Variant CJD: Reflections a Quarter of a Century on
Abstract
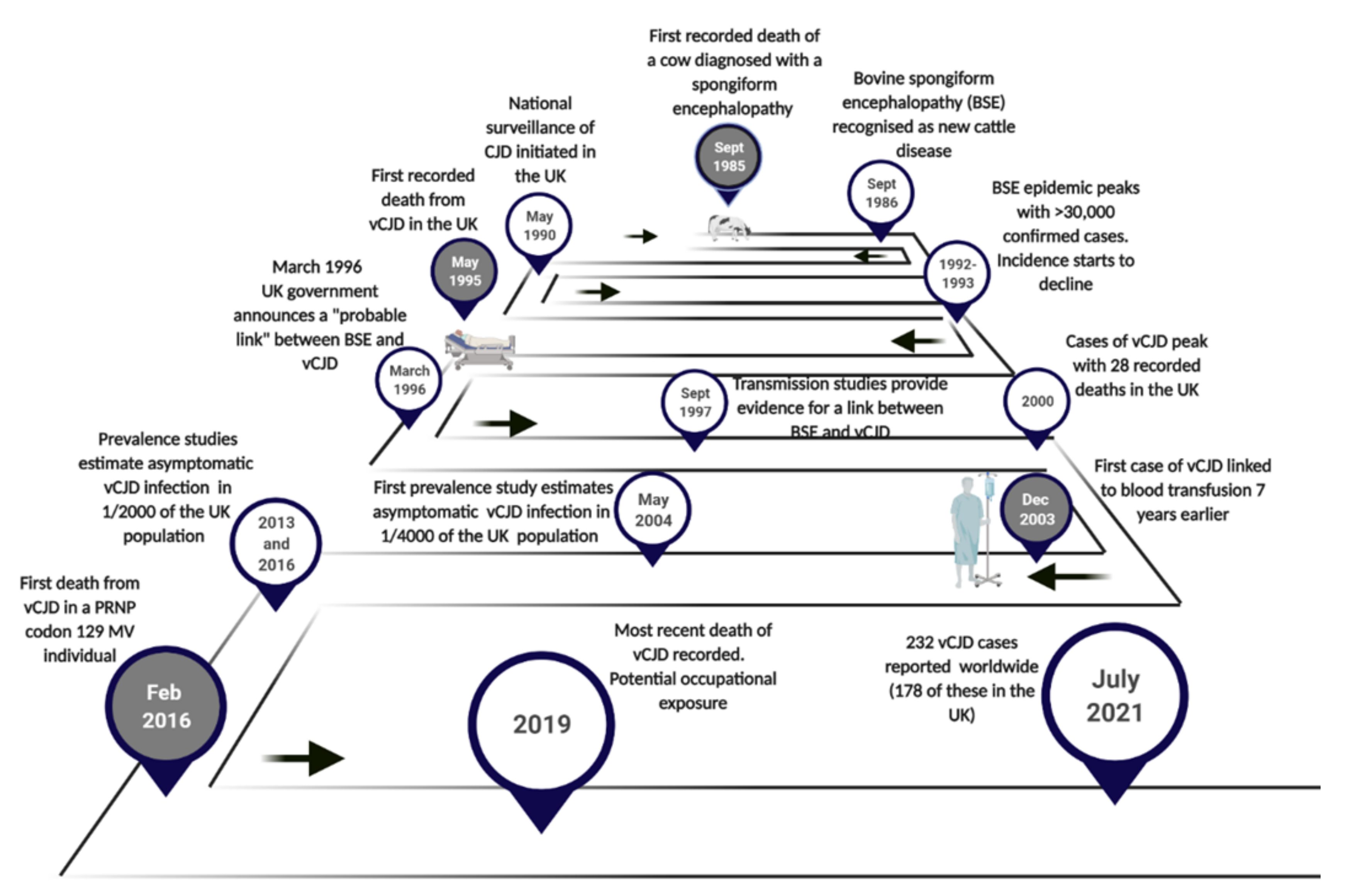
1. Introduction
2. The Emergence of vCJD
3. BSE and vCJD, A Single Strain of Agent
3.1. Animal Models of vCJD
3.2. Biochemical Properties of PrPSc in vCJD
4. Epidemiology
4.1. Primary Cases of vCJD
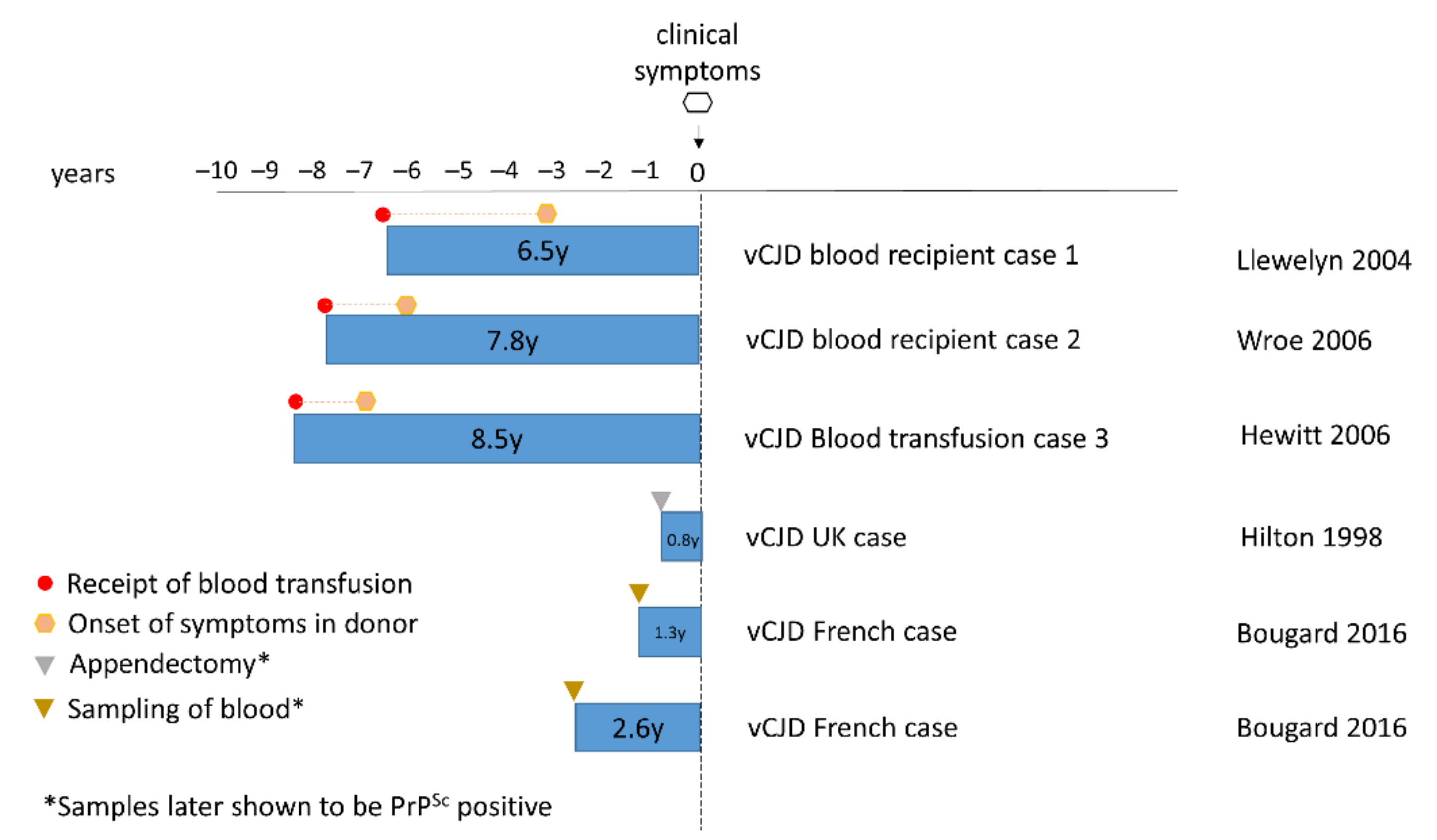
4.2. Secondary Human-to-Human Transmission of vCJD in the UK
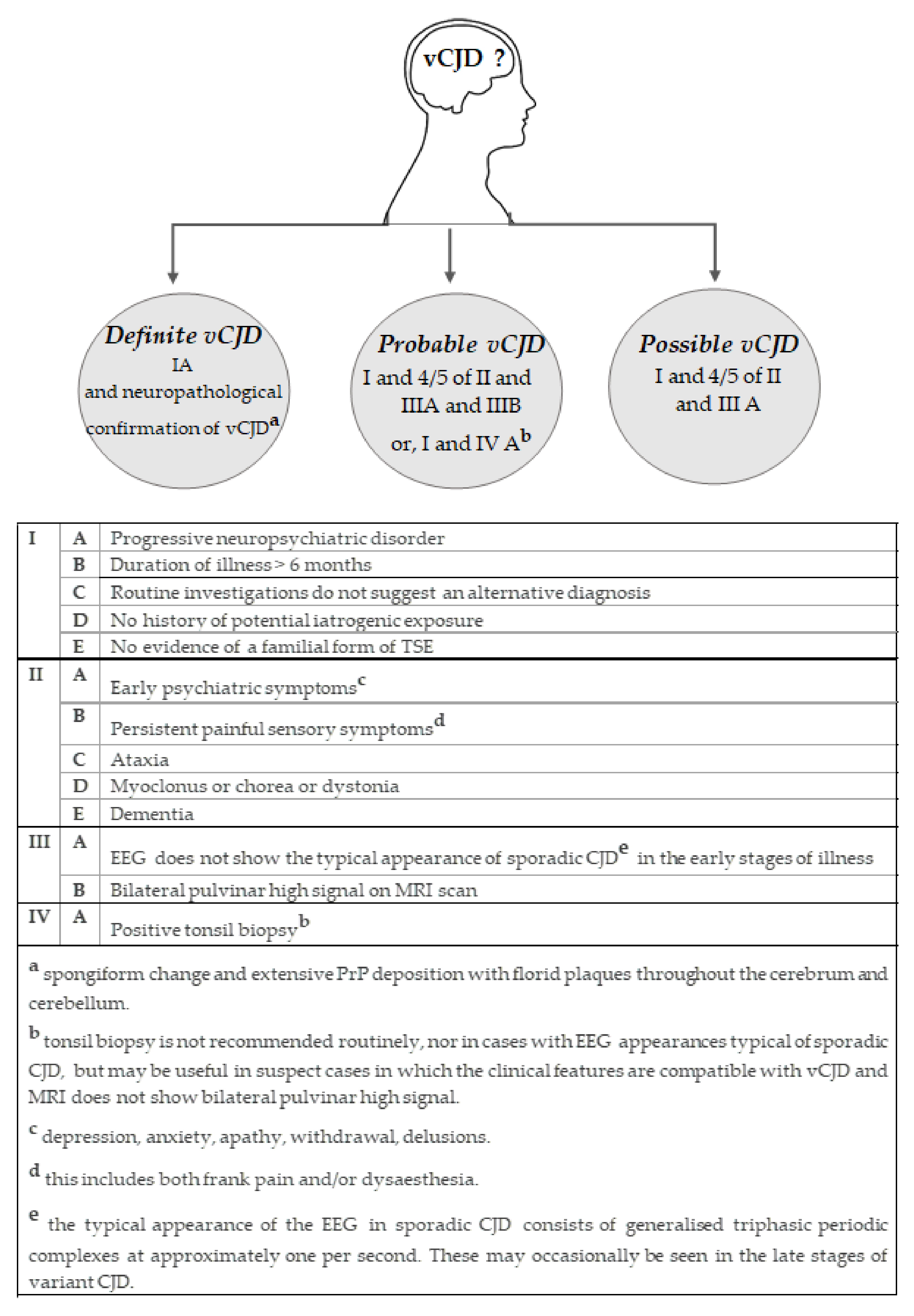
5. Diagnosis
5.1. Clinical Features
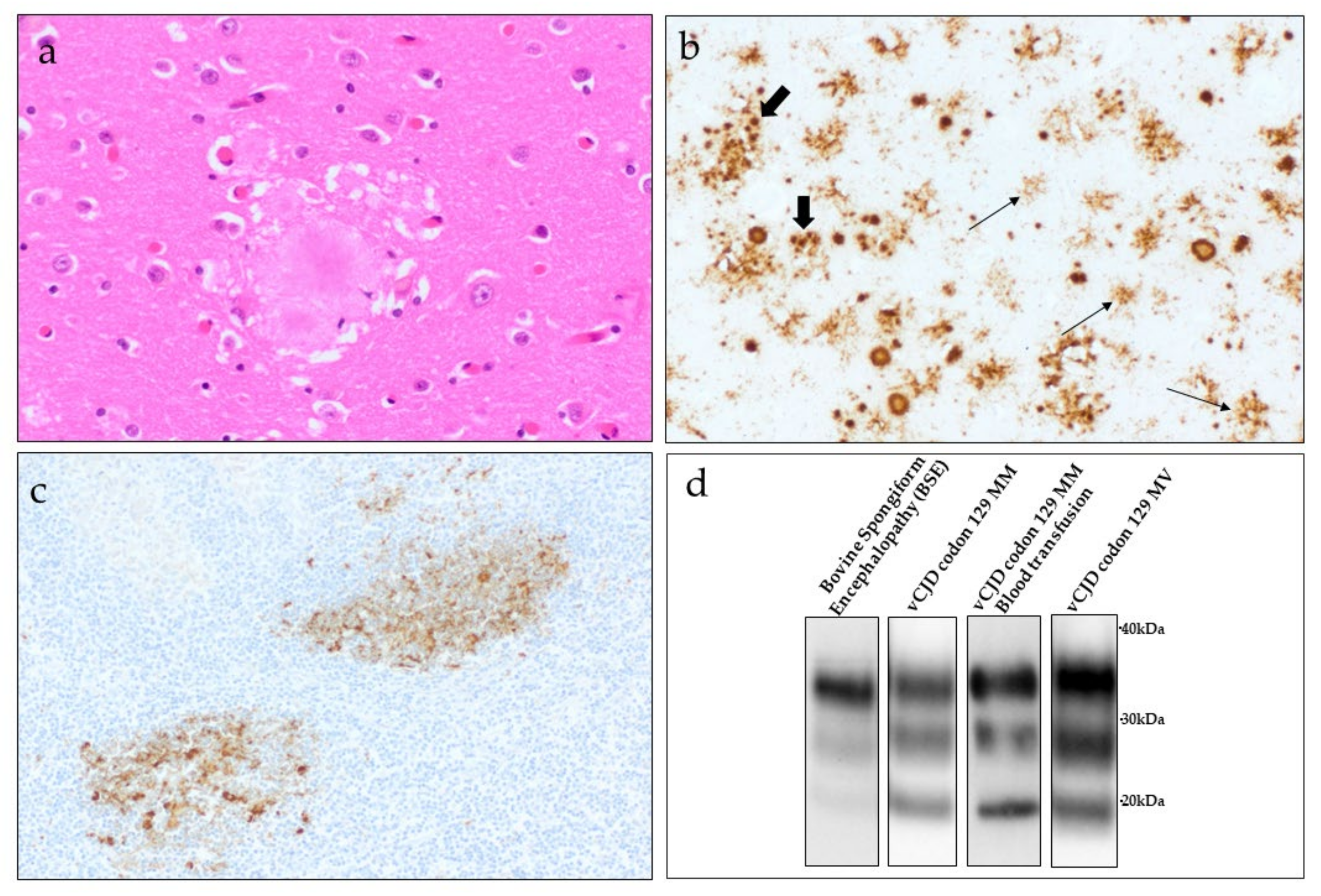
5.2. Neuropathology
5.3. Peripheral Pathology
5.4. Biochemical Features
6. Current Public Health Concerns from vCJD
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef]
- Bolton, D.C.; McKinley, M.P.; Prusiner, S.B. Identification of a protein that purifies with the scrapie prion. Science 1982, 218, 1309–1311. [Google Scholar] [CrossRef] [PubMed]
- McKinley, M.P.; Bolton, D.C.; Prusiner, S.B. A protease-resistant protein is a structural component of the scrapie prion. Cell 1983, 35, 57–62. [Google Scholar] [CrossRef]
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar] [CrossRef] [PubMed]
- Ladogana, A.; Puopolo, M.; Croes, E.A.; Budka, H.; Jarius, C.; Collins, S.; Klug, G.M.; Sutcliffe, T.; Giulivi, A.; Alperovitch, A.; et al. Mortality from Creutzfeldt-Jakob disease and related disorders in Europe, Australia, and Canada. Neurology 2005, 64, 1586–1591. [Google Scholar] [CrossRef]
- Prusiner, S.B. Novel proteinaceous particles cause scrapie. Science 1982, 216, 136–144. [Google Scholar] [CrossRef]
- Will, R.G.; Ironside, J.W.; Zeidler, M.; Cousens, S.N.; Estibeiro, K.; Alperovitch, A.; Poser, S.; Pocchiari, M.; Hofman, A.; Smith, P.G. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 1996, 347, 921–925. [Google Scholar] [CrossRef]
- Wells, G.A.; Scott, A.C.; Johnson, C.T.; Gunning, R.F.; Hancock, R.D.; Jeffrey, M.; Dawson, M.; Bradley, R. A novel progressive spongiform encephalopathy in cattle. Vet. Rec. 1987, 121, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Active TSE Disease Surveillance Statistics, Gov UK. Available online: https://www.gov.uk/government/publications/active-tse-surveillance-statistics (accessed on 1 July 2021).
