Abstract
Trypanosomatids are diverse and can infect several host species, including small mammals (rodents and marsupials). Between 2012 and 2014, 91 small mammals were surveyed for trypanosomatid infection in the Estação Biológica FIOCRUZ Mata Atlântica (EFMA), an Atlantic Forest area in Rio de Janeiro that presents different levels of conserved and degraded areas. Blood, skin, liver, and spleen samples were submitted to parasitological, serological, and molecular assays to detect the infection and determine the taxonomic status of their parasites. Sixty-eight individuals (74.7%; n = 91) were infected by trypanosomatids, including fourteen mixed infected by different trypanosomatid parasites. These hosts were infected by: T. cruzi DTU TcI (n = 12), T. cruzi DTU TcIV (n = 2), T. janseni (n = 15), T. dionisii (n = 1), and T. rangeli A (n = 1) detected in blood or tissue cultures, in addition to T. cruzi DTU TcI (n = 9) and Leishmania sp. (n = 1) only by the molecular diagnosis. Serological diagnosis was positive in 38 (71.6%) individuals for T. cruzi, the same amount for Leishmania spp., and 23 (43.3%) individuals were mixed infected. These data indicate a remarkable richness of trypanosomatid species/genotypes infecting small mammals, even in a disturbed area with low mammal species diversity—as is the case of the EFMA—reinforcing the generalist aspect of these parasites.
1. Introduction
The Trypanosomatidae family (Protozoa: Trypanosomatida) comprises parasites from plants, invertebrates, and vertebrate animals that, according to their life cycles, can be classified as monoxenic or heteroxenic [,]. At least twenty-four genera are recognized within this family, Refs. [,,] with the genera Trypanosoma and Leishmania being the most studied because of their medical and veterinary importance []. For example, the more than twenty species of Leishmania described as responsible for different clinical forms of human leishmaniasis []; Trypanosoma evansi [], which is the causative agent of an equine disease called “mal-de-cadeiras” or “surra”; and Trypanosoma cruzi, the causative agent of Chagas disease, a heterogeneous parasite that can be classified into seven discrete typing units (DTUs): TcI-TcVI and Tcbat [,]. More than twenty Leishmania species described as responsible for different clinical forms of human leishmaniasis []; Trypanosoma evansi [], which is the causative agent of an equine disease called “mal-de-cadeiras” or “surra”; Trypanosoma cruzi, the causative agent of Chagas disease, a heterogeneous parasite that can be classified into seven discrete typing units (DTUs): TcI-TcVI and Tcbat [,].
Rodents and marsupials are some of the hosts that can be involved in the transmission cycle of several trypanosomatid species. They have an important role in the maintenance of these parasites in the wild environment, acting as hosts and, in some scenarios, as reservoirs []. Rodents are the most diverse of all mammalian groups worldwide, and in South America, the subfamily Sigmodontinae encompasses 56% of rodent species []. Reports of trypanosomatid infections in rodents are extensive and diverse [,], and probably related to the different types of environments in which they explore, such as forests, open fields, grasslands, and both rural and urban areas. Indeed, reports of infections by different Leishmania species, by distinct DTUs of T. cruzi, and by other Trypanosoma species have been described in several rodent species [,,,].
Marsupials are known to be some of the most ancient hosts of trypanosomatid parasites in the Americas. Apart from the Leishmania species and T. cruzi, they were recently described to be infected by other Trypanosoma species, such as Trypanosoma dionisii, Trypanosoma cascavelli, and Trypanosoma lainsoni, previously associated with other vertebrate hosts: respectively, bats, snakes, and rodents []. In addition, new Trypanosoma species and/or genotypes have also been described in these hosts, such as Trypanosoma janseni and Trypanosoma sp. DID, as was named this recently described taxonomic unit. This indicates that, although marsupials are the most commonly studied hosts, unknown parasites are still quite often described for this group [,,]. Among marsupials, the common opossums of the genus Didelphis stand out as potential reservoirs for different Trypanosoma and Leishmania species, in addition to being considered bioaccumulators of T. cruzi DTU TcI, supporting their role as reservoirs [].
Distinct parasitological, molecular, and serological assays are employed to diagnose trypanosomatid infection in their hosts [,]. Parasitological diagnoses are the only method that can indicate the presence of trypanosomatids in tissues, i.e., the potential of a host to be a source of infection for vectors. In addition, cultures are the only tool that allows the isolation and morphological description of these parasites []. Molecular assays are sensible and specific, especially when using conserved molecular targets that, once genomically sequenced, are able to identify parasite species and even subpopulations that do not grow in culture media [,,]. Serological diagnoses are very sensitive, but have limited specificity and are dependent on the availability of positive and negative controls for reactions and conjugates specific to the investigated mammalian species [,]. The association of these different diagnostic methods is necessary to identify hosts and to define their putative role in the transmission of such parasites [].
The Atlantic Forest is one of the most diverse Brazilian biomes, even though it is also the most degraded due to anthropic actions. Its territorial extension originally covered the entire Brazilian coast and, currently, only 11% to 16% of the original forests remain, most of them restricted to governmental protected areas [,]. One of these environmental conservation units is the Parque Estadual da Pedra Branca (PEPB: Pedra Branca State Park) located at the Pedra Branca Massif, which is the largest urban forest in the Americas encompassing an area of 12,491.72 hectares in the West Zone of the municipality of Rio de Janeiro []. For this reason, several initiatives were proposed, aiming to mitigate the effects of human occupation in this environment, such as the implementation of a biological station named Estação Biológica FIOCRUZ Mata Atlântica (EFMA: Fiocruz Atlantic Forest Biological Station). The EFMA is a part of the campus FIOCRUZ Mata Atlântica (CFMA—FIOCRUZ Atlantic Forest Campus), and is currently an environmentally protected area surrounded by low-income communities [,,]. In this area, several scientific research projects have been developed, including the monitoring of fauna [] and its parasites [,].
In EFMA, infections by trypanosomatids were described in different hosts, such as bats, dogs, marsupials, and humans [,,,]. Remarkably, two new Trypanosoma species were described in this area—T. janseni and Trypanosoma caninum, [,]—showing that this area, although relatively small, may still present unknown trypanosomatid diversity. In this study, we evaluated trypanosomatid infections in rodents and marsupials collected in areas from EFMA with different habitat characteristics according to the level of anthropic influence. Infections were detected, employing parasitological, molecular, and serological assays, and parasites were identified by DNA sequence analysis.
2. Results
2.1. Small Mammals and Their Sampling Areas
The species Didelphis aurita (Wied-Neuwied, 1826) widely prevailed in the study area (n = 70), followed by Akodon cursor (Winge, 1887) (n = 7), Rattus rattus (Linnaeus, 1758) (n = 7), Marmosa paraguayana (Tate, 1931) (n = 4), Oligoryzomys nigripes (Olfers, 1918) (n = 2), Monodelphis americana (Müller, 1776) (n = 1), and Metachirus myosurus (Temminck, 1824) (n = 1). The most captured species, D. aurita, was collected in all expeditions: 19 in July 2012, 11 in November 2012, 9 in April 2013, 15 in July 2013, 15 in November 2013, and 5 in April 2014, including the 4 recaptures. A significantly larger number of small mammals captured was observed in peridomicile area A1 (n = 51) than in the other areas; namely, transition area A2 (n = 32) and preserved forest area A3 (n = 11) (χ2 = 12.372, p = 1.2607E-05, df = 2).
2.2. Infection Rates of Trypanosomatids
Despite the differences observed in the number of collected individuals, we did not observe a significant difference in trypanosomatid prevalence among the different environments: A1 (36/50, 72%, confidence interval: 57.5–83.7), A2 (23/30, 76.7%, CI: 57.7–90.1), and A3 (11/9, 81.8%, CI: 48.2–97.7) (χ2 = 0.07819, p = 0.96166, df = 2) (Table 1). Seventy-five specimens of marsupials and sixteen specimens of rodents collected were analyzed for trypanosomatids, totaling ninety-one individuals. Considering all of the host species, the total trypanosomatid prevalence was 74.7% (CI: 64.5–83.3). Trypanosomatid prevalence was similar for marsupials (76%, CI: 64.7–85.1) and rodents (68.7%, CI: 41.3–88.9), without significant difference (χ2 = 0.054569, p = 0.8153, df = 1). No significant difference was observed in trypanosomatid prevalence between male (73.6%, CI: 59.7–84.7) and female (76.3%, CI: 59.8–88.5) hosts (χ2 = 0.01261, p = 0.91059, df = 1).

