Ticks, Human Babesiosis and Climate Change
Abstract
:1. Introduction
2. Life Cycles and Ecology of the Human Babesiosis Vectors
3. The Roles of Reservoir Hosts of Human Babesiosis
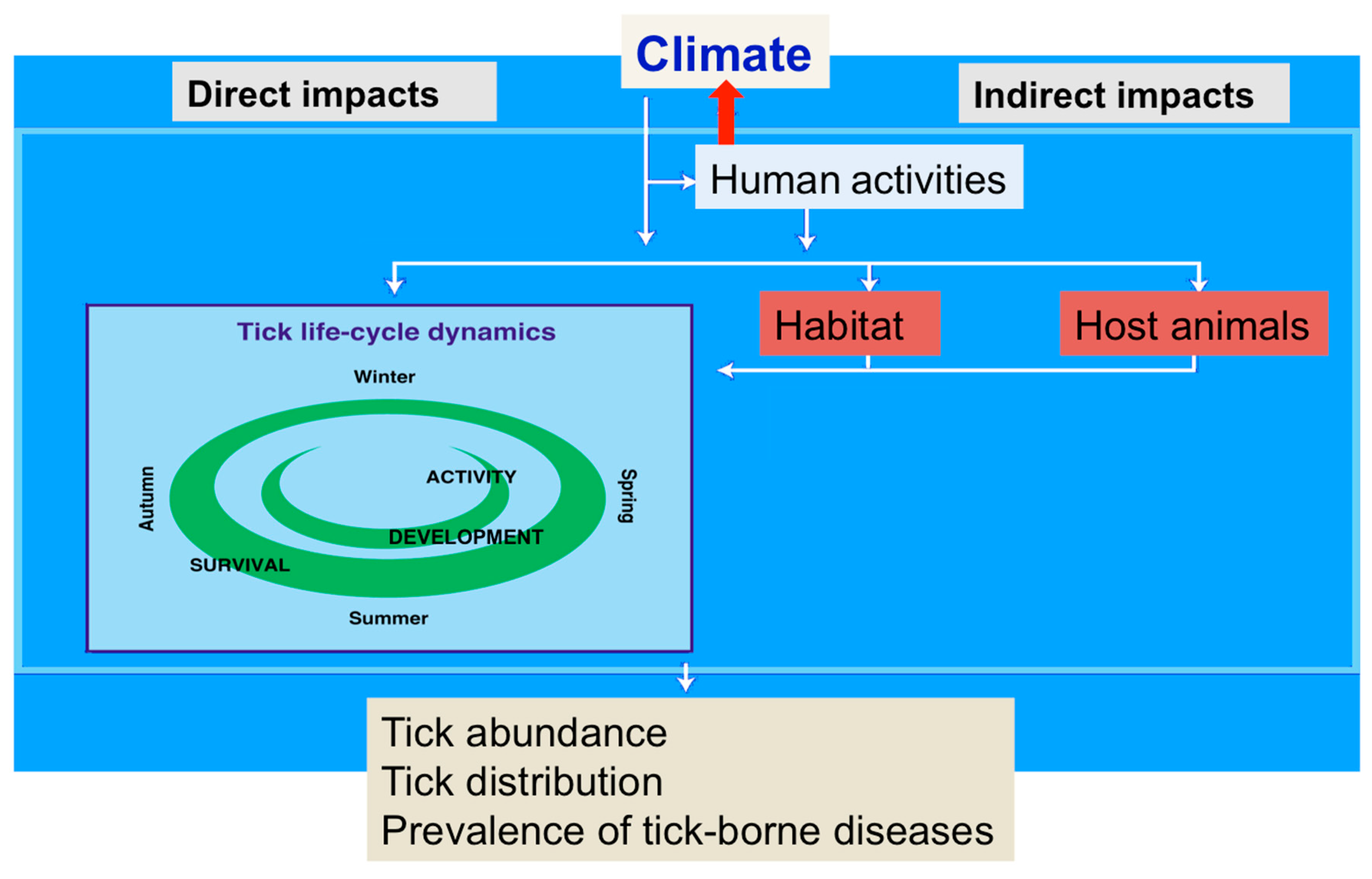
4. Expected Impacts of Climate Change on the Vectors
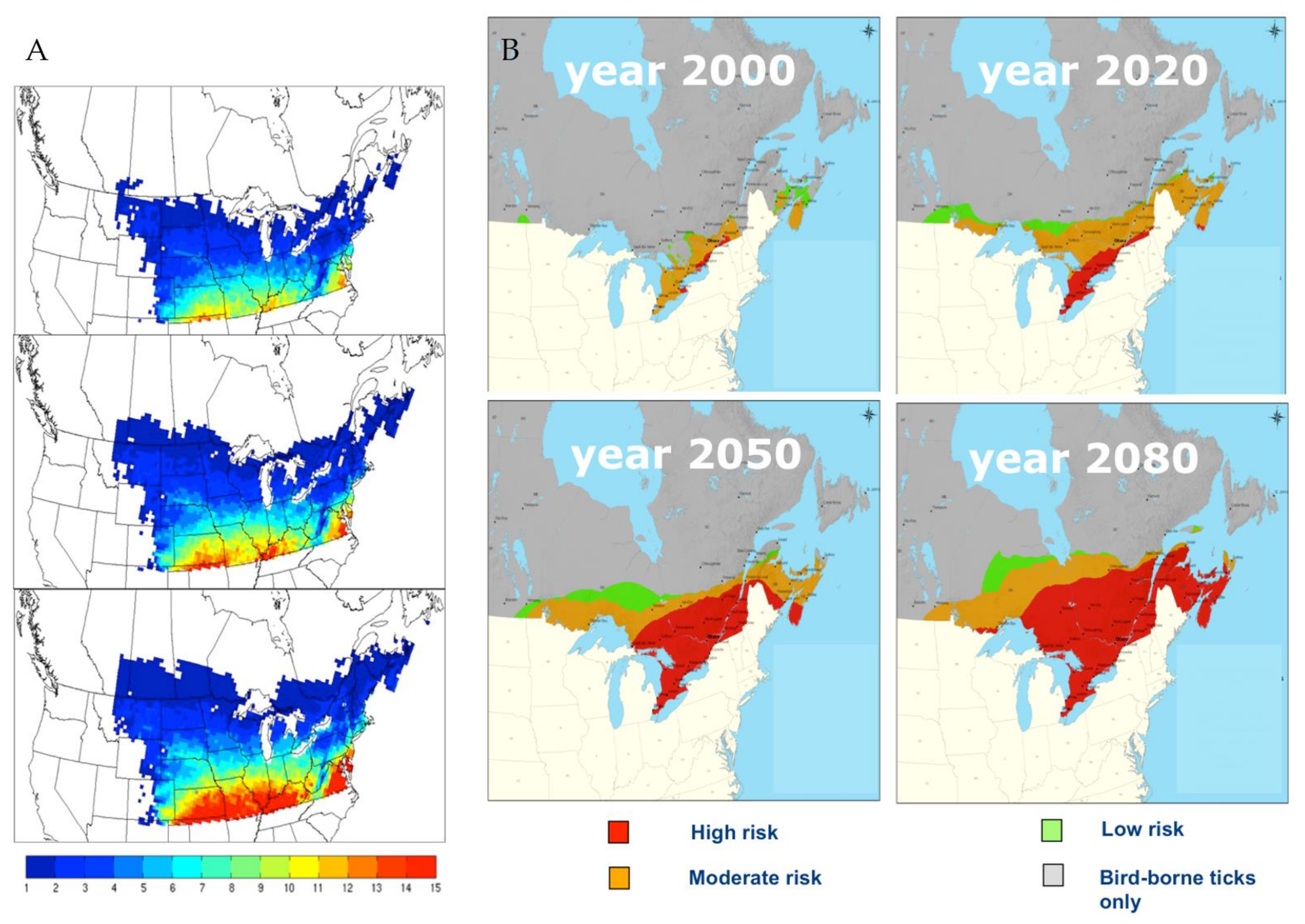
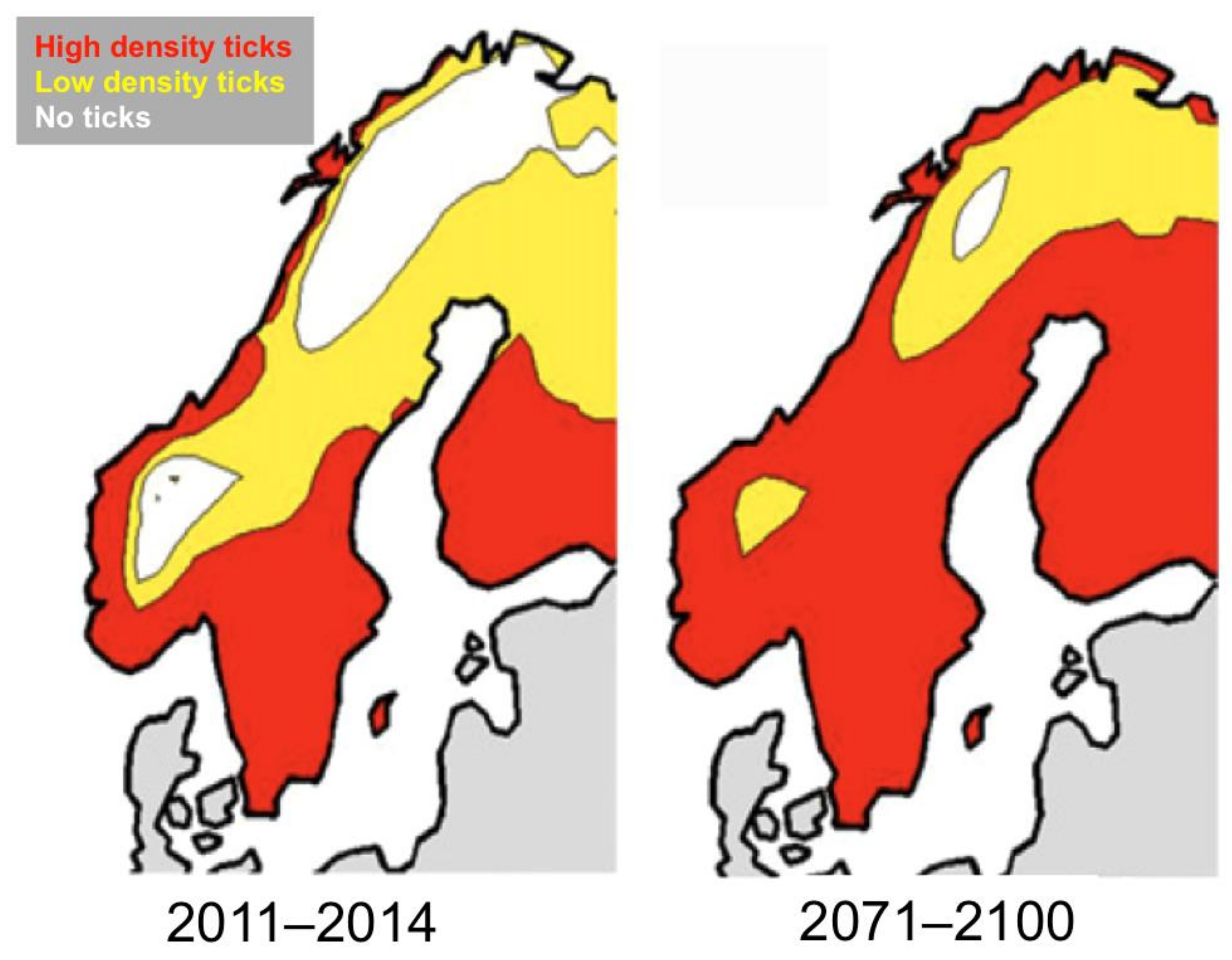
5. Projected Effects of Climate Change on Tick and Babesia spp. Distributions
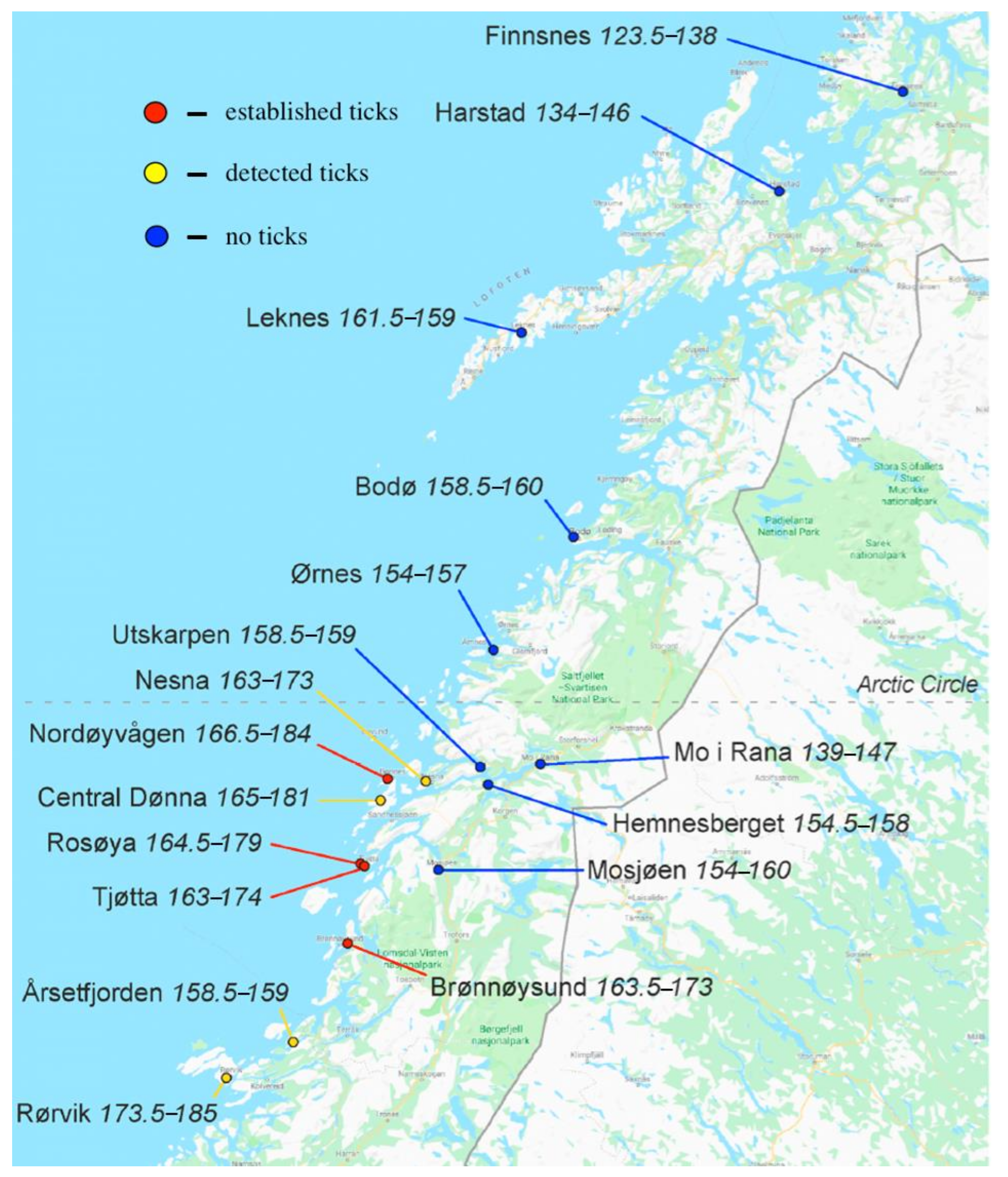
6. Observed Effects of Climate Changes
6.1. Climate Effects on Ticks
6.2. Climate Effects on Reservoir Hosts
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rocklöv, J.; Dubrow, R. Climate change: An enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 2020, 21, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.R.; Hoberg, E.P.; Boeger, W.A. The Stockholm Paradigm: Climate Change and Emerging Disease; University of Chicago Press: Chicago, IL, USA, 2019; p. 400. [Google Scholar]
- Kramer, K.; Degen, B.; Buschbom, J.; Hickler, T.; Thuiller, W.; Sykes, M.T.; Winter, W. Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change—Range, abundance, genetic diversity and adaptive response. For. Ecol. Manag. 2010, 259, 2213–2222. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Levi, T.; Keesing, F.; Oggenfuss, K.; Canham, C.D. Tick-borne disease risk in a forest food web. Ecology 2018, 99, 1562–1573. [Google Scholar] [CrossRef]
- Bregnard, C.; Rais, O.; Voordouw, M.J. Climate and tree seed production predict the abundance of the European Lyme disease vector over a 15-year period. Parasit. Vectors 2020, 13, 408. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, E.; Tälleklint, L.; Polfeldt, T. Impact of climatic change on the northern latitude limit and population density of the disease-transmitting European tick Ixodes ricinus. Environ. Health Perspect. 2000, 108, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Lobo, C.A.; Singh, M.; Rodriguez, M. Human babesiosis: Recent advances and future challenges. Curr. Opin. Hematol. 2020, 27, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, A.; Zintl, A.; Montero, E.; Hunfeld, K.-P.; Gray, J. Human babesiosis in Europe. Pathogens 2021, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Zhou, D.; Liu, J.; Cheng, Z.; Zhang, L.; Wang, L.; Wang, Z.; Yang, D.; Wang, S.; Chai, T. Detection of Babesia divergens using molecular methods in anemic patients in Shandong Province, China. Parasitol. Res. 2011, 109, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.F.; Zheng, Y.C.; Jiang, R.R.; Li, H.; Huo, Q.B.; Jiang, B.G.; Sun, Y.; Jia, N.; Wang, Y.W.; Ma, L.; et al. Epidemiological, clinical, and laboratory characteristics of 48 cases of “Babesia venatorum” infection in China: A descriptive study. Lancet Infect. Dis. 2015, 15, 196–203. [Google Scholar] [CrossRef]
