.C.M.; Corbellini, L.G. Seroprevalence of Brucella ovis in rams and associated flock level risk factors in the state of Rio Grande do Sul, Brazil. Prev. Vet. Med. 2015, 121, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Blasco, J.M. Brucelosis ovina. Ovis 2002, 82, 113. [Google Scholar]
- Petrović, M.; Špičić, S.; Potkonjak, A.; Lako, B.; Kostov, M.; Cvetnić, Ž. First evidence of Brucella ovis infection in ram sin the Pirot Municipality, Serbia. Vet. Italy 2014, 50, 259–268. [Google Scholar]
- Picard-Hagen, N.; Berthelot, X.; Champion, J.L.; Eon, L.; Lyazrhi, F.; Marois, M.; Peglion, M.; Schuster, A.; Trouche, C.; Garin-Bastuji, B. Contagious epididymitis due to Brucella ovis: Relationship between sexual function, serology and bacterial shedding in semen. BMC Vet. Res. 2015, 11, 125. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW). Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): Ovine epididymitis (Brucella ovis). EFSA J. 2017, 15, 10. [Google Scholar] [CrossRef]
- Gaffuri, A.; Garbarino, C.; Carrara, M.; Sala, L.; Bassi, S.; Messa, G.L.; Cerri, D. Regional control program for monitoring and eradication of Brucella ovis in Lombardia region( August 1995–December 1998): Consideration and evaluation of problems faund in its application. Atti. Fe. Me. S. P. Rum. 1999, VII, 99–103. [Google Scholar]
- Grilló, M.J.; Marín, C.M.; Barberán, M.; Blasco, J.M. Experimental Brucella ovis infection in pregnant ewes. Vet. Rec. 1999, 144, 555–558. [Google Scholar] [CrossRef]
- Vitale, M.; Migliore, S.; La Giglia, M.; Alberti, P.; Di Marco Lo Presti, V.; Langeveld, J.P. Scrapie incidence and PRNP polymorphisms: Rare small ruminant breeds of Sicily with TSE protecting genetic reservoirs. BMC Vet. Res. 2016, 12, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AQIS (Australian Quarantine and Inspection Service), An Analysis of the Disease Risks, Other Than Scrapie, Associated with the Importation of Ovine and Caprine Semen and Embryos from Canada, the USA and Member States of the European Union. Final Report. 2000; 67p. Available online: http://www.agriculture.gov.au/SiteCollectionDocuments/ba/memos/2000/animal/00-038b.pdf (accessed on 9 September 2021).
- Morgan, W.J.B.; McCullough, N.B. Genus Brucella Meyer and Shaw. In Bergey’s Manual of Determinative Bacteriology, 8th ed.; Buchanan, R.E., Gibbons, N.E., Eds.; Williams & Wilkins: Baltimore, MA, USA, 1974; pp. 278–282. [Google Scholar]
- Bricker, B.J.; Halling, S.M. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J. Clin. Microbiol. 1994, 32, 2660–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bricker, B.J.; Hallings, S.M. Enhancement of the Brucella AMOS PCR assay for the differentiation of Brucella abortus vaccine strains S19 and RB51. J. Clin. Microbiol. 1995, 33, 1640–1642. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galluzzo, P.; Migliore, S.; Cascio, S.; Barreca, S.; Alfano, M.; Tagliarini, A.; Candela, A.; Piraino, C.; Galuppo, L.; Condorelli, L.; et al. Diagnostic Findings in a Confirmed Outbreak of Brucella ovis Infection in a Traditional Sheep Farm in Sicily (South-Italy). Pathogens 2021, 10, 1472. https://doi.org/10.3390/pathogens10111472
Galluzzo P, Migliore S, Cascio S, Barreca S, Alfano M, Tagliarini A, Candela A, Piraino C, Galuppo L, Condorelli L, et al. Diagnostic Findings in a Confirmed Outbreak of Brucella ovis Infection in a Traditional Sheep Farm in Sicily (South-Italy). Pathogens. 2021; 10(11):1472. https://doi.org/10.3390/pathogens10111472
Chicago/Turabian StyleGalluzzo, Paola, Sergio Migliore, Silvana Cascio, Santino Barreca, Marilena Alfano, Antonina Tagliarini, Anna Candela, Chiara Piraino, Lucia Galuppo, Lucia Condorelli, and et al. 2021. "Diagnostic Findings in a Confirmed Outbreak of Brucella ovis Infection in a Traditional Sheep Farm in Sicily (South-Italy)" Pathogens 10, no. 11: 1472. https://doi.org/10.3390/pathogens10111472
APA StyleGalluzzo, P., Migliore, S., Cascio, S., Barreca, S., Alfano, M., Tagliarini, A., Candela, A., Piraino, C., Galuppo, L., Condorelli, L., Hussein, H. A., Tittarelli, M., & Chiarenza, G. (2021). Diagnostic Findings in a Confirmed Outbreak of Brucella ovis Infection in a Traditional Sheep Farm in Sicily (South-Italy). Pathogens, 10(11), 1472. https://doi.org/10.3390/pathogens10111472