Table 1.
Rodents and marsupials captured in three environments (peridomicile—A1, transition—A2, and preserved forest—A3) at EFMA, Rio de Janeiro (RJ), Brazil, between 2012 and 2014, and their infection rates by trypanosomatids.
Seventy-four percent (74.3%; 52/70) of the captured D. aurita were infected by any trypanosomatid parasite, detected by at least one of the employed diagnostic tests (Table 1). Four of seven specimens of A. cursor were found to be infected by trypanosomatids. All specimens of R. rattus, M. myosurus, and M. paraguayana analyzed were infected, and none of the O. nigripes were infected with trypanosomatids (Table 1). M. americana was not surveyed for trypanosomatid infection because it was found dead in the trap. No significant difference was observed in trypanosomatid prevalence for D. aurita between males (70.4%, CI: 54.8–83.2) and females (80.8%, CI: 60.6–93.4) (χ2 = 0.13239, p = 0.71596, df = 1) or between young (75%, CI: 58.8–87.3) and adult (73.3%, CI: 54.1–87.7) individuals (χ2 = 0.0036831, p = 0.95161, df = 1).
2.3. Parasitological and Molecular Diagnosis
Eighty-five individuals were tested by fresh blood examination; of these, four (4.7%), all D. aurita, were positive for the presence of flagellates, two from A1: LBCE 17667 and 18228, and two from A2: LBCE 17674 and 18209. Two of them were also positive in other diagnostic tests: LBCE 18228 and 18209 were positive in serology for both T. cruzi and Leishmania sp., while the latter was also positive in hemoculture that was characterized as T. cruzi, DTU TcIV.
Twenty-five individuals (27.8%; n = 90) were positive in hemocultures: twenty-three D. aurita, one M. paraguayana, and one A. cursor. Of these, ten hemocultures were cryopreserved, and fifteen were characterized as employing culture sediments. The following parasites were characterized using the 18S rDNA molecular target: T. cruzi DTU TcI (n = 12), T. cruzi DTU TcIV (n = 2), T. janseni (n = 9), T. dionisii (n = 1), and T. rangeli lineage A (n = 1) (Table 2).

Table 2.
Small mammal (rodents and marsupials) infection by Trypanosomatids in parasitological and molecular assays at EFMA, Rio de Janeiro (RJ), Brazil, between 2012 and 2014.
Two hundred and five samples of tissue fragments of spleen (n = 61), liver (n = 61), and skin (n = 83) were examined by culture, and six of them (2.9%) were positive: five from spleen and one from liver. These tissues were derived from five individuals, because one individual of the species D. aurita was also positive in the liver, in addition to the spleen. All of these tissue samples were characterized as T. janseni using the 18S rDNA molecular target, and all of them were cryopreserved, except for one spleen sample that was characterized as employing the culture sediment (Table 2).
Concerning Leishmania sp. infection, only one spleen fragment derived from A. cursor (LBCE 18231) was positive in kDNA-PCR, but Leishmania species identification was not achieved because HSP70 (234)-PCR was negative.
The 18S molecular target was also tested directly on DNA extracted from host tissues, and eighteen of them were positive: nine spleen, six skin, and three liver samples. These positive tissues were derived from twelve individuals, six of whom presented at least two positive tissues, with the spleen always associated with another tissue: skin in M. myosurus (n = 1), D. aurita (n = 1), M. paraguayana (n = 2), A. cursor (n = 1), and liver in another individual from M. paraguayana (n = 1) (Table 2).
From these eighteen samples, nine were successfully characterized at the species level, all as T. cruzi DTU TcI, two of them employing the 24S molecular target, and the other nine samples that could not be characterized at the species level were defined as infected by Trypanosomatidae (Table 2).
2.4. Phylogenetic Analysis of Trypanosomatids Characterized at the Species Level
For the construction of the phylogenetic tree and analysis of the genetic distance between trypanosomatids characterized in this study, fourteen representative sequences of the thirty-nine samples sequenced at the species level were used: T. cruzi DTU TcI (6), T. cruzi DTU TcIV (2), T. dionisii (1), T. rangeli (1), and T. janseni (4) (Figure 1; Table 2).
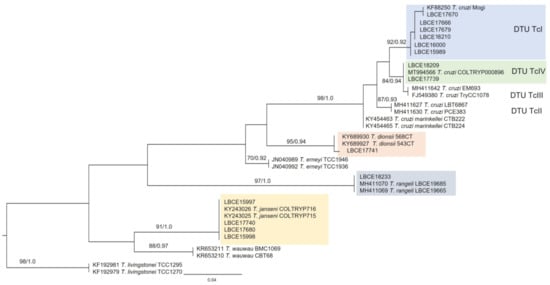
Figure 1.
Phylogenetic analysis of 18S rDNA gene sequences by maximum likelihood (ML) and Bayesian (BI) inference analyses. The analysis indicates the phylogenetic position of trypanosomatids characterized as T. cruzi DTU TcI, T. cruzi DTU TcIV, T. dionisii, T. rangeli, and T. janseni. The maximum likelihood bootstrap values and Bayesian posterior probabilities are shown near the nodes. The numbers in the nodes indicate support per 5000 bootstrap in ML parsing. The scale bar shows the number of nucleotide substitutions per site. Trypanosoma livingstonei was used as an outgroup.
The reference sequences used in the phylogenetic tree construction come from the GenBank database and are presented with their respective accession numbers (Figure 1). These sequences were selected according to the percentage of identity and coverage among the generated gene sequences in this study with the gene sequences from GenBank.
2.5. Serological Diagnosis
Serological diagnosis was performed in 88 individuals, among which 53 (60.2%) were positive. Seropositivity was detected in 38 (71.6%, CI: 32.66–54.18) animals for both T. cruzi and Leishmania sp., of which 23 (43.3%, CI: 29.84–57.72) individuals had mixed infections (Table 3).

Table 3.
T. cruzi and Leishmania spp. infection in small mammals detected by indirect immunofluorescent assay test (IFAT) at EFMA, Rio de Janeiro (RJ), Brazil, between 2012 and 2014.
2.6. Recaptures
During the expeditions, four D. aurita individuals were recaptured, showing different results in the employed diagnostic assays:
- LBCE 17825: captured twice in A2. It was positive in serology for T. cruzi and Leishmania spp., and T. cruzi DTU TcI was isolated from the blood. Four months later, only serological infection was observed, with the same IFAT titers (1/40 T. cruzi; 1/80 Leishmania spp.);
- LBCE 17674: captured twice in A2. In the first capture, it was not positive for any of the diagnostic assays, and showed positivity in the fresh blood examination only in the second capture (nine months later);
- LBCE 18232: captured twice in A1 (three months interval). It was positive in serology, for T. cruzi and Leishmania spp. The IFAT titers were different for Leishmania spp. (1:40 and 1:80), while the IFAT titers for T. cruzi remained the same (1:160);
- LBCE 18255: first captured in A2, and four months later in A1 it was positive in serology for T. cruzi, showing different IFAT titers in the first (1:160) and second capture (1:80), while it was serologically positive for Leishmania spp. only in the first capture (1:40). In the second capture, T. janseni was isolated in the blood culture.
3. Discussion
D. aurita was the most abundant small mammal in the samplings from EFMA, probably because capture expeditions occurred during their reproductive period, described to begin in July/August and to finish in March []. As a consequence, most of the isolated parasites in this study were derived from this species, which certainly influenced the ecological pattern of the local enzooty. The sex and age of these hosts did not seem to significantly influence the prevalence of trypanosomatids. These individuals of D. aurita were found infected and may move among the three sampling areas, highlighting their potential for parasite dispersion [,].