- World Organization for Animal Health. Bovine Spongiform Encephalopathy (BSE). Available online: https://www.oie.int/en/disease/bovine-spongiform-encephalopathy/ (accessed on 1 July 2021).
- Wells, G.A.; Wilesmith, J.W. The neuropathology and epidemiology of bovine spongiform encephalopathy. Brain Pathol. 1995, 5, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.G.; Bradley, R. Bovine spongiform encephalopathy (BSE) and its epidemiology. Br. Med. Bull. 2003, 66, 185–198. [Google Scholar] [CrossRef]
- Capobianco, R.; Casalone, C.; Suardi, S.; Mangieri, M.; Miccolo, C.; Limido, L.; Catania, M.; Rossi, G.; Di Fede, G.; Giaccone, G.; et al. Conversion of the BASE prion strain into the BSE strain: The origin of BSE? PLoS Pathog. 2007, 3, e31. [Google Scholar] [CrossRef]
- Torres, J.M.; Andréoletti, O.; Lacroux, C.; Prieto, I.; Lorenzo, P.; Larska, M.; Baron, T.; Espinosa, J.C. Classical bovine spongiform encephalopathy by transmission of H-type prion in homologous prion protein context. Emerg. Infect. Dis. 2011, 17, 1636–1644. [Google Scholar] [CrossRef]
- Béringue, V.; Andréoletti, O.; Le Dur, A.; Essalmani, R.; Vilotte, J.L.; Lacroux, C.; Reine, F.; Herzog, L.; Biacabé, A.G.; Baron, T.; et al. A bovine prion acquires an epidemic bovine spongiform encephalopathy strain-like phenotype on interspecies transmission. J. Neurosci. 2007, 27, 6965–6971. [Google Scholar] [CrossRef]
- Huor, A.; Espinosa, J.C.; Vidal, E.; Cassard, H.; Douet, J.Y.; Lugan, S.; Aron, N.; Marín-Moreno, A.; Lorenzo, P.; Aguilar-Calvo, P. The emergence of classical BSE from atypical/Nor98 scrapie. Proc. Natl. Acad. Sci. USA 2019, 116, 26853–26862. [Google Scholar] [CrossRef]
- The European Commission for Food Safety. TSE Route Map 2. Available online: https://ec.europa.eu/food/system/files/2016-10/biosafety_food-borne-disease_tse_road-map2.pdf (accessed on 1 July 2021).
- Aldhous, P. BSE: Spongiform encephalopathy found in cat. Nature 1990, 345, 194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pearson, G.R.; Gruffydd-Jones, T.J.; Wyatt, J.M.; Hope, J.; Chong, A.; Scott, A.C.; Dawson, M.; Wells, G.A. Feline spongiform encephalopathy. Vet. Rec. 1991, 128, 532. [Google Scholar] [CrossRef]