- Zhou, X.; Xia, S.; Yin, S.Q.; Zhou, X.N. Emergence of babesiosis in China-Myanmar border areas. Parasit. Vectors 2015, 8, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamoto-Niikura, A.; Morikawa, S.; Hanaki, K.I.; Holman, P.J.; Ishihara, C. Ixodes persulcatus ticks as vectors for the Babesia microti U.S. lineage in Japan. Appl. Environ. Microbiol. 2016, 82, 6624–6632. [Google Scholar] [CrossRef] [Green Version]
- Zamoto-Niikura, A.; Tsuji, M.; Qiang, W.; Morikawa, S.; Hanaki, K.I.; Holman, P.J.; Ishihara, C. The Babesia divergens Asia lineage is maintained through enzootic cycles between Ixodes persulcatus and sika deer in Hokkaido, Japan. Appl. Environ. Microbiol. 2018, 84, e02491-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swei, A.; O’Connor, K.E.; Couper, L.I.; Thekkiniath, J.; Conrad, P.A.; Padgett, K.A.; Burns, J.; Yoshimizu, M.H.; Gonzales, B.; Munk, B.; et al. Evidence for transmission of the zoonotic apicomplexan parasite Babesia duncani by the tick Dermacentor albipictus. Int. J. Parasitol. 2019, 49, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Zheng, Y.C.; Jiang, J.F.; Jiang, R.R.; Jiang, B.G.; Wei, R.; Liu, H.B.; Huo, Q.B.; Sun, Y.; Chu, Y.L.; et al. Human babesiosis caused by a Babesia crassa-like pathogen: A case series. Clin. Infect. Dis. 2018, 167, 1110–1119. [Google Scholar] [CrossRef]
- Goethert, H.K.; Telford, S.R., III. Enzootic transmission of Babesia divergens among cottontail rabbits on Nantucket Island, Massachusetts. Am. J. Trop. Med. Hyg. 2003, 69, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Belozerov, V.N. Diapause and biological rhythms in ticks. In Physiology of Ticks; Obenchain, F.D., Galun, R., Eds.; Pergamon Press: Oxford, UK, 1982; pp. 469–500. [Google Scholar]
- Gray, J.S.; Kahl, O.; Lane, R.S.; Levin, M.L.; Tsao, J.I. Diapause in ticks of the medically important Ixodes ricinus species complex. Ticks Tick Borne Dis. 2016, 7, 992–1003. [Google Scholar] [CrossRef] [Green Version]
- Korenberg, E.I. Comparative ecology and epidemiology of Lyme disease and tick-borne encephalitis in the former Soviet Union. Parasitol. Today 1994, 10, 157–160. [Google Scholar] [CrossRef]