In parasitological diagnoses, four individuals of D. aurita were considered positive in the fresh blood exam, and twenty-five individuals had trypanosomatids isolated by blood cultures: D. aurita (n = 23), M. paraguayana (n = 1), and A. cursor (n = 1). This indicates that they may be competent to be a source of infection for vectors and/or infect other mammals if predated [,]. T. cruzi DTU TcI prevailed (n = 12) and was detected exclusively in D. aurita. T. cruzi DTU TcIV, was detected in individuals of D. aurita and M. paraguayana, and had previously been reported in bat species in the EFMA [], indicating a broad host distribution in this area. Both DTUs were previously described in the Atlantic Forest: T. cruzi TcI is the most ubiquitous genotype, and was found infecting distinct mammalian orders in several areas from the Brazilian Southeastern Atlantic Forest: São Paulo, Espírito Santo, and Rio de Janeiro states. The T. cruzi TcIV genotype is widely reported in other Brazilian biomes and was also detected in Atlantic Forest fragments from Espírito Santo and other locations in Rio de Janeiro state. Moreover, T. cruzi DTUs TcII and TcIII were also previously reported in Atlantic Forest areas [,,,,].
Trypanosoma janseni was first described at EFMA in spleen and liver cultures from an individual of D. aurita: LBCE 17665 []. This parasite has also been detected in other studies in blood clots from D. aurita and in dogs [,]. In this study, this parasite was isolated in spleen cultures (n = 3) and, for the first time, in one hemoculture. These samples come from three individuals of D. aurita, where one of these individuals was infected both in the spleen and the blood. This parasite was also detected in the spleen (n = 1) and blood (n = 8) of nine other individuals of D. aurita, but these cultures were not established. Later, in expeditions conducted in June and November 2017, T. janseni was once more detected in the other four individuals captured close to A3, more precisely above 100 m height (data not shown). These data indicate that this parasite is established in all three sampling environments at EFMA, and that these hosts can be a source of T. janseni infection for its potential (and still unknown) vectors.
T. dionisii is usually associated with bat species, and was previously reported in bats from EFMA []. Recently, this parasite has also been detected in other distinct groups of hosts, such as marsupials and even humans [,]. In this study, we detected T. dionisii in a non-bat species for the first time in EFMA; in this case, D. aurita. This reinforces the idea that this parasite is probably more generalist than previously recognized.
Trypanosoma rangeli has genetic heterogeneity, and can be grouped into five genotypes (A, B, C, D, and E) [,]. In this study, infection by T. rangeli lineage A was detected in blood samples from only one D. aurita, and this can be explained by the low parasitemia that this parasite presents in parasitological diagnoses, as reported by Dario et al. (2021) []. This parasite can be found in several species of mammalian hosts, having a large geographical distribution that has been reported in several locations [,], including other areas of the Atlantic Forest [,,,]. Despite this, this is the first time that lineage A has been reported in the state of Rio de Janeiro, where only lineages D and E were previously reported [].
In the molecular diagnosis directly in tissues, trypanosomatid infections were detected in eighteen tissue samples, with the spleen having the largest number of positive samples, followed by the liver and skin. Of these, nine were characterized as T. cruzi DTU TcI, and the other nine were maintained as Trypanosomatidae because it was not possible to characterize them at the species level. This is probably related to the quality of the amplified DNA and the presence of host DNA in the tissue samples, as these had poor and/or unspecific bands in the agarose gel, even after the two steps of DNA amplification by nested-PCR, hindering the purification and sequencing processes. When sequenced, these samples had electropherograms with very high and/or very low peaks, indicating that the DNA used in these reactions was not viable to generate good sequencing and, consequently, characterize the parasites present in these tissues at the species level. Positivity in 18S PCR added important information because these samples belonged to four individuals who were negative in other diagnostic assays, highlighting the efficiency of the molecular diagnosis in detecting trypanosomatid infections.
Molecular detection and parasite characterization from host tissue samples also allowed the detection of T. cruzi DTU TcI in other hosts in addition to D. aurita, such as A. cursor and M. paraguayana. DTU TcI was the most prevalent parasite subpopulation infecting small mammals at EFMA, and in fact, this DTU is the most common in the wild transmission cycle []. It is important to note that DTU TcI was detected mainly in individuals of D. aurita (and only isolated in this species). This may be related to the fact that the genus Didelphis can present great infectivity potential and high levels of parasitemia, especially when infected by T. cruzi belonging to this DTU [,]. Regarding the phylogenetic analysis of these characterized trypanosomatids, it is possible to observe through their phylogenetic positions that all these species and their related taxonomic units belong to the Trypanosoma cruzi clade.
Serological diagnosis (IFAT) presented the highest number of positive samples (n = 53) in comparison to the other diagnostic methods. According to Roque and Jansen (2014) [], serology indicates host exposure to the parasite at some moment in its lifetime, but is not able to confirm the maintenance of the parasite in the host or its potential to act as a source of infection for vectors. Animals that had a positive diagnosis only by serological assays are considered to have low potential to transmit the parasite to vectors.
The A. cursor spleen sample (LBCE 18231) that was positive in kDNA-PCR was negative in HSP70 (234)-PCR, probably because the kDNA molecular target has more copies (estimated 10–20 thousand mini-circle copies) of genome compared to the other molecular targets, such as the HSP70 molecular target (234), increasing chances of amplification [,,].
Twenty-five individuals were positive in at least two of the diagnostic assays performed. Among them, fourteen were mixed infections with more than one different trypanosomatid species. These infections can modulate the infections detected in the serological diagnosis, where cross reactions can interfere with the titers of detected parasites, and in the parasitological diagnosis; for example, in fresh blood examination, where only parasites with high parasitemia will be able to be detected. This high rate of mixed infections is probably related to their ecological habits (diet, for example), because most of these small mammals are omnivorous and may prey on insects infected by these parasites. In addition, it is worth mentioning that there was a significant difference in prevalence rates considering the different diagnostic methods used, which reinforces the importance of combining the use of these diagnostic assays. In addition to improving the detection of different trypanosomatid species, the etiological agent was confirmed in some infections previously detected only by serology and fresh blood examination.
Trypanosoma cruzi is a paninfective parasite that is able to infect all nucleated mammalian cells in addition to blood. The most common tissue in which it is detected is blood, but other tissues, such as the liver and spleen, can also present parasites, probably in amastigote nests []. Trypanosoma janseni is less known, and was first described in spleen and liver fragments [], but was isolated in blood for the first time in the present study. These parasites, as well as T. rangeli and T. dionisii, share the same mammalian host in the area (sometimes the same individual, as presented), but almost nothing is known concerning the consequences of the mixed infection in the course of infection or in the success to be transmitted. Understanding the influence of mixed infection on parasite fitness in natural conditions is a quite difficult aspect that parasitologists must face.
Among the four individuals of D. aurita that were recaptured, three were positive in the serological diagnosis for T. cruzi and Leishmania spp. in both captures, showing that the antibody levels were maintained over time (up to nine months). Three of them had positive results by the parasitological diagnoses:
- positive in the fresh blood examination in the second capture, probably showing an increase in parasitemia or infection by other trypanosomatid parasites;
- positive blood culture for T. cruzi DTU TcI only in the first capture, with the expected decrease in parasitemia in the late phase of infection, as this parasite was not detected in the second capture; and
- positive blood culture for T. janseni only in the second capture, probably because that host became infected after the first capture. The latter was recaptured after four months in a different area. This result indicates that individuals of D. aurita can move across different areas in the study site. This is not a surprising finding considering that D. aurita commonly covers long distances during its lifetime [,].
This study showed that even in an area that has high levels of human disturbance and low richness of mammalian species, as is the case of EFMA, it was possible to detect a remarkable richness of trypanosomatid species, especially when using different diagnostic methods. In addition, some of the infected small mammals displayed infection patterns (detectable parasitemia) that highlighted their potential to act as reservoirs in space and time. D. aurita, which presented high levels of infection, moved across areas, potentially allowing parasite dispersion. This fact corroborates the nonsignificant difference observed in trypanosomatid prevalence among peridomicile, transition, and preserved forest environments. Furthermore, all rodent species captured are either synanthropic (R. rattus) or opportunistic (A. cursor and O. nigripes), the two latter occurring in several kinds of habitats, including rural and urban areas. The urban expansion that has been occurring in the surroundings of EFMA is also an important factor that directly affects small mammal richness and the transmission of their parasites, especially considering the dwellings and domestic animals present in the area, representing an interface region between urban and sylvatic environments.