- Willoughby, K.; Kelly, D.F.; Lyon, D.G.; Wells, G.A. Spongiform encephalopathy in a captive puma (Felis concolor). Vet. Rec. 1992, 131, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, J.K.; Cunningham, A.A. Epidemiological observations on spongiform encephalopathies in captive wild animals in the British Isles. Vet. Rec. 1994, 135, 296–303. [Google Scholar] [CrossRef] [PubMed]
- National CJD Research & Surveillance Unit, University of Edinburgh. Available online: http://www.cjd.ed.ac.uk (accessed on 1 July 2021).
- Will, R.G.; Alperovitch, A.; Poser, S.; Pocchiari, M.; Hofman, A.; Mitrova, E.; de Silva, R.; D’Alessandro, M.; Delasnerie-Laupretre, N.; Zerr, I.; et al. Descriptive epidemiology of Creutzfeldt-Jakob disease in six European countries, 1993–1995. EU Collaborative Study Group for CJD. Ann. Neurol. 1998, 43, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yanagawa, H.; Hoshi, K.; Yoshino, H.; Urata, J.; Sato, T. Incidence rate of Creutzfeldt-Jakob disease in Japan. Int. J. Epidemiol. 1999, 28, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Puopolo, M.; Ladogana, A.; Almonti, S.; Daude, N.; Bevivino, S.; Petraroli, R.; Poleggi, A.; Quanguo, L.; Pocchiari, M. Mortality trend from sporadic Creutzfeldt-Jakob disease (CJD) in Italy, 1993–2000. J. Clin. Epidemiol. 2003, 56, 494–499. [Google Scholar] [CrossRef]
- Heinemann, U.; Krasnianski, A.; Meissner, B.; Varges, D.; Kallenberg, K.; Schulz-Schaeffer, W.J.; Steinhoff, B.J.; Grasbon-Frodl, E.M.; Kretzschmar, H.A.; Zerr, I. Creutzfeldt-Jakob disease in Germany: A prospective 12-year surveillance. Brain 2007, 130, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Brandel, J.P.; Peckeu, L.; Haïk, S. The French surveillance network of Creutzfeldt-Jakob disease. Epidemiological data in France and worldwide. Transfus. Clin. Biol. 2013, 20, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Case Western Reserve University. National Prion Disease Pathology Surveillance Center. Available online: https://case.edu/medicine/pathology/divisions/prion-center (accessed on 1 July 2021).
- Palmer, C.M. A week that shook the meat industry: The effects on the UK beef industry of the BSE crisis. Br. Food J. 1996, 98, 17–25. [Google Scholar] [CrossRef]
- British Beef Ban, BBC News. Available online: http://news.bbc.co.uk/1/hi/uk/4785610.stm (accessed on 1 July 2021).