- Arsnoe, I.; Tsao, J.I.; Hickling, G.J. Nymphal Ixodes scapularis questing behavior explains geographic variation in Lyme borreliosis risk in the eastern United States. Ticks Tick Borne Dis. 2019, 10, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S. The ecology of Lyme borreliosis vectors. Exp. Appl. Acarol. 1998, 22, 249–258. [Google Scholar] [CrossRef]
- Gilbert, L.; Aungier, J.; Tomkins, J.L. Climate of origin affects tick (Ixodes ricinus) host-seeking behavior in response to temperature: Implications for resilience to climate change? Ecol. Evol. 2014, 4, 1186–1198. [Google Scholar] [CrossRef]
- Ogden, N.H.; Beard, C.B.; Ginsberg, H.; Tsao, J. Possible effects of climate change on ixodid ticks and the pathogens they transmit: What has been projected and what has been observed. J. Med. Entomol. 2020, 58, 1536–1545. [Google Scholar] [CrossRef]
- Dautel, H.; Kämmer, D.; Kahl, O. How an extreme weather spell in winter can influence vector tick abundance and tick-borne disease incidence. In Ecology and Prevention of Lyme Borreliosis; Braks, M.A.H., Van Wierer, S.E., Takken, W., Sprong, H., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; pp. 335–350. [Google Scholar]
- MacLeod, J. Ixodes ricinus in relation to its physical environment. II. The factors governing survival and activity. Parasitology 1935, 27, 123–144. [Google Scholar] [CrossRef]
- Földvári, G.; Rigó, K.; Jablonszky, M.; Biró, N.; Majoros, G.; Molnár, V.; Tóth, M. Ticks and the city: Ectoparasites of the Northern white-breasted hedgehog (Erinaceus roumanicus) in an urban park. Ticks Tick Borne Dis. 2011, 2, 231–234. [Google Scholar] [CrossRef]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Špitalská, E.; et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: New hazards and relevance for public health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef] [PubMed]
- Goethert, H.K.; Telford, S.R., III. What is Babesia microti? Parasitology 2003, 127, 301–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hersh, M.H.; Tibbetts, M.; Strauss, M.; Ostfeld, R.S.; Keesing, F. Reservoir competence of wildlife host species for Babesia microti. Emerg. Infect. Dis. 2012, 18, 1951–1957. [Google Scholar] [CrossRef]
- Gray, J.S.; von Stedingk, L.-V.; Gürtelschmid, M.; Granström, M. Transmission studies on Babesia microti in Ixodes ricinus ticks and gerbils. J. Clin. Microbiol. 2002, 40, 1258–1263. [Google Scholar] [CrossRef] [Green Version]