In this area of EFMA, D. aurita proved to be an important reservoir for T. cruzi and T. janseni, and presented detectable parasitemia for T. dionisii and T. rangeli, as demonstrated by positive hemocultures. These ancient trypanosomatid hosts can be found near human dwellings and serve as a source of infection for vectors in this area. It was already reported that these animals may present (and eliminate) metacyclic infective forms of T. cruzi in their scent glands, but this trait was not yet observed for other Trypanosoma species. The consumption of opossum meat by the local population has not been reported, which would also represent a potential risk for human infection due to the manipulation of infected blood. Except for T. cruzi, infection by the other Trypanosoma species observed in EFMA was not observed (T. janseni) or is not described as pathogenic for humans (T. rangeli and T. dionisii). Infection by Leishmania sp. was observed in only one rodent species, but it is worth mentioning that there are human and canine cases of Tegumentary Leishmaniasis in the area [,,], demonstrating the risk of occurrence of mixed infections by distinct trypanosomatid parasites (as observed in the local fauna), also in humans. All these factors may favor the establishment of zoonotic transmission involving some of these trypanosomatids, and highlight the importance of long term monitoring of zoonosis in wild and synanthropic reservoirs in the region.
4. Materials and Methods
4.1. Study Area
The study was conducted in EFMA (22°56′25″ S, 43°24′18″ W), which is partially located in PEPB, Rio de Janeiro, Brazil (22°56′23″ S, 43°24′12″ W). EFMA became a biological station administered by Fundação Oswaldo Cruz in 2016, being the first biological station in Rio de Janeiro and the first administered by the Brazilian Ministry of Health, representing a unique potential for research and interdisciplinary actions of environmental health, urban planning, and public policies [,].
EFMA comprises preserved areas with secondary vegetation of Atlantic Forest surrounded by degraded areas partially occupied by low-income communities. The study was performed in three environments, according to distinct levels of human disturbance (Figure 2):
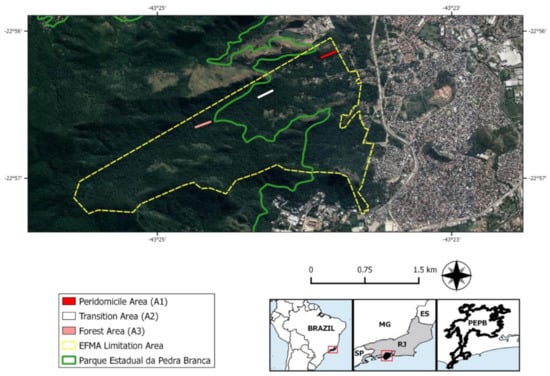
Figure 2.
Map indicating the limits of FIOCRUZ Atlantic Forest Biological Station (EFMA) (yellow surrounded area), partially inserted in Pedra Branca State Park (PEPB) (green surrounded area). The three environments of small mammal captures are marked by colored lines: peridomicile (A1) in red, transition (A2) in white, and preserved forest (A3) in pink. Satellite images of the collection environments were obtained using Terra Incognita™ software, version 2.45. Vector layers of the municipal limit of the Rio de Janeiro state were obtained from Brazilian Institute of Geography and Statistics, the limit of PEPB was obtained from Ministry of Environment, and limits of EFMA areas were assigned by EFMA team.
- peridomicile (A1): representing areas adjacent to human dwellings;
- transition (A2): disturbed forest and reforestation areas between the peridomicile and the preserved forest; and
- preserved forest (A3): the most preserved and distant area from the human dwellings composed of a secondary forest of ombrophilous dense vegetation. See Gentile et al. (2018) [] for a better description of the study areas.
4.2. Small Wild Mammal Capture and Identification
Rodents and marsupials were captured using Sherman™ (Model XLK; 3 × 3.75 × 12 in.; Tallahassee, FL, USA) and Tomahawk™ (Model 201; 16 × 5 × 5 in.; Hazlehurst, WI, USA) live-traps placed on the floor and baited with a mixture of banana, bacon, peanut butter, and oats. Traps were settled in six linear transects, two in each area. Each transect had 20 capture points spaced 10 m apart. The captures occurred in six expeditions conducted in July and November 2012, April, July, and November 2013, and April 2014. The total capture effort was 1200 trap-nights/sampling section (totaling 7200 trap-nights during the six sampling sections). For every captured animal, the trap was identified, placed in a plastic bag, and taken to a field laboratory.
The identification of small mammal species was carried out using external and cranial morphological characters, with the exception of the genus Akodon Meyen, 1833, whose species were identified by their diploid number after karyotyping []. Lactating females, young animals, and specimens that exceeded the limit of the capture license were tagged with earrings and released at their capture points. Some of these individuals were later recaptured. Euthanized small mammals were taxidermized and deposited as voucher specimens in the scientific collection (Coleção de Mamíferos do Museu Nacional) of the Department of Vertebrates at the National Museum of Rio de Janeiro (Museu Nacional, Universidade Federal do Rio de Janeiro) [].
4.3. Field Procedures
Blood samples were obtained through cardiac puncture of the captured small mammals after anesthesia with ketamine hydrochloride (10–30 mg/kg), associated with xylazine (2 mg/kg) for marsupials (1:1) or associated with acepromazine (5–10 mg/kg) for rodents (9:1). The collected blood samples were employed for parasitological analysis (fresh blood examination and hemoculture) and serology.
A drop of approximately 5 µL of blood was placed between the slide and coverslip for fresh blood examination. For hemoculture, approximately 0.6–0.8 mL of blood from each animal was divided into two tubes containing NNN (Nicolle, Novy, and McNeal) and LIT (Liver Infusion Triptose) culture medium []. For serology, blood was centrifuged (4000 G/5 min) to obtain serum, which was stored at −20 °C.
The captured small mammals previously anesthetized were euthanized with the intracardiac use of potassium chloride 19.1% for the collection of fragments of spleen, liver, and skin tissues []. Young and lactant D. aurita and individuals exceeding the limit of the capture license were examined, had blood samples collected, were marked by ear-tags, and were released at their trapping points. When possible (depending on the size of the animal), skin samples, with subsequent suturing, were collected.
The collected tissues were stored in tubes containing:
- sterile saline (sodium chloride-NaCl at 58.44 g/mol), antibiotics, and antifungals (10 mg streptomycin, 25 µL amphotericin B, and 10,000 IU penicillin per mL, Sigma™, St, Louis, MO, USA commercial solution) for culture; and
- absolute ethanol that was stored in a freezer at −20 °C for subsequent molecular diagnosis.
4.4. Parasitological Procedures
Fresh blood examination was performed in the field laboratory by the observation of a drop of blood on microscope slides using optical microscopy (400×). The samples that presented flagellates morphologically compatible with trypanosomatids were considered positive [].
Hemocultures were observed every two weeks for up to five months []. The tissue samples were maintained in saline solution at 4 °C for 24 h and then transferred to culture tubes containing NNN medium and Schneider liquid medium []. The tissue cultures were observed twice a week for one month.
Positive cultures were amplified and cryopreserved in the Trypanosoma Collection of Wild and Domestic Mammals and Vectors (Coleção de Trypanosoma de Mamíferos Silvestres, Domésticos e Vetores -ColTryp). The positive cultures that were not able to grow and amplify in culture medium were centrifuged to obtain the sediments.
4.5. Serological Diagnosis
The serum samples from rodents and marsupials were tested by IFAT (indirect immunofluorescent antibody test) to detect the presence of anti- T. cruzi IgG and anti- Leishmania sp. IgG [] in twofold serial dilutions. Antigens were prepared using a mix of L. braziliensis (IOC/L566; MHOM/BR/1975/M2903) and L. infantum (IOC/L579; MHOM/BR/1974/PP75) or a mix of T. cruzi DTUs TcI (TcI - M000/BR/1974/F; []) and TcII (MHOM/BR/1950/Y; []) for the diagnosis of Leishmania spp. and T. cruzi, respectively. Rodents were tested using a commercial anti-rat IgG conjugated with fluorescein isothiocyanate (Sigma™, St. Louis, MO, USA). Marsupial sera were tested using an intermediate anti-Didelphis sp. IgG that was produced in rabbits and a commercial anti-rabbit IgG conjugated with fluorescein isothiocyanate (Sigma™, St. Louis, MO, USA) []. The adopted cutoff values were 1/40 for marsupials and 1/10 for rodents [].
4.6. Molecular Diagnosis
For the molecular characterization of cultures, positive samples were incubated with proteinase K and sodium dodecyl sulfate (SDS) []. Genomic DNA from these samples was extracted using the standard phenol–chloroform method [].