- US to Lift Ban on UK Beef Exports, Gov UK. Available online: https://www.gov.uk/government/news/us-to-lift-ban-on-uk-beef-exports (accessed on 1 July 2021).
- Collinge, J.; Sidle, K.C.; Meads, J.; Ironside, J.; Hill, A.F. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature 1996, 383, 685–690. [Google Scholar] [CrossRef]
- Padilla, D.; Béringue, V.; Espinosa, J.C.; Andreoletti, O.; Jaumain, E.; Reine, F.; Herzog, L.; Gutierrez-Adan, A.; Pintado, B.; Laude, H. Sheep and goat BSE propagate more efficiently than cattle BSE in human PrP transgenic mice. PLoS Pathog. 2011, 7, 1001319. [Google Scholar] [CrossRef] [PubMed]
- Plinston, C.; Hart, P.; Hunter, N.; Manson, J.C.; Barron, R.M. Increased susceptibility of transgenic mice expressing human PrP to experimental sheep bovine spongiform encephalopathy is not due to increased agent titre in sheep brain tissue. J. Gen. Virol. 2014, 95, 1855–1859. [Google Scholar] [CrossRef]
- Lasmézas, C.I.; Deslys, J.P.; Demaimay, R.; Adjou, K.T.; Lamoury, F.; Dormont, D.; Robain, O.; Ironside, J.; Hauw, J.J. BSE transmission to macaques. Nature 1996, 381, 743–744. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.E.; Will, R.G.; Ironside, J.W.; McConnell, I.; Drummond, D.; Suttie, A.; McCardle, L.; Chree, A.; Hope, J.; Birkett, C.; et al. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 1997, 389, 498–501. [Google Scholar] [CrossRef]
- Dickinson, A.G.; Mackay, J.M. Genetical control of the incubation period in mice of the neurological disease, scrapie. Heredity 1964, 19, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.G.; Meikle, V.M. Host-genotype and agent effects in scrapie incubation: Change in allelic interaction with different strains of agent. Mol. Gen. Genet. 1971, 112, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.; Dickinson, A.G. Scrapie in mice: Agent-strain differences in the distribution and intensity of grey matter vacuolation. J. Comp. Pathol. 1973, 83, 29–40. [Google Scholar] [CrossRef]
- Bruce, M.; McConnell, I.; Fraser, H.; Dickinson, A.G. The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: Implications for the nature of the agent and host control of pathogenesis. J. Gen. Virol. 1991, 72, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.E. Scrapie strain variation and mutation. Br. Med. Bull. 1993, 49, 822–838. [Google Scholar] [CrossRef]
- Fraser, H.; McConnell, I.; Wells, G.A.; Dawson, M. Transmission of bovine spongiform encephalopathy to mice. Vet. Rec. 1988, 123, 472. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.; Bruce, M.E.; Chree, A.; McConnell, I.; Wells, G.A. Transmission of bovine spongiform encephalopathy and scrapie to mice. J. Gen. Virol. 1992, 73, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.; Pearson, G.R.; McConnell, I.; Bruce, M.E.; Wyatt, J.M.; Gruffydd-Jones, T.J. Transmission of feline spongiform encephalopathy to mice. Vet. Rec. 1994, 134, 449. [Google Scholar] [CrossRef]
- Bruce, M.; Chree, A.; McConnell, I.; Foster, J.; Pearson, G.; Fraser, H. Transmission of bovine spongiform encephalopathy and scrapie to mice: Strain variation and the species barrier. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 343, 405–411. [Google Scholar] [CrossRef]
- Brown, D.A.; Bruce, M.E.; Fraser, J.R. Comparison of the neuropathological characteristics of bovine spongiform encephalopathy (BSE) and variant Creutzfeldt-Jakob disease (vCJD) in mice. Neuropathol. Appl. Neurobiol. 2003, 29, 262–272. [Google Scholar] [CrossRef]
- Ritchie, D.L.; Boyle, A.; McConnell, I.; Head, M.W.; Ironside, J.W.; Bruce, M.E. Transmissions of variant Creutzfeldt-Jakob disease from brain and lymphoreticular tissue show uniform and conserved bovine spongiform encephalopathy-related phenotypic properties on primary and secondary passage in wild-type mice. J. Gen. Virol. 2009, 90, 3075–3082. [Google Scholar] [CrossRef]
- Hill, A.F.; Desbruslais, M.; Joiner, S.; Sidle, K.C.; Gowland, I.; Collinge, J.; Doey, L.J.; Lantos, P. The same prion strain causes vCJD and BSE. Nature 1997, 389, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.E.; Will, R.G.; Ironside, J.W.; Fraser, H. Comparison of the biological characteristics of BSE and CJD in mice. In Alzheimers Disease and Related Disorders; EdsIqbal, K., Swaab, D.F., Winblad, B., Wisniewski, H.M., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1999; pp. 553–559. [Google Scholar]
- Scott, M.R.; Will, R.; Ironside, J.; Nguyen, H.O.; Tremblay, P.; DeArmond, S.J.; Prusiner, S.B. Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc. Natl. Acad. Sci. USA 1999, 96, 15137–15142. [Google Scholar] [CrossRef] [PubMed]
- Asante, E.A.; Linehan, J.M.; Desbruslais, M.; Joiner, S.; Gowland, I.; Wood, A.L.; Welch, J.; Hill, A.F.; Lloyd, S.E.; Wadsworth, J.D.; et al. BSE prions propagate as either variant CJD-like or sporadic CJD-like prion strains in transgenic mice expressing human prion protein. EMBO J. 2002, 21, 6358–6366. [Google Scholar] [CrossRef]
- Wadsworth, J.D.; Asante, E.A.; Desbruslais, M.; Linehan, J.M.; Joiner, S.; Gowland, I.; Welch, J.; Stone, L.; Lloyd, S.E.; Hill, A.F.; et al. Human prion protein with valine 129 prevents expression of variant CJD phenotype. Science 2004, 306, 1793–1796. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.T.; Hart, P.; Aitchison, L.; Baybutt, H.N.; Plinston, C.; Thomson, V.; Tuzi, N.L.; Head, M.W.; Ironside, J.W.; Will, R.G.; et al. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol. 2006, 5, 393–398. [Google Scholar] [CrossRef]
- Asante, E.A.; Linehan, J.M.; Gowland, I.; Joiner, S.; Fox, K.; Cooper, S.; Osiguwa, O.; Gorry, M.; Welch, J.; Houghton, R.; et al. Dissociation of pathological and molecular phenotype of variant Creutzfeldt-Jakob disease in transgenic human prion protein 129 heterozygous mice. Proc. Natl. Acad. Sci. USA 2006, 103, 10759–10764. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.E.; McConnell, I.; Will, R.G.; Ironside, J.W. Detection of variant Creutzfeldt-Jakob disease infectivity in extraneural tissues. Lancet 2001, 358, 208–209. [Google Scholar] [CrossRef]