- Herwaldt, B.L.; Cacció, S.; Gherlinzoni, F.; Aspöck, H.; Slemenda, S.B.; Piccaluga, P.; Martinelli, G.; Edelhofer, R.; Hollenstein, U.; Poletti, G.; et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg. Infect. Dis. 2003, 9, 942–948. [Google Scholar] [CrossRef]
- Bonnet, S.; Jouglin, M.; L’Hostis, M.; Chauvin, A. Babesia sp. EU1 from roe deer and transmission within Ixodes ricinus. Emerg. Infect. Dis. 2007, 13, 1208–1210. [Google Scholar] [CrossRef]
- Bonnet, S.; Brisseau, N.; Hermouet, A.; Jouglin, M.; Chauvin, A. Experimental in vitro transmission of Babesia sp. (EU1) by Ixodes ricinus. Vet. Res. 2009, 40, 21. [Google Scholar] [CrossRef] [Green Version]
- Fanelli, A. A historical review of Babesia spp. associated with deer in Europe: Babesia divergens/Babesia divergens-like, Babesia capreoli, Babesia venatorum, Babesia cf. odocoilei. Vet. Parasitol. 2021, 294, 109433. [Google Scholar] [CrossRef] [PubMed]
- Zintl, A.; Mulcahy, G.; Skerrett, H.E.; Taylor, S.M.; Gray, J.S. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin. Microbiol. Rev. 2003, 16, 622–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monaghan, A.J.; Moore, S.M.; Sampson, K.M.; Beard, C.B.; Eisen, R.J. Climate change influences on the annual onset of Lyme disease in the United States. Ticks Tick Borne Dis. 2015, 6, 615–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perret, J.L.; Guigoz, E.; Rais, O.; Gern, L. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland). Parasitol. Res. 2000, 86, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Radojevic, M.; Wu, X.; Duvvuri, V.R.; Leighton, P.A.; Wu, J. Estimated effects of projected climate change on the basic reproductive number of the tick vector of Lyme disease Ixodes scapularis. Environ. Health Perspect. 2014, 122, 631–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPherson, M.; García-García, A.; Cuesta-Valero, F.J.; Belltrami, H.; Hansen-Ketchum, P.; MacDougall, D.; Ogden, N.H. Expansion of the Lyme disease vector Ixodes scapularis in Canada inferred from CMIP5 climate projections. Environ. Health Perspect. 2017, 125, 057008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogden, N.H.; Lindsay, R.L.; Hanincová, K.; Barker, I.K.; Bigras-Poulin, M.; Charron, D.F.; Heagy, A.; Francis, C.M.; O’Callaghan, C.J.; Schwartz, I.; et al. The role of migratory birds in introduction and range expansion of Ixodes scapularis ticks, and Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl. Environ. Microbiol. 2008, 74, 1780–1790. [Google Scholar] [CrossRef] [Green Version]