Spleen, skin, and liver fragments from small mammals were previously rehydrated (washing three times with Milli-Q water) to remove ethanol and subjected to DNA extraction using the commercial Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA) following the manufacturer’s instructions. At the end of the extraction, the DNA was suspended in 100 μL of DNA hydration solution (DNA Rehydration Solution) and stored in a freezer at −20 °C until use []. Experimentally infected and uninfected hamster tissues from previous studies were used as positive and negative controls, respectively.
Four molecular targets were used in tissue samples for the diagnosis of trypanosomatids:
- kDNA target, used to detect Leishmania spp. Infections;
- HSP70 (234) target, employed in Leishmania spp. kDNA-positive samples;
- the 18S rDNA target for the detection of Trypanosomatidae, and for the characterization of all positive culture samples; and
- the 24S rDNA target for 18S-positive samples in which characterization was not possible due to the low quality of the DNA sequences obtained.
For Leishmania spp. diagnosis, DNA amplification was carried out using the commercial PCR pureTaq Beads kit (Illustra PureTaq Ready-To-Go PCR Beads™, GE Healthcare, Chicago, IL, USA), with primers targeting the conserved region of Leishmania sp. kDNA minicircle, with 120 base pairs (bp): forward (5’-GGG(G/T)AGGGGCGTTCT(C/G)CGAA-3′) and reverse (5’-(C/G)(C/G)(C/G)G)(A/T)CTAT(A/T)TTACACCAACCCC-3′), as described by Degrave et al. (1994) []. The reactions were carried out in an Eppendorf Nexus™ Thermocycler (Eppendorf, Stevenage, England), under the following conditions: 94 °C for 5 min, 30 cycles of 94 °C for 1 min, 60 °C for 1 min, and 72 °C for 30 sec; followed by a final extension of 72 °C for 5 min. Electrophoresis was conducted in 8% polyacrylamide mini-gels and revealed by the Silver Stain Plus™ kit (Bio Rad, Hercules, CA, USA), following the manufacturer’s instructions. A molecular weight of 50 bp (DNA Step Ladder) (Promega, Madison, WI, USA) was used. The same tissues from experimentally infected and uninfected hamsters from previous studies were also used as positive and negative controls, respectively, in PCR and electrophoresis.
The HSP70 (234pb) PCR was conducted with the following primers: forward (5’-GGACGAGATCGAGCGCATGGT-3′) and reverse (5’-TCCTTCGACGCCTCCTGGTTG-3′) according to Da Graça et al. (2012) [] with some modifications. These modifications included a concentration of MgCl2 of 2.5 mM instead of 2 mM, the annealing temperature in the cycling reduced from 59 °C to 58 °C for 1 min, and 10 min of final extension instead of 5 min. The samples were amplified in a Veriti™ thermocycler (Applied Biosystems, Waltham, MA, USA). After electrophoresis, the 8% polyacrylamide minigels were also stained with silver nitrate using a specific kit (DNA Silver Staining, GE Healthcare, Chicago, IL, USA). The molecular weight used was also the 50 bp marker (DNA Step Ladder) (Promega, Madison, WI, USA). The positive DNA extraction controls were used as a positive control of the reaction, and the reaction mix was used as a negative control.
Nested-PCR using the molecular target for a partial region of 18S rDNA (approximately 600 bp) consisted of two rounds, and was conducted according to Smith et al. (2008) [] with the following changes:
- a final volume of 25 µL was used, containing 13.5 µL of ultrapure water, 8.5 µL of Go Taq Master Mix (Promega, Madison, WI, USA), and 2 µL of DNA in both rounds;
- in the first round, 0.5 µL of the primers TRY R and F (16 pmol) (Eurofins Genomics™, Val Fleuri, Luxembourg, Luxembourg) were used, and in the second round, 0.5 µL of the primers SSU R and F (16 pmol) (Eurofins Genomics™, Val Fleuri, Luxembourg, Luxembourg) were used; and
- in the cycling condition, the initial denaturation occurred at 95 °C for 15 min, in a Swift™ Max Pro Thermal Cycler 16 thermal cycler (model SWT-MXP-BLC-1).
Positive products derived from 18S PCR were purified with an Illustra™ GFX™ PCR DNA/Gel Band Purification kit (GE Healthcare, Chicago, IL, USA) or Wizard SV Gel kit and Clean-up System PCR kit (Promega, Madison, WI, USA) following the manufacturer’s instructions. The products were then sequenced with the BigDyeTM Terminator v3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Waltham, MA, USA) in an ABI3730 DNA Analyzer Automatic Sequencer (Applied) by the PDTIS/FIOCRUZ Sequencing Platform.
Tissue samples that were positive by 18S nested-PCR but presented very weak bands and/or many unspecific bands were subjected to new DNA extraction using the Qiagen—Dneasy™, (Hilden, North Rhine-Westphalia, Germany) Blood and Tissue Kit following the manufacturer’s instructions. The obtained DNA was then used in novel Nested-PCRs with the following modifications aiming to improve the quality of the amplified material: ≥90.00 ng/µL was applied to 2 µL of DNA/< 90.00 ng/µL, and was applied 5 µL of DNA, 8.5 µL, or 5.5 µL of ultrapure water (depending on the volume of the DNA sample used in the reaction), 12.5 µL of Go Taq Master Mix, and 1.0 µL of primers R and F (TRY/SSU) at 20 pmol, with the following cycling condition: initial denaturation at 94 °C for 3 min, followed by 35 cycles at 94 °C for 30 s, 55 °C for 60 s, and 72 °C for 90 s. The final extension was at 72 °C for 10 min. From these samples, three could be characterized at the species level.
The PCRs targeting the 24S rRNA, which corresponds to the large ribosomal subunit and can amplify from 200 to 300 bp, were used to detect trypanosomatid infections in the nine samples that could not be characterized at the species level by the 18S molecular target. The reactions occurred following Arruda et al. (1990) [] with modifications: 5 µL of DNA, 12.5 µL of GoTaq Master Mix, and 2 µL of primers (Invitrogen™, Waltham, MA, USA) F D75 (5′-GCAGATCTTGGTTGGCGTAG-3′) and R D76 (5′-GGTTCTCTGTTGCCCCTTTT-3′) at 10 pmol and 3.5 µL of ultrapure water. The cycling conditions were as follows: 1 cycle at 94 °C for 15 min, 3 cycles at 94 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min; 3 cycles at 94 °C for 1 min, 58 °C for 1 min, 72 °C for 1 min; 3 cycles at 94 °C for 1 min, 56 °C for 1 min, 72 °C for 1 min; 3 cycles at 94 °C for 1 min, 54 °C for 1 min, 72 °C for 1 min; 35 cycles at 94 °C for 1 min, 52 °C for 1 min, 72 °C for 1 min; 1 cycle at 72 °C for 10 min.
The purification and sequencing of the PCR products from the 24S rRNA molecular target were performed as described for the 18S molecular target. For 18S and 24S molecular targets, the following reaction controls were used: T. cruzi DNA (Y strain) was used as a positive control, and ultrapure water was used as a negative control. Electrophoresis for 18S and 24S molecular targets occurred as follows: the amplified products were applied (5 μL) in a 2% TBE (Tris-Borato-EDTA) agarose gel and stained with GelRed Biotium. The gels were visualized on the Gel Logic 212 Pro photo documenter using the Carestream MISE program, using a molecular weight marker of 100 base pairs (bp) as a reference (Ludwig Biotecnologia, Alvorada, Rio Grande do Sul, Brazil).
4.7. Phylogenetic Analyses
The obtained consensus sequences were manually edited using the SeqMan-DNA Star Program [], and compared for similarity with sequences deposited in the GenBank database from the National Center for Biotechnology Information (NCBI) using the BLAST algorithm (Basic Local Alignment Search Tool). For species identification, the following values were adopted: cover (≥97%), identity (≥97%), and E-value (0.0).
Phylogenetic analyses were performed using maximum likelihood (ML) and Bayesian inference (BI) to confirm the characterization of the trypanosomatid species from the 18S rDNA gene and assess their phylogenetic positions. The BI analysis occurred in MrBayes (Version 3.1.1) [], which is included in TOPALi v.2.5 software. Two runs were performed with 1,000,000 generations, a sample frequency of 10 and a burning of 25%, using the model Hasegawa Kishino Yano + gamma distribution (HKY + G) with rate variation between sites.
The ML analysis was performed using the jModelTest v.2 program, which indicated the SYM+G4 model (Symmetrical Model plus 4 gamma distributed sites) using the Akaike information criterion (AICc Score) []. The construction of the ML tree was performed in the IQ-Tree [,] which is available in PhyloSuite software. To branch support, ultrafast bootstrapping [] of 5000 replications with 1000 maximum interactions and a minimum interaction coefficient of 0.99 was performed. To visualize the ML tree and its bootstrap values, FigTree software was used. The genetic distance was analyzed in the Mega X program, and representative sequences were used for the construction of the phylogenetic tree, among all the sequences obtained in this study.
4.8. Statistical Analysis
Marsupials and rodents that were positive in any of the performed diagnostic assays (parasitological, serological, and/or molecular) were considered infected by trypanosomatids. Characterizations at the species level and/or subpopulation in the infected tissues were obtained by DNA sequence analysis.
Prevalence rates of trypanosomatids were calculated as the proportion of the number of infected animals in relation to the total number of animals analyzed according to Bush et al. (1997) [] for each host species and considering all of the hosts analyzed. For marsupial D. aurita, trypanosomatid prevalence was investigated in relation to host sex and age. Chi-squared contingency tests were performed to evaluate differences in the number of animals captured, and in trypanosomatid prevalence among areas, between rodents and marsupials, male and female hosts, and young and adult hosts. These analyses were carried out using Past software version 3.09 [] considering a significance level of 5%.
4.9. Ethics Statement
Small mammals were captured in accordance with the Normative Instruction of the Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA nº154/2007 Licenses 13373-1 e 19037-1), Chico Mendes Institute for Biodiversity and Conservation (ICMBIO, license number 13373), and Environmental Institute of Rio de Janeiro state (INEA, license number 020/2011). Procedures with animals were previously approved by the Animal Use Ethics Committee of the FIOCRUZ (CEUA/FIOCRUZ), license LW81-12.
Author Contributions
Conceptualization, R.G., A.M.J. and A.L.R.R.; methodology, A.P.B., J.H.d.S.B., C.V.L., E.S.P., R.G., S.C.d.C.X. and A.L.R.R.; formal analysis, A.P.B., J.H.d.S.B., C.V.L., R.G., S.C.d.C.X., A.M.J. and A.L.R.R.; investigation, A.P.B., E.S.P., R.G. and A.L.R.R.; resources, R.G., A.M.J. and A.L.R.R.; writing—original draft preparation, A.P.B. and A.L.R.R.; writing—review and editing, A.P.B., J.H.d.S.B., C.V.L., R.G., S.C.d.C.X., A.M.J. and A.L.R.R.; visualization, A.P.B. and J.H.d.S.B.; supervision, A.L.R.R.; project administration, A.L.R.R.; funding acquisition, R.G., A.M.J. and A.L.R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior: CAPES)—Finance Code 001, CNPq/Universal (425293/2018-1), JCNE/FAPERJ (E-26/202.794/2019), and APQ1/FAPERJ (E-26/111.296/2014). R.G. received researcher grants from CNPq (304355/2018-6).
Institutional Review Board Statement
The sampling procedures reported in this study were authorized by Normative Instruction of the Brazilian Institute of the Environment and Renewable Natural Resources (IBAMA nº154/2007 Licenses 13373-1 e 19037-1), Chico Mendes Institute for Biodiversity and Conservation (ICMBIO, license number 13373), and Environmental Institute of Rio de Janeiro state (INEA, license number 020/2011). Procedures with animals were previously approved by the Animal Use Ethics Committee of the FIOCRUZ (CEUA/FIOCRUZ), license LW81-12.
Informed Consent Statement
Not applicable.
Data Availability Statement
The sequences that were generated in this study are openly available on GenBank database (https://www.ncbi.nlm.nih.gov/genbank/) (accessed on 15 October 2021). Access numbers for 18S rDNA and 24S rDNA are provided in Table 2.
Acknowledgments
We want to thank several people who participated in the field expeditions: (i) Sócrates Fraga da Costa-Neto, and all members of the Laboratório de Biologia e Parasitologia de Mamíferos Silvestres Reservatórios, IOC/Fiocruz; (ii) José Luiz Passos Cordeiro, Martha Brandão, and all members of the Estação Biológica Fiocruz Mata Atlântica/Fiocruz; (iii) Fabiano Figueiredo, Arthur Velho, Renato Ornellas, and all members of Laboratório de Dermatozoonoses, INI/Fiocruz; and (iv) members of Laboratório de Hantaviroses e Rickettsioses, IOC/FIOCRUZ. We also thank the FIOCRUZ sequencing platform team for their support in the sample sequencing process, and Paulo Sérgio D’Andrea for the ICMBio and INEA licenses. We would also like to thank the entire LABTRIP team, especially Carlos Ruiz Ardé and Marcos Antônio dos Santos Lima for technical support in the cultures, Ivo Guimarães Neto, Bruna Sender, André Pereira and Larissa Costa for some of the performed experiments, and Maria Augusta Dario for support with the phylogenetic analyses.
Conflicts of Interest
The authors declare they have no conflict of interest.
References
- D’Avila-Levy, C.M.; Boucinha, C.; Kostygov, A.; Santos, H.L.C.; Morelli, K.A.; Grybchuk-Ieremenko, A.; Duval, L.; Votýpka, J.; Yurchenko, V.; Grellier, P.; et al. Exploring the environmental diversity of kinetoplastid flagellates in the high-throughput DNA sequencing era. Memórias Inst. Oswaldo Cruz 2015, 110, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Votýpka, J.; D’Avila-Levy, C.M.; Grellier, P.; Maslov, D.A.; Lukeš, J.; Yurchenko, V. New approaches to systematics of Trypanosomatidae: Criteria for taxonomic (re)description. Trends Parasitol. 2015, 31, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Kaufer, A.; Ellis, J.; Stark, D.; Barratt, J. The evolution of trypanosomatid taxonomy. Parasites Vectors 2017, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Maslov, D.A.; Opperdoes, F.R.; Kostygov, A.Y.; Hashimi, H.; Lukeš, J.; Yurchenko, V. Recent advances in trypanosomatid research: Genome organization, expression, metabolism, taxonomy and evolution. Parasitology 2018, 146, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Page, A.M.; Canning, E.U.; Barker, R.J.; Nicholas, J.P. A new species of Rhynchoidomonas Patton, 1910 (Kinetoplastida: Trypanosomatina) from Operophtera brumata (Lepidoptera: Geometridae). Syst. Parasitol. 1986, 8, 101–105. [Google Scholar] [CrossRef]
- Akhoundi, M.; Downing, T.; Votýpka, J.; Kuhls, K.; Luke, J.; Cannet, A.; Ravel, C.; Marty, P.; Delaunay, P.; Kasbari, M.; et al. Leishmania infections: Molecular targets and diagnosis. Mol. Asp. Med. 2017, 57, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Ereqat, S.; Nasereddin, A.; Al-Jawabreh, A.; Al-Jawabreh, H.; Al-Laham, N.; Abdeen, Z. Prevalence of Trypanosoma evansi in livestock in Palestine. Parasites Vectors 2020, 13, 21. [Google Scholar] [CrossRef]
- Roman, F.; Iñiguez, A.M.; Yeo, M.; Jansen, A.M. Multilocus sequence typing: Genetic diversity in Trypanosoma cruzi I (TcI) isolates from Brazilian didelphids. Parasites Vectors 2018, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B. Trypanosoma cruzi: Um parasita, dois parasitas ou vários parasitas da doença de Chagas? Rev. Biol. 2011, 6, 44–48. [Google Scholar] [CrossRef]
- Jansen, A.M.; Xavier, S.C.D.C.; Roque, A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasites Vectors 2018, 11, 502. [Google Scholar] [CrossRef]
- Patton, J.L.; Pardiñas, U.F.J.; D’elía, G. Mammals of South America: Rodents; The University of Chicago Press: Chicago, IL, USA, 2015; Volume 2, p. 1384. [Google Scholar]
- Cássia-Pires, R.; Boite, M.C.; D’Andrea, P.S.; Herrera, H.M.; Cupolillo, E.; Jansen, A.M.; Roque, A.L.R. Distinct Leishmania Species Infecting Wild Caviomorph Rodents (Rodentia: Hystricognathi) from Brazil. PLoS Negl. Trop. Dis. 2014, 8, e3389. Available online: www.plosntds.org (accessed on 15 October 2021). [CrossRef]
- Roque, A.L.R.; Jansen, A.M. Reservatórios do Trypanosoma cruzi e sua relação com os vetores. In Vetores da doença de chagas no Brasil, Curitiba: Sociedade Brasileira de Zoologia; Zoologia: Guias e Manuais de Identificação, Series; Galvão, C., Ed.; Sociedade Brasileira de Zoologia: Curitiba, Brazil, 2014; pp. 75–87. ISBN 978-85-98203-09-6. [Google Scholar]
- Caldart, E.T.; Freire, R.L.; Ferreira, F.P.; Ruffolo, B.B.; Sbeghen, M.R.; Mareze, M.; Garcia, J.L.; Mitsuka-Breganó, R.; Navarro, I.T. Leishmania in synanthropic rodents (Rattus rattus): New evidence for the urbanization of Leishmania (Leishmania) amazonensis. Braz. J. Vet. Parasitol. 2017, 26, 17–27. [Google Scholar] [CrossRef]
- Morales, E.A.; Mayor, P.; Bowler, M.; Aysanoa, E.; Pérez-Velez, E.S.; Pérez, J.; Ventocilla, J.A.; Baldeviano, C.C.; Lescano, A.G. Prevalence of Trypanosoma cruzi and Other Trypanosomatids in Frequently-Hunted Wild Mammals from the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2017, 97, 1482–1485. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Lima, L.; Xavier, S.C.C.; Herrera, H.M.; Rocha, F.L.; Roque, A.L.R.; Teixeira, M.M.G.; Jansen, A.M. Uncovering Trypanosoma spp. diversity of wild mammals by the use of DNA from blood clots. Parasites Wildl. 2019, 8, 171–181. [Google Scholar] [CrossRef]
- Lopes, C.M.T.; Barreto, R.F.S.M.; Pavan, M.G.; Pereira, C.S.; Roque, A.L.R. Trypanosoma janseni n. sp. (Trypanosomatida: Trypanosomatidae) isolated from Didelphis aurita (Mammalia: Didelphidae) in the Atlantic Rainforest of Rio de Janeiro, Brazil: Integrative taxonomy and phylogeography within the Trypanosoma cruzi clade. Memórias Inst. Oswaldo Cruz 2018, 113, 45–55. [Google Scholar] [CrossRef]
- Tahir, D.; Davousta, B.; Heuc, K.; Lamourb, T.; Demarc, M.; Mariéa, J.L.; Blanchet, D. Molecular and serological investigation of Trypanosoma cruzi infection in dogs in French Guiana. Vet. Parasitol. Reg. Stud. Rep. 2018, 12, 106–109. [Google Scholar] [CrossRef]
- Barros, J.H.S.; Toma, H.K.; Madeira, M.D.F. Molecular study of Trypanosoma caninum isolates based on different genetic markers. Parasitol. Res. 2015, 114, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.K.; Godfrey, S.S.; Thompson, R.C.A. Trypanosomes of Australian mammals: A review. Int. J. Parasitol. Parasites Wildl. 2014, 3, 57–66. [Google Scholar] [CrossRef]
- Jansen, A.M.; Xavier, S.C.; Roque, A.L. The multiple and complex and changeable scenarios of the Trypanosoma cruzi transmission cycle in the sylvatic environment. Acta Trop. 2015, 151, 1–15. [Google Scholar] [CrossRef] [PubMed]
- SOS Mata Atlântica. Available online: https://www.sosma.org.br/noticias/desmatamento-da-mata-atlantica-cresce-em-dez-estados/ (accessed on 22 September 2021).
- Rezende, C.L.; Scarano, F.R.; Assad, E.D.; Joly, C.A.; Metzgerf, J.P.; Strassburg, B.B.N.; Tabarelli, M.; Fonseca, G.A.; Mittermeier, R.A. From hotspot to hopespot: An opportunity for the Brazilian Atlantic Forest. Perspect. Ecol. Conserv. 2018, 16, 208–214. [Google Scholar] [CrossRef]
- INSTITUTO ESTADUAL DO AMBIENTE (INEA). BIODIVERSIDADE E ÁREAS PROTEGIDAS/Unidades de Conservação. Parque Estadual da Pedra Branca. Available online: http://www.inea.rj.gov.br/biodiversidade-territorio/conheca-as-unidades-de-conservacao/parque-estadual-da-pedra-branca/ (accessed on 22 September 2021).
- De Souza, N.A.; Da Silva, J.B.; Godoy, R.E.; De Souza, F.J.M.; De Andrade-Coelho, C.A.; Da Silva, V.C.; De Azevedo, A.C.R.; Rangel, E.F. Studies on Phlebotominae (Diptera: Psychodidae) in the Campus FIOCRUZ Mata Atlântica, Jacarepaguá, in the City of Rio de Janeiro, Brazil. Rev. Soc. Bras. Med. Trop. 2015, 48, 26–32. [Google Scholar] [CrossRef]
- Gentile, R.; Cardoso, T.S.; Costa-Neto, S.F.; Teixeira, B.R.; D’Andrea, P.S. Community structure and population dynamics of small mammals in an urban-sylvatic interface area in Rio de Janeiro, Brazil. Zoologia 2018, 35, e13465. [Google Scholar] [CrossRef]
- Rangel, D.A.; Lisboa, C.V.; Novaes, R.L.M.; Silva, B.A.; Souza, R.D.F.; Jansen, A.M.; Moratelli, R.; Roque, A.L.R. Isolation and characterization of trypanosomatids, including Crithidia mellificae, in bats from the Atlantic Forest of Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 2019, 13, e0007527. [Google Scholar] [CrossRef]
- Costa-Neto, S.F.; Cardoso, T.S.; Boullosa, R.G.; Maldonado, A., Jr.; Gentile, R. Metacommunity structure of the helminths of the black-eared opossum Didelphis aurita in peri-urban, sylvatic and rural environments in south-eastern Brazil. J. Helminthol. 2019, 93, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.F.; Sousa, M.A.; Barros, J.H.S.; Figueiredo, F.B.; Fagundes, A.; Schubach, A.; De Paula, A.A.; Faissal, B.N.S.; Fonseca, T.S.; Thoma, H.K.; et al. Trypanosoma caninum n. sp. (Protozoa: Kinetoplastida) isolated from intact skin of domestic dog (Canis familiaris) captured in Rio de Janeiro, Brazil. Parasitology 2009, 136, 411–423. [Google Scholar] [CrossRef]
- Smith, A.; Clark, P.; Averis, S.; Lymbery, A.J.; Wayne, A.F.; Morris, K.D.; Thompsom, R.C.A. Trypanosomes in a declining species of threatened Australian marsupial, the brush-tailed bettong Bettongia penicillata (Marsupialia: Potoridae). Parasitology 2008, 135, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Brandão, E.M.V.; Xavier, S.C.C.; Carvalhaes, J.G.; D’Andrea, P.S.; Lemos, F.G.; Azevedo, F.C.; Cássia-Pires, R.; Jansen, A.M.; Roque, A.L.R. Trypanosomatids in Small Mammals of an Agroecosystem in Central Brazil: Another Piece in the Puzzle of Parasite Transmission in an Anthropogenic Landscape. Pathogens 2019, 8, 190. [Google Scholar] [CrossRef]
- Dario, M.A.; Rodrigues, M.S.; Barros, J.H.; Xavier, S.C.D.C.; D’andrea P., S.; Roque, A.L.; Jansen, A.M. Ecological scenario and Trypanosoma cruzi DTU characterization of a fatal acute Chagas disease case transmitted orally (Espírito Santo state, Brazil). Parasites Vectors 2016, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Dario, M.A.; Lisboa, C.V.; Costa, L.M.; Moratelli, R.; Nascimento, M.P.; Costa, L.P.; Leite, Y.L.R.; Llewellyn, M.S.; Xavier, S.C.D.C.; Roque, A.L.R.; et al. High Trypanosoma spp. diversity is maintained by bats and triatomines in Espírito Santo state, Brazil. PLoS ONE 2017, 12, e0188412. [Google Scholar] [CrossRef]
- Dario, M.A.; Moratelli, R.; Schwabl, P.; Jansen, A.M.; Llewellyn, M.S. Small subunit ribosomal metabarcoding reveals extraordinary trypanosomatid diversity in Brazilian bats. PLoS Negl. Trop. Dis. 2017, 11, e0005790. [Google Scholar] [CrossRef]
- Malavazi, P.F.N.S.; Daudt, C.; Melchior, L.A.K.; Meneguetti, D.U.O.; Xavier, S.C.C.; Jansen, A.M.; Souza, S.F.; Roque, A.R.L. Trypanosomes of vectors and domestic dogs in Trypanosoma cruzi transmission areas from Brazilian southwestern amazon: New mammalian host for Trypanosoma janseni. Acta Trop. 2020, 210, 105504. [Google Scholar] [CrossRef]
- Da Silva, F.M.; Junqueira, A.C.V.; Campaner, M.; Rodrigues, A.C.; Crisante, G.; Ramirez, L.E.; Caballero, Z.C.E.; Monteiro, F.A.; Coura, J.R.; Añez, N.; et al. Comparative phylogeography of Trypanosoma rangeli and Rhodnius (Hemiptera: Reduviidae) supports a long coexistence of parasite lineages and their sympatric vectors. Mol. Ecol. 2007, 16, 3361–3373. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.M.; Marcili, A.; Lima, L.; Cavazzana, M.J.R.; Ortiz, P.A.; Campaner, M.; Takeda, G.F.; Paiva, F.; Nunes, V.L.B.; Camargo, E.P.; et al. Trypanosoma rangeli isolates of bats from Central Brazil: Genotyping and phylogenetic analysis enable description of a new lineage using spliced-leader gene sequences. Acta Trop. 2009, 109, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Dario, M.A.; Pavan, M.G.; Rodrigues, M.S.; Lisboa, C.V.; Kluyber, D.; Desbiez, A.L.J.; Herrera, H.M.; Roque, A.L.R.; Lima, L.; Teixeira, M.M.G.; et al. Trypanosoma rangeli genetic, mammalian hosts and geographical diversity from five Brazilian biomes. Pathogens 2021, 10, 736. [Google Scholar] [CrossRef]
- Álvarez, O.E.; Ortiz, P.A.; Lima, L.; Costa-Martins, A.G.; Serrano, M.G.; Herder, S.; Buck, G.A.; Camargo, E.P.; Hamilton, P.B.; Stevens, J.R.; et al. Trypanosoma rangeli is phylogenetically closer to Old World trypanosomes than to Trypanosoma cruzi. Int. J. Parasitol. 2018, 48, 569–584. [Google Scholar] [CrossRef]
- Ramirez, L.E.; Machado, M.I.; Maywald, P.G.; Matos, A.; Chiari, E.; Silva, E.L. Primeira evidência de Trypanosoma rangeli no sudeste do Brasil, região endêmica para doença de Chagas. Rev. Soc. Bras. Med. Trop. 1998, 31, 99–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brandão, E.M.V.; Xavier, S.C.C.; Rocha, F.L.; Lima, C.F.M.; Candeias, Í.Z.; Lemos, F.G.; Azevedo, F.C.; Jansen, A.M.; Roque, A.L.R. Wild and Domestic Canids and Their Interactions in the Transmission Cycles of Trypanosoma Cruzi and Leishmania spp. in an Area of the Brazilian Cerrado. Pathogens 2020, 9, 818. [Google Scholar] [CrossRef]
- Cássia-Pires, R.; De Melo, M.D.F.A.D.; Barbosa, R.D.H.; Roque, A.L.R. Multiplex PCR as a tool for the diagnosis of Leishmania spp. kDNA and the gapdh housekeeping gene of mammal hosts. PLoS ONE 2017, 12, e0173922. [Google Scholar] [CrossRef]
- Folgueira, C.; Quijada, L.; Soto, M.; Abanades, D.R.; Alonso, C.; Requena, J.M. The Translational Efficiencies of the Two Leishmania infantum HSP70 mRNAs, Differing in Their 3-Untranslated Regions, Are Affected by Shifts in the Temperature of Growth through Different Mechanisms. J. Biol. Chem. 2005, 280, 35172–35183. [Google Scholar] [CrossRef]
- Gentile, R.; Cerqueira, R. Movement patterns of five species of small mammals in a Brazilian restinga. J. Trop. Ecol. 1995, 11, 671–677. [Google Scholar] [CrossRef]
- Agência FIOCRUZ de Notícias. Fiocruz inaugura primeira estação biológica do município do Rio. 11 October 2016. Available online: https://portal.fiocruz.br/noticia/fiocruz-inaugura-primeira-estacao-biologica-do-municipio-do-rio (accessed on 22 September 2021).
- Agência FIOCRUZ de Notícias. Fundação promove seminários sobre biodiversidade e saúde. 11 July 2017. Available online: https://agencia.fiocruz.br/fundacao-promove-seminarios-sobre-biodiversidade-e-saude (accessed on 22 September 2021).
- Neves, D.P. Parasitologia Humana, 11th ed.; Atheneu: São Paulo, Brazil, 2004; p. 494. [Google Scholar]
- Gomes, Y.M. Diagnóstico Etiológico. In Doença de Chagas; Malta, J. (Org), Editora Savier: São Paulo, Brazil, 1996; pp. 119–132. [Google Scholar]
- Rocha, F.L.; Roque, A.L.R.; De Lima, J.S.; Cheida, C.C.; Lemos, F.G.; Azevedo, F.C.; Arrais, R.C.; Bilac, D.; Herrera, H.M.; Mourão, G.; et al. Trypanosoma cruzi Infection in Neotropical Wild Carnivores (Mammalia: Carnivora): At the Top of the T. cruzi Transmission Chain. PLoS ONE 2013, 8, e67463. [Google Scholar] [CrossRef]
- Camargo, M.E. Fluorescent antibody test for the serodiagnosis of American trypanosomiasis. Technical modification employing preserved culture forms of Trypanosoma cruzi in a slide test. Rev. Inst. Med. Trop. São Paulo 1966, 8, 227–235. [Google Scholar] [PubMed]
- Deane, M.P.; Sousa, M.A.; Pereira, N.M.; Gonçalves, A.; Momem, H.; Morel, C. Trypanosoma cruzi: Inoculation schedules and re-isolation methods demonstrated by schizodeme and zymodeme analyses. J. Protozool. 1984, 31, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.M.; Moriearty, P.L.; Castro, B.G.; Deane, M.P. Trypanosoma cruzi in the opossum Didelphis marsupialis: An indirect fluorescent antibody test for the diagnosis and follow-up of natural and experimental infections. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 474–477. [Google Scholar] [CrossRef]
- Roque, A.L.R.; Xavier, S.C.; da Rocha, M.G.; Duarte, A.C.; D’Andrea, P.S.; Jansen, A.M. Trypanosoma cruzi transmission cycle among wild and domestic mammals in three areas of orally transmitted Chagas disease outbreaks. Am. J. Trop. Med. Hyg. 2008, 79, 742–749. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; p. 1626. [Google Scholar]
- Degrave, W.; Fernandes, O.; Campbell, D.; Bozza, M.; Lopes, U. Use of molecular probes and PCR for detection and typing of Leishmania—A mini-review. Memórias Inst. Oswaldo Cruz 1994, 89, 463–469. [Google Scholar] [CrossRef]
- Da Graça, G.C.; Volpini, A.C.; Romero, G.A.S.; Oliveira Neto, M.P.; Hueb, M.; Porrozzi, R.; Boité, M.C.; Cupolillo, E. Development and validation of PCR-based assays for diagnosis of American cutaneous leishmaniasis and identification of the parasite species. Memórias Inst. Oswaldo Cruz 2012, 107, 664–674. [Google Scholar] [CrossRef]
- Arruda, M.V.; Reinach, F.C.; Colli, W.; Zingales, B. Sequence of the 24Set ribosomal RNA gene and characterization of a corresponding pseudogene from Trypanos. Cruzi. Mol. Biochem. Parasitol. 1990, 40, 35–42. [Google Scholar] [CrossRef]
- LASERGENE. User’s Guide: A Manual for the Lasergene System; [s.l.] Biocomputing Software for Windows; DNASTAR, Inc: Madison, WI, USA, 1994. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. MRBAYES 3: Bayesian Phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Chernomor, O.; von Haeseler, A.; Minh, B.Q. Terrace aware data structure forphylogenomic inference from supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).