- Bishop, M.T.; Diack, A.B.; Ritchie, D.L.; Ironside, J.W.; Will, R.G.; Manson, J.C. Prion infectivity in the spleen of a PRNP heterozygous individual with subclinical variant Creutzfeldt-Jakob disease. Brain 2013, 136, 1139–1145. [Google Scholar] [CrossRef]
- Diack, A.B.; Boyle, A.; Plinston, C.; Hunt, E.; Bishop, M.T.; Will, R.G.; Manson, J.C. Variant Creutzfeldt-Jakob disease strain is identical in individuals of two PRNP codon 129 genotypes. Brain 2019, 142, 1416–1428. [Google Scholar] [CrossRef]
- Boyle, A.; Plinston, C.; Laing, F.; Mackenzie, G.; Will, R.G.; Manson, J.C.; Diack, A.B. No Adaptation of the Prion Strain in a Heterozygous Case of Variant Creutzfeldt-Jakob Disease. Emerg. Infect. Dis. 2020, 26, 1300–1303. [Google Scholar] [CrossRef]
- Diack, A.B.; Ritchie, D.; Bishop, M.; Pinion, V.; Brandel, J.P.; Haik, S.; Tagliavini, F.; Van Duijn, C.; Belay, E.D.; Gambetti, P.; et al. Constant transmission properties of variant Creutzfeldt-Jakob disease in 5 countries. Emerg. Infect. Dis. 2012, 18, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Diack, A.B.; Boyle, A.; Ritchie, D.; Plinston, C.; Kisielewski, D.; de Pedro-Cuesta, J.; Rábano, A.; Will, R.G.; Manson, J.C. Similarities of Variant Creutzfeldt-Jakob Disease Strain in Mother and Son in Spain to UK Reference Case. Emerg. Infect. Dis. 2017, 23, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.T.; Ritchie, D.L.; Will, R.G.; Ironside, J.W.; Head, M.W.; Thomson, V.; Bruce, M.; Manson, J.C. No major change in vCJD agent strain after secondary transmission via blood transfusion. PLoS ONE 2008, 3, e2878. [Google Scholar] [CrossRef] [PubMed]
- Bessen, R.A.; Marsh, R.F. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J. Virol. 1994, 68, 7859–7868. [Google Scholar] [CrossRef]
- Telling, G.C.; Parchi, P.; DeArmond, S.J.; Cortelli, P.; Montagna, P.; Gabizon, R.; Mastrianni, J.; Lugaresi, E.; Gambetti, P.; Prusiner, S.B. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 1996, 274, 2079–2082. [Google Scholar] [CrossRef] [PubMed]
- Somerville, R.A.; Chong, A.; Mulqueen, O.U.; Birkett, C.R.; Wood, S.C.; Hope, J. Biochemical typing of scrapie strains. Nature 1997, 386, 564. [Google Scholar] [CrossRef] [PubMed]
- Safar, J.; Wille, H.; Itri, V.; Groth, D.; Serban, H.; Torchia, M.; Cohen, F.E.; Prusiner, S.B. Eight prion strains have PrP(Sc) molecules with different conformations. Nat. Med. 1998, 4, 1157–1165. [Google Scholar] [CrossRef]
- Caughey, B.; Raymond, G.J.; Bessen, R.A. Strain-dependent differences in beta-sheet conformations of abnormal prion protein. J. Biol. Chem. 1998, 273, 32230–32235. [Google Scholar] [CrossRef]
- Peretz, D.; Williamson, R.A.; Legname, G.; Matsunaga, Y.; Vergara, J.; Burton, D.R.; DeArmond, S.J.; Prusiner, S.B.; Scott, M.R. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 2002, 34, 921–932. [Google Scholar] [CrossRef]
- Parchi, P.; Giese, A.; Capellari, S.; Brown, P.; Schulz-Schaeffer, W.; Windl, O.; Zerr, I.; Budka, H.; Kopp, N.; Piccardo, P.; et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann. Neurol. 1999, 46, 224–233. [Google Scholar] [CrossRef]
- Parchi, P.; Capellari, S.; Chen, S.G.; Petersen, R.B.; Gambetti, P.; Kopp, N.; Brown, P.; Kitamoto, T.; Tateishi, J.; Giese, A.; et al. Typing prion isoforms. Nature 1997, 386, 232–234. [Google Scholar] [CrossRef]
- Variant CJD Cases Worldwide Creutzfeldt-Jakob Disease International Surveillance Network. Available online: https://www.eurocjd.ed.ac.uk/data_tables (accessed on 1 July 2021).
- Riverol, M.; Palma, J.A.; Alañá, M.; Guerrero-Márquez, C.; Luquin, M.R.; Rábano, A. Variant Creutzfeldt-Jakob disease occurring in mother and son. J. Neurol. Neurosurg. Psychiatry 2012, 83, 235–236. [Google Scholar] [CrossRef]
- Jansen, G.H.; Voll, C.L.; Robinson, C.A.; Gervais, R.; Sutcliffe, T.; Bergeron, C.; Coulthart, M.B.; Giulivi, A. First case of variant Creutzfeldt-Jakob disease in Canada. Can. Commun. Dis. Rep. 2003, 29, 117–120. [Google Scholar]
- Yamada, M. Variant CJD Working Group, Creutzfeldt-Jakob Disease Surveillance Committee, Japan. The first Japanese case of variant Creutzfeldt-Jakob disease showing periodic electroencephalogram. Lancet 2006, 367, 874. [Google Scholar] [CrossRef]
- Holman, R.C.; Belay, E.D.; Christensen, K.Y.; Maddox, R.A.; Minino, A.M.; Folkema, A.M.; Haberling, D.L.; Hammett, T.A.; Kochanek, K.D.; Sejvar, J.J.; et al. Human prion diseases in the United States. PLoS ONE 2010, 5, e8521. [Google Scholar] [CrossRef]
- Brandel, J.P.; Heath, C.A.; Head, M.W.; Levavasseur, E.; Knight, R.; Laplanche, J.L.; Langeveld, J.P.; Ironside, J.W.; Hauw, J.J.; Mackenzie, J.; et al. Variant Creutzfeldt-Jakob disease in France and the United Kingdom: Evidence for the same agent strain. Ann. Neurol. 2009, 65, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Fuh, J.L.; Wang, S.J.; Lirng, J.F.; Yang, C.C.; Cheng, S.J. Probable variant Creutzfeldt–Jakob disease in Asia: A case report from Taiwan and review of two prior cases. Psychiatry Clin. Neurosci. 2010, 64, 652–658. [Google Scholar] [CrossRef]
- Maheshwari, A.; Fischer, M.; Gambetti, P.; Parker, A.; Ram, A.; Soto, C.; Concha-Marambio, L.; Cohen, Y.; Belay, E.D.; Maddox, R.A.; et al. Recent US Case of Variant Creutzfeldt-Jakob Disease-Global Implications. Emerg. Infect. Dis. 2015, 21, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Juan, P.; Cousens, S.N.; Will, R.G.; van Duijn, C.M. Source of variant Creutzfeldt-Jakob disease outside United Kingdom. Emerg. Infect. Dis. 2007, 13, 1166–1169. [Google Scholar] [CrossRef]
- Chadeau-Hyam, M.; Tard, A.; Bird, S.; Le Guennec, S.; Bemrah, N.; Volatier, J.L.; Alpérovitch, A. Estimation of the exposure of the French population to the BSE agent: Comparison of the 1980-95 consumption of beef products containing mechanically recovered meat in France and the UK, by birth cohort and gender. Stat. Methods Med. Res. 2003, 12, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Creutzfeldt-Jakob Disease Surveillance in the UK, 29th Annual Report 2019. National CJD Research & Surveillance Unit, University of Edinburgh. Available online: https://www.cjd.ed.ac.uk/sites/default/files/Report28.pdf (accessed on 1 July 2021).