- Jaenson, T.G.; Lindgren, E. The range of Ixodes ricinus and the risk of contracting Lyme borreliosis will increase northwards when the vegetation period becomes longer. Ticks Tick Borne Dis. 2011, 2, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Porretta, D.; Mastrantonio, V.; Amendolia, S.; Gaiarsa, S.; Epis, S.; Genchi, C.; Bandi, C.; Otranto, D.; Urbanelli, S. Effects of global changes on the climatic niche of the tick Ixodes ricinus inferred by species distribution modelling. Parasit. Vectors 2013, 6, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkishe, A.A.; Peterson, A.T.; Samy, A.M. Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus. PLoS ONE 2017, 12, e0189092. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L. Altitudinal patterns of tick and host abundance: A potential role for climate change in regulating tick-borne diseases? Oecologia 2010, 162, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, J.S.; Holford, T.R.; Fish, D. Effect of climate change on Lyme disease risk in North America. EcoHealth 2005, 2, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Ogden, N.H.; Linday, L.R. Effects of climate and climate change on vectors and vector-borne diseases: Ticks are different. Trends Parasitol. 2016, 32, 646–656. [Google Scholar] [CrossRef]
- Simon, J.A.; Marrotte, R.R.; Desrosiers, N.; Fiset, J.; Gaitan, J.; Gonzalez, A.; Koffi, J.K.; Lapointe, F.-J.; Leighton, P.A.; Lindsay, L.R.; et al. Climate change, habitat fragmentation, ticks and the white-footed mouse drive occurrence of B. burgdorferi, the agent of Lyme disease, at the northern limit of its distribution. Evol. Appl. 2014, 7, 750–764. [Google Scholar] [CrossRef] [Green Version]
- Dunn, J.M.; Krause, P.J.; Davis, S.; Vannier, E.G.; Fitzpatrick, M.C.; Rollend, L.; Belperron, A.A.; States, S.L.; Stacey, A.; Bockenstedt, L.K.; et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS ONE 2014, 9, e115494. [Google Scholar] [CrossRef]
- Gray, J.S. Ixodes ricinus seasonal activity: Implications of global warming indicated by revisiting tick and weather data. Int. J. Med. Microbiol. 2008, 298 (Suppl. 1), 19–24. [Google Scholar] [CrossRef]
- Walter, K.S.; Pepin, K.M.; Webb, C.T.; Gaff, H.D.; Krause, P.J.; Pitzer, V.E.; Diuk-Wasser, M.A. Invasion of two tick-borne diseases across New England: Harnessing human surveillance data to capture underlying ecological invasion processes. Proc. R. Soc. B 2016, 283, 20160834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhav, N.K.; Brownstein, J.S.; Tsao, J.I.; Fish, D. A dispersal model for the range expansion of blacklegged tick (Acari: Ixodidae). J. Med. Entomol. 2004, 41, 842–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilhelmsson, P.; Pawełczyk, O.; Jaenson, T.G.T.; Waldenström, J.; Olsen, B.; Forsberg, P.; Lindgren, P.E. Three Babesia species in Ixodes ricinus ticks from migratory birds in Sweden. Parasit. Vectors 2021, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans; Oxford University Press: Oxford, UK, 1992; p. 768. [Google Scholar]
- Leighton, P.; Koffi, J.; Pelcat, Y.; Lindsay, L.R.; Ogden, N.H. Predicting the speed of tick invasion: An empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J. Appl. Ecol. 2012, 49, 457–464. [Google Scholar] [CrossRef]
- Clow, K.; Ogden, N.H.; Lindsay, L.R.; Michel, P.; Pearl, D.; Jardine, C. The influence of abiotic and biotic factors on the invasion of Ixodes scapularis in Ontario, Canada. Ticks Tick Borne Dis. 2017, 8, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Clow, K.; Leighton, P.A.; Ogden, N.H.; Lindsay, L.R.; Michel, P.; Pearl, D.; Jardine, C. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS ONE 2017, 12, e0189393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasmi, S.; Ogden, N.H.; Lindsay, L.R.; Burns, S.; Fleming, S.; Badcock, J.; Hanan, S.; Gaulin, C.; Leblanc, M.A.; Russell, C.; et al. Surveillance for Lyme disease in Canada: 2009–2015. Can. Commun. Dis. Rep. 2017, 43, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.; Jaenson, D.G.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit. Vectors 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hvidsten, D.; Frafjord, K.; Gray, J.S.; Henningsson, A.J.; Jenkins, A.; Kristiansen, B.E.; Lager, M.; Rognerud, B.; Slåtsve, A.M.; Stordal, F.; et al. The distribution limit of the common tick, Ixodes ricinus, and some associated pathogens in north-western Europe. Ticks Tick Borne Dis. 2020, 11, 101388. [Google Scholar] [CrossRef] [PubMed]
- Korotkov, Y.; Kozlova, T.; Kozlovskaya, L. Observations on changes in abundance of questing Ixodes ricinus, castor bean tick, over a 35-year period in the eastern part of its range (Russia, Tula region). Med. Vet. Entomol. 2015, 29, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Danielová, V.; Kríz, B.; Jirsa, A.; Nozicka, J. Shift of the tick Ixodes ricinus and tick-borne encephalitis to higher altitudes in central Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Materna, J.; Honig, V.; Metelka, L.; Danielová, V.; Harcarik, J.; Kliegrová, S.; Grubhoffer, L. Vertical distribution of the tick Ixodes ricinus and tick-borne pathogens in the northern Moravian mountains correlated with climate warming (Jeseniky Mts., Czech Republic). Cent. Eur. J. Public Health 2009, 17, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Vozmediano, A.; Krawczyk, A.I.; Sprong, H.; Rossi, L.; Ramassa, E.; Tomassone, L. Ticks climb the mountains: Ixodid tick infestation and infection by tick-borne pathogens in the Western Alps. Ticks Tick Borne Dis. 2020, 11, 101489. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M. Influence of the microclimate on the vertical distribution of the tick Ixodes ricinus (L) in Central Europe. Acarologia 1993, 34, 105–113. [Google Scholar]
- Anonymous. Consensus conference on Lyme disease. Can. J. Infect. Dis. 1991, 2, 49–54. [Google Scholar]
- Mysterud, A.; Jore, S.; Østerås, O.; Viljugrein, H. Emergence of tick-borne diseases at northern latitudes in Europe: A comparative approach. Sci. Rep. 2017, 7, 16316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jore, S.; Viljugrein, H.; Hofshagen, M.; Brun-Hansen, H.; Kristoffersen, A.B.; Nygård, K.; Brun, E.; Ottesen, P.; Sævik, B.K.; Ytrehus, B. Multi-source analysis reveals latitudinal and altitudinal shifts in range of Ixodes ricinus at its northern distribution limit. Parasit. Vectors 2011, 4, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, N.; Phipps, L.; McFadzean, H.; Barlow, A.M. An outbreak of bovine babesiosis in February, 2019, triggered by above average winter temperatures in southern England and co-infection with Babesia divergens and Anaplasma phagocytophilum. Parasit. Vectors 2020, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.; Capewell, P.; Loney, C.; Katzer, F.; Shiels, B.R.; Weir, W. Sheep as host species for zoonotic Babesia venatorum, United Kingdom. Emerg. Infect. Dis. 2019, 25, 2257–2260. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, S.F.; Drews, S.J.; Yi, Q.L.; Bloch, E.M.; Ogden, N.H.; Koffi, J.K.; Lindsay, L.R.; Gregoire, Y.; Delage, G. Risk of transfusion-transmitted Babesia microti in Canada. Transfusion 2021, 61, 2958–2968. [Google Scholar] [CrossRef]
- Stein, E.; Elbadawi, L.I.; Kazmierczak, J.; Davis, J.P. Babesiosis surveillance, Wisconsin, 2001–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 687. [Google Scholar] [CrossRef] [Green Version]
- Drews, S.J.; Van Caeseele, P.; Bullard, J.; Lindsay, L.R.; Gaziano, T.; Zeller, M.P.; Lane, D.; Ndao, M.; Allen, V.G.; Boggild, A.K.; et al. Babesia microti in a Canadian blood donor and lookback in a red blood cell recipient. Vox Sang. 2021, in press. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J. Coinfection by Ixodes tick-borne pathogens: Ecological, epidemiological, and clinical consequences. Trends Parasitol. 2016, 32, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolkacz, K.; Bednarska, M.; Alsarraf, M.; Dwuznik, D.; Grzybek, M.; Welc-Faleciak, R.; Behnke, J.M.; Bajer, A. Prevalence, genetic identity and vertical transmission of Babesia microti in three naturally infected species of vole, Microtus spp. (Cricetidae). Parasit. Vectors 2017, 10, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tufts, D.M.; Diuk-Wasser, M.A. Transplacental transmission of tick-borne Babesia microti in its natural host Peromyscus leucopus. Parasit. Vectors 2018, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- Roy-Dufresne, E.; Logan, T.; Simon, J.A.; Chmura, G.L.; Millien, V. Poleward expansion of the white-footed mouse (Peromyscus leucopus) under climate change: Implications for the spread of Lyme disease. PLoS ONE 2013, 8, e80724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goethert, H.K.; Lubelcyzk, C.; LaCombe, E.; Holman, M.; Rand, P.; Smith, R.P., Jr.; Telford, S.R., III. Enzootic Babesia microti in Maine. J. Parasitol. 2003, 89, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, J.E.; Tran, A.D.; Freimark, L.; Schaffner, S.F.; Goethert, H.; Andersen, K.G.; Bazner, S.; Li, A.; McGrath, G.; Sloan, L.; et al. A global map of genetic diversity in Babesia microti reveals strong population structure and identifies variants associated with clinical relapse. Nat. Microbiol. 2016, 1, 16079. [Google Scholar] [CrossRef] [Green Version]
- Matuschka, F.R.; Spielman, A. The emergence of Lyme disease in a changing environment in North America and central Europe. Exp. Appl. Acarol. 1986, 2, 337–353. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gray, J.S.; Ogden, N.H. Ticks, Human Babesiosis and Climate Change. Pathogens 2021, 10, 1430. https://doi.org/10.3390/pathogens10111430
Gray JS, Ogden NH. Ticks, Human Babesiosis and Climate Change. Pathogens. 2021; 10(11):1430. https://doi.org/10.3390/pathogens10111430
Chicago/Turabian StyleGray, Jeremy S., and Nicholas H. Ogden. 2021. "Ticks, Human Babesiosis and Climate Change" Pathogens 10, no. 11: 1430. https://doi.org/10.3390/pathogens10111430
APA StyleGray, J. S., & Ogden, N. H. (2021). Ticks, Human Babesiosis and Climate Change. Pathogens, 10(11), 1430. https://doi.org/10.3390/pathogens10111430