- Brandel, J.P.; Vlaicu, M.B.; Culeux, A.; Belondrade, M.; Bougard, D.; Grznarova, K.; Denouel, A.; Plu, I.; Bouaziz-Amar, E.; Seilhean, D.; et al. Variant Creutzfeldt-Jakob Disease Diagnosed 7.5 Years after Occupational Exposure. N. Engl. J. Med. 2020, 383, 83–85. [Google Scholar] [CrossRef]
- Ward, H.J.; Everington, D.; Cousens, S.N.; Smith-Bathgate, B.; Leitch, M.; Cooper, S.; Heath, C.; Knight, R.S.; Smith, P.G.; Will, R.G. Risk factors for variant Creutzfeldt-Jakob disease: A case-control study. Ann. Neurol. 2006, 59, 111–120. [Google Scholar] [CrossRef]
- Watson, N.; Brandel, J.P.; Green, A.; Hermann, P.; Ladogana, A.; Lindsay, T.; Mackenzie, J.; Pocchiari, M.; Smith, C.; Zerr, I.; et al. The importance of ongoing international surveillance for Creutzfeldt-Jakob disease. Nat. Rev. Neurol. 2021, 17, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Jaunmuktane, Z.; Joiner, S.; Campbell, T.; Morgan, C.; Wakerley, B.; Golestani, F.; Rudge, P.; Mead, S.; Jäger, H.R.; et al. Variant Creutzfeldt-Jakob Disease in a Patient with Heterozygosity at PRNP Codon 129. N. Engl. J. Med. 2017, 376, 292–294. [Google Scholar] [CrossRef]
- Kaski, D.; Mead, S.; Hyare, H.; Cooper, S.; Jampana, R.; Overell, J.; Knight, R.; Collinge, J.; Rudge, P. Variant CJD in an individual heterozygous for PRNP codon 129. Lancet 2009, 374, 2128. [Google Scholar] [CrossRef]
- Jones, M.; Peden, A.H.; Prowse, C.V.; Gröner, A.; Manson, J.C.; Turner, M.L.; Ironside, J.W.; MacGregor, I.R.; Head, M.W. In vitro amplification and detection of variant Creutzfeldt-Jakob disease PrPSc. J. Pathol. 2007, 213, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, K.; Zou, S.; Schonberger, L.B.; Sullivan, M.; Kessler, D.; Notari, E., IV; Fang, C.T.; Dodd, R.Y. Lack of evidence of transfusion transmission of Creutzfeldt-Jakob disease in a US surveillance study. Transfusion 2009, 49, 977–984. [Google Scholar] [CrossRef]
- Puopolo, M.; Ladogana, A.; Vetrugno, V.; Pocchiari, M. Transmission of sporadic Creutzfeldt-Jakob disease by blood transfusion: Risk factor or possible biases. Transfusion 2011, 51, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Head, M.W.; Ritchie, D.; Smith, N.; McLoughlin, V.; Nailon, W.; Samad, S.; Masson, S.; Bishop, M.; McCardle, L.; Ironside, J.W. Peripheral tissue involvement in sporadic, iatrogenic, and variant Creutzfeldt-Jakob disease: An immunohistochemical, quantitative, and biochemical study. Am. J. Pathol. 2004, 164, 143–153. [Google Scholar] [CrossRef]
- Hill, A.F.; Zeidler, M.; Ironside, J.; Collinge, J. Diagnosis of new variant Creutzfeldt-Jakob disease by tonsil biopsy. Lancet 1997, 349, 99–100. [Google Scholar] [CrossRef]
- Hilton, D.A.; Fathers, E.; Edwards, P.; Ironside, J.W.; Zajicek, J. Prion immunoreactivity in appendix before clinical onset of variant Creutzfeldt-Jakob disease. Lancet 1998, 352, 703–704. [Google Scholar] [CrossRef]
- Joiner, S.; Linehan, J.; Brandner, S.; Wadsworth, J.D.; Collinge, J. Irregular presence of abnormal prion protein in appendix in variant Creutzfeldt-Jakob disease. J. Neurol. Neurosurg. Psychiatry 2002, 73, 597–598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Houston, F.; Foster, J.D.; Chong, A.; Hunter, N.; Bostock, C.J. Transmission of BSE by blood transfusion in sheep. Lancet 2000, 356, 999–1000. [Google Scholar] [CrossRef]
- Hunter, N.; Foster, J.; Chong, A.; McCutcheon, S.; Parnham, D.; Eaton, S.; MacKenzie, C.; Houston, F. Transmission of prion diseases by blood transfusion. J. Gen. Virol. 2002, 83, 2897–2905. [Google Scholar] [CrossRef] [PubMed]
- Houston, F.; McCutcheon, S.; Goldmann, W.; Chong, A.; Foster, J.; Sisó, S.; González, L.; Jeffrey, M.; Hunter, N. Prion diseases are efficiently transmitted by blood transfusion in sheep. Blood 2008, 112, 4739–4745. [Google Scholar] [CrossRef] [PubMed]
- Llewelyn, C.A.; Hewitt, P.E.; Knight, R.S.; Amar, K.; Cousens, S.; Mackenzie, J.; Will, R.G. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet 2004, 363, 417–421. [Google Scholar] [CrossRef]
- Hewitt, P.E.; Llewelyn, C.A.; Mackenzie, J.; Will, R.G. Creutzfeldt-Jakob disease and blood transfusion: Results of the UK Transfusion Medicine Epidemiological Review study. Vox Sang. 2006, 91, 221–230. [Google Scholar] [CrossRef]
- Wroe, S.J.; Pal, S.; Siddique, D.; Hyare, H.; Macfarlane, R.; Joiner, S.; Linehan, J.M.; Brandner, S.; Wadsworth, J.D.; Hewitt, P.; et al. Clinical presentation and pre-mortem diagnosis of variant Creutzfeldt-Jakob disease associated with blood transfusion: A case report. Lancet 2006, 368, 2061–2067. [Google Scholar] [CrossRef]
- Peden, A.H.; Head, M.W.; Ritchie, D.L.; Bell, J.E.; Ironside, J.W. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 2004, 364, 527–529. [Google Scholar] [CrossRef]
- Garske, T.; Ghani, A.C. Uncertainty in the Tail of the Variant Creutzfeldt-Jakob Disease Epidemic in the UK. PLoS ONE 2010, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Valleron, A.J.; Boelle, P.Y.; Will, R.; Cesbron, J.Y. Estimation of epidemic size and incubation time based on age characteristics of vCJD in the United Kingdom. Science 2001, 294, 1726–1728. [Google Scholar] [CrossRef]
- Ghani, A.C.; Ferguson, N.M.; Donnelly, C.A.; Anderson, R.M. Short-term projections for variant Creutzfeldt-Jakob disease onsets. Stat. Methods Med. Res. 2003, 12, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Urwin, P.J.; Mackenzie, J.M.; Llewelyn, C.A.; Will, R.G.; Hewitt, P.E. Creutzfeldt-Jakob disease and blood transfusion: Updated results of the UK Transfusion Medicine Epidemiology Review Study. Vox Sang. 2016, 110, 310–316. [Google Scholar] [CrossRef]
- McCutcheon, S.; Alejo Blanco, A.R.; Houston, E.F.; de Wolf, C.; Tan, B.C.; Smith, A.; Groschup, M.H.; Hunter, N.; Hornsey, V.S.; MacGregor, I.R.; et al. All clinically-relevant blood components transmit prion disease following a single blood transfusion: A sheep model of vCJD. PLoS ONE 2011, 8, e23169. [Google Scholar] [CrossRef] [PubMed]
- Head, M.W.; Yull, H.M.; Ritchie, D.L.; Bishop, M.T.; Ironside, J.W. Pathological investigation of the first blood donor and recipient pair linked by transfusion-associated variant Creutzfeldt-Jakob disease transmission. Neuropathol. Appl. Neurobiol. 2009, 35, 433–436. [Google Scholar] [CrossRef] [PubMed]
- El Tawil, S.; Mackay, G.; Davidson, L.; Summers, D.; Knight, R.; Will, R. Variant Creutzfeldt-Jakob disease in older patients. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1279–1280. [Google Scholar] [CrossRef]
- Comoy, E.E.; Mikol, J.; Jaffré, N.; Lebon, V.; Levavasseur, E.; Streichenberger, N.; Sumian, C.; Perret-Liaudet, A.; Eloit, M.; Andreoletti, O.; et al. Experimental transfusion of variant CJD-infected blood reveals previously uncharacterised prion disorder in mice and macaque. Nat. Commun. 2017, 2, 1268. [Google Scholar] [CrossRef] [PubMed]
- Peden, A.; McCardle, L.; Head, M.W.; Love, S.; Ward, H.J.; Cousens, S.N.; Keeling, D.M.; Millar, C.M.; Hill, F.G.; Ironside, J.W. Variant CJD infection in the spleen of a neurologically asymptomatic UK adult patient with haemophilia. Haemophilia 2010, 16, 296–304. [Google Scholar] [CrossRef]
- Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO), Gov UK. Available online: https://www.gov.uk/government/groups/advisory-committee-on-the-safety-of-blood-tissues-and-organs (accessed on 1 July 2021).
- Edgeworth, J.A.; Farmer, M.; Sicilia, A.; Tavares, P.; Beck, J.; Campbell, T.; Lowe, J.; Mead, S.; Rudge, P.; Collinge, J.; et al. Detection of prion infection in variant Creutzfeldt-Jakob disease: A blood-based assay. Lancet 2011, 377, 487–493. [Google Scholar] [CrossRef]
- Jackson, G.S.; Burk-Rafel, J.; Edgeworth, J.A.; Sicilia, A.; Abdilahi, S.; Korteweg, J.; Mackey, J.; Thomas, C.; Wang, G.; Schott, J.M.; et al. Population screening for variant Creutzfeldt-Jakob disease using a novel blood test: Diagnostic accuracy and feasibility study. JAMA Neurol. 2014, 71, 421–428. [Google Scholar] [CrossRef]
- Bougard, D.; Brandel, J.P.; Bélondrade, M.; Béringue, V.; Segarra, C.; Fleury, H.; Laplanche, J.L.; Mayran, C.; Nicot, S.; Green, A.; et al. Detection of prions in the plasma of presymptomatic and symptomatic patients with variant Creutzfeldt-Jakob disease. Sci. Transl. Med. 2016, 8, 370ra182. [Google Scholar] [CrossRef] [PubMed]
- Lacroux, C.; Comoy, E.; Moudjou, M.; Perret-Liaudet, A.; Lugan, S.; Litaise, C.; Simmons, H.; Jas-Duval, C.; Lantier, I.; Béringue, V.; et al. Preclinical detection of variant CJD and BSE prions in blood. PLoS Pathog. 2014, 10, e1004202. [Google Scholar] [CrossRef] [PubMed]
- Concha-Marambio, L.; Chacon, M.A.; Soto, C. Preclinical Detection of Prions in Blood of Nonhuman Primates Infected with Variant Creutzfeldt-Jakob Disease. Emerg. Infect. Dis. 2020, 26, 34–43. [Google Scholar] [CrossRef]
- Concha-Marambio, L.; Pritzkow, S.; Moda, F.; Tagliavini, F.; Ironside, J.W.; Schulz, P.E.; Soto, C. Detection of prions in blood from patients with variant Creutzfeldt-Jakob disease. Sci. Transl. Med. 2016, 8, 370ra183. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, R.; Sano, K.; Satoh, K.; Nishida, N. Real-time quaking-induced conversion: A highly sensitive assay for prion detection. Prion 2011, 5, 150–153. [Google Scholar] [CrossRef]
- Wilham, J.M.; Orrú, C.D.; Bessen, R.A.; Atarashi, R.; Sano, K.; Race, B.; Meade-White, K.D.; Taubner, L.M.; Timmes, A.; Caughey, B. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 2010, 6, e1001217. [Google Scholar] [CrossRef]
- Peden, A.H.; McGuire, L.I.; Appleford, N.E.J.; Mallinson, G.; Wilham, J.M.; Orrú, C.D.; Caughey, B.; Ironside, J.W.; Knight, R.S.; Will, R.G.; et al. Sensitive and specific detection of sporadic Creutzfeldt-Jakob disease brain prion protein using real-time quaking-induced conversion. J. Gen. Virol. 2012, 93, 438–449. [Google Scholar] [CrossRef]
- Orrú, C.D.; Wilham, J.M.; Raymond, L.D.; Kuhn, F.; Schroeder, B.; Raeber, A.J.; Caughey, B. Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. MBio 2011, 2, e00078-11. [Google Scholar] [CrossRef]
- Orrú, C.D.; Groveman, B.R.; Raymond, L.D.; Hughson, A.G.; Nonno, R.; Zou, W.; Ghetti, B.; Gambetti, P.; Caughey, B. Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains. PLoS Pathog. 2015, 11, e1004983. [Google Scholar] [CrossRef]
- Green, A.J.E.; Zanusso, G. Prion protein amplification techniques. Handb. Clin. Neurol. 2018, 153, 357–370. [Google Scholar] [CrossRef]
- Diagnostic Criteria for Human Prion Diseases, National CJD Research & Surveillance Unit. Available online: http://www.cjd.ed.ac.uk/sites/default/files/criteria_0.pdf (accessed on 1 July 2021).
- Heath, C.A.; Cooper, S.A.; Murray, K.; Lowman, A.; Henry, C.; MacLeod, M.A.; Stewart, G.E.; Zeidler, M.; MacKenzie, J.M.; Ironside, J.W.; et al. Validation of diagnostic criteria for variant Creutzfeldt-Jakob disease. Ann. Neurol. 2010, 67, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Barbot, C.; Castro, L.; Oliveira, C.; Carpenter, S. Variant Creutzfeldt-Jakob disease: The first confirmed case from Portugal shows early onset, long duration and unusual pathology. J. Neurol. Neurosurg. Psychiatry 2010, 81, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.D.; Bird, S.M. UK dietary exposure to BSE in beef mechanically recovered meat: By birth cohort and gender. J. Cancer Epidemiol. Prev. 2002, 7, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Boëlle, P.Y.; Cesbron, J.Y.; Valleron, A.J. Epidemiological evidence of higher susceptibility to vCJD in the young. BMC Infect. Dis. 2004, 4, 26. [Google Scholar] [CrossRef]
- Zeidler, M.; Johnstone, E.C.; Bamber, R.W.; Dickens, C.M.; Fisher, C.J.; Francis, A.F.; Goldbeck, R.; Higgo, R.; Johnson-Sabine, E.C.; Lodge, G.J.; et al. New variant Creutzfeldt-Jakob disease: Psychiatric features. Lancet 1997, 350, 908–910. [Google Scholar] [CrossRef]
- Spencer, M.D.; Knight, R.S.; Will, R.G. First hundred cases of variant Creutzfeldt-Jakob disease: Retrospective case note review of early psychiatric and neurological features. BMJ 2002, 324, 1479–1482. [Google Scholar] [CrossRef]
- Macleod, M.A.; Stewart, G.E.; Zeidler, M.; Will, R.; Knight, R. Sensory features of variant Creutzfeldt-Jakob disease. J. Neurol. 2002, 249, 706–711. [Google Scholar] [CrossRef]
- Zeidler, M.; Stewart, G.E.; Barraclough, C.R.; Bateman, D.E.; Bates, D.; Burn, D.J.; Colchester, A.C.; Durward, W.; Fletcher, N.A.; Hawkins, S.A.; et al. New variant Creutzfeldt-Jakob disease: Neurological features and diagnostic tests. Lancet 1997, 350, 903–907. [Google Scholar] [CrossRef]
- Collie, D.A.; Summers, D.M.; Sellar, R.J.; Ironside, J.W.; Cooper, S.; Zeidler, M.; Knight, R.; Will, R.G. Diagnosing variant Creutzfeldt-Jakob disease with the pulvinar sign: MR imaging findings in 86 neuropathologically confirmed cases. AJNR Am. J. Neuroradiol. 2003, 24, 1560–1569. [Google Scholar]
- Steinhoff, B.J.; Zerr, I.; Glatting, M.; Schulz-Schaeffer, W.; Poser, S.; Kretzschmar, H.A. Diagnostic value of periodic complexes in Creutzfeldt-Jakob disease. Ann. Neurol. 2004, 56, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, M.; Ebert, E.; Stoeck, K.; Karch, A.; Collins, S.; Calero, M.; Sklaviadis, T.; Laplanche, J.L.; Golanska, E.; Baldeiras, I.; et al. Validation of 14-3-3 Protein as a Marker in Sporadic Creutzfeldt-Jakob Disease Diagnostic. Mol. Neurobiol. 2016, 53, 2189–2199. [Google Scholar] [CrossRef]
- Green, A.J.E. RT-QuIC: A new test for sporadic CJD. Pract. Neurol. 2019, 19, 49–55. [Google Scholar] [CrossRef]
- Binelli, S.; Agazzi, P.; Giaccone, G.; Will, R.G.; Bugiani, O.; Franceschetti, S.; Tagliavini, F. Periodic electroencephalogram complexes in a patient with variant Creutzfeldt-Jakob disease. Ann. Neurol. 2006, 59, 423–427. [Google Scholar] [CrossRef]
- Green, A.J.; Thompson, E.J.; Stewart, G.E.; Zeidler, M.; McKenzie, J.M.; MacLeod, M.A.; Ironside, J.W.; Will, R.G.; Knight, R.S. Use of 14-3-3 and other brain-specific proteins in CSF in the diagnosis of variant Creutzfeldt-Jakob disease. J. Neurol. Neurosurg. Psychiatry 2001, 70, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Ironside, J.W.; McCardle, L.; Horsburgh, A.; Lim, Z.; Head, M.W. Pathological diagnosis of variant Creutzfeldt-Jakob disease. Apmis 2002, 110, 79–87. [Google Scholar] [CrossRef]
- Head, M.W.; Ironside, J.W.; Ghetti, B.; Jeffrey, M.; Piccardo, P.; Will, R.G. Prion diseases. In Greenfield’s Neuropathology, 9th ed.; Love, S., Budka, H., Ironside, J.W., Perry, A., Eds.; CRC Press: Boca Raton, FL, USA, 2015; Volume 2, pp. 1016–1086. [Google Scholar]
- Ironside, J.W.; Sutherland, K.; Bell, J.E.; McCardle, L.; Barrie, C.; Estebeiro, K.; Zeidler, M.; Will, R.G. A new variant of Creutzfeldt-Jakob disease: Neuropathological and clinical features. Cold Spring Harb. Symp. Quant. Biol. 1996, 61, 523–530. [Google Scholar]
- Takashima, S.; Tateishi, J.; Taguchi, Y.; Hirade, S.; Inoue, H.; Matsui, Y.; Furukawa, H. Creutzfeldt-Jakob disease with a widespread presence of kuru-type plaques after cadaveric dural graft replacement. An autopsy case. Rinsho Shinkeigaku Clin. Neurol. 1997, 37, 824–828. (In Japanese) [Google Scholar]
- Shimizu, S.; Hoshi, K.; Muramoto, T.; Homma, M.; Ironside, J.W.; Kuzuhara, S.; Sato, T.; Yamamoto, T.; Kitamoto, T. Creutzfeldt-Jakob disease with florid-type plaques after cadaveric dura mater grafting. Arch. Neurol. 1999, 56, 357–362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kretzschmar, H.A.; Sethi, S.; Földvári, Z.; Windl, O.; Querner, V.; Zerr, I.; Poser, S. Iatrogenic Creutzfeldt-Jakob disease with florid plaques. Brain Pathol. 2003, 13, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Haïk, S.; Faucheux, B.A.; Sazdovitch, V.; Privat, N.; Kemeny, J.L.; Perret-Liaudet, A.; Hauw, J.J. The sympathetic nervous system is involved in variant Creutzfeldt-Jakob disease. Nat. Med. 2003, 9, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Peden, A.H.; Ritchie, D.L.; Head, M.W.; Ironside, J.W. Detection and localization of PrPSc in the skeletal muscle of patients with variant, iatrogenic, and sporadic forms of Creutzfeldt-Jakob disease. Am. J. Pathol. 2006, 168, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Peden, A.H.; Ritchie, D.L.; Uddin, H.P.; Dean, A.F.; Schiller, K.A.F.; Head, M.W.; Ironside, J.W. Abnormal prion protein in the pituitary in sporadic and variant Creutzfeldt-Jakob disease. J. Gen. Virol. 2007, 88, 1068–1072. [Google Scholar] [CrossRef]
- Notari, S.; Moleres, F.J.; Hunter, S.B.; Belay, E.D.; Schonberger, L.B.; Cali, I.; Parchi, P.; Shieh, W.J.; Brown, P.; Zaki, S.; et al. Multiorgan detection and characterization of protease-resistant prion protein in a case of variant CJD examined in the United States. PLoS ONE 2010, 5, e8765. [Google Scholar] [CrossRef] [PubMed]
- Douet, J.Y.; Lacroux, C.; Aron, N.; Head, M.W.; Lugan, S.; Tillier, C.; Huor, A.; Cassard, H.; Arnold, M.; Beringue, V.; et al. Distribution and Quantitative Estimates of Variant Creutzfeldt-Jakob Disease Prions in Tissues of Clinical and Asymptomatic Patients. Emerg. Infect. Dis. 2017, 23, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Douet, J.Y.; Huor, A.; Cassard, H.; Lugan, S.; Aron, N.; Arnold, M.; Vilette, D.; Torres, J.M.; Ironside, J.W.; Andreoletti, O. Wide distribution of prion infectivity in the peripheral tissues of vCJD and sCJD patients. Acta Neuropathol. 2021, 141, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Moda, F.; Gambetti, P.; Notari, S.; Concha-Marambio, L.; Catania, M.; Park, K.W.; Maderna, E.; Suardi, S.; Haïk, S.; Brandel, J.P.; et al. Prions in the urine of patients with variant Creutzfeldt-Jakob disease. N. Engl. J. Med. 2014, 371, 530–539. [Google Scholar] [CrossRef]
- Barria, M.A.; Lee, A.; Green, A.J.; Knight, R.; Head, M.W. Rapid amplification of prions from variant Creutzfeldt-Jakob disease cerebrospinal fluid. J. Pathol. Clin. Res. 2018, 4, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Bougard, D.; Bélondrade, M.; Mayran, C.; Bruyère-Ostells, L.; Lehmann, S.; Fournier-Wirth, C.; Knight, R.S.; Will, R.G.; Green, A.J.E. Diagnosis of Methionine/Valine Variant Creutzfeldt-Jakob Disease by Protein Misfolding Cyclic Amplification. Emerg. Infect. Dis. 2018, 24, 1364–1366. [Google Scholar] [CrossRef] [PubMed]
- Head, M.W.; Bunn, T.J.; Bishop, M.T.; McLoughlin, V.; Lowrie, S.; McKimmie, C.S.; Williams, M.C.; McCardle, L.; MacKenzie, J.; Knight, R.; et al. Prion protein heterogeneity in sporadic but not variant Creutzfeldt-Jakob disease: UK cases 1991–2002. Ann. Neurol. 2004, 55, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Collinge, J.; Whitfield, J.; McKintosh, E.; Beck, J.; Mead, S.; Thomas, D.J.; Alpers, M.P. Kuru in the 21st century--an acquired human prion disease with very long incubation periods. Lancet 2006, 367, 2068–2074. [Google Scholar] [CrossRef]
- Brown, P.; Brandel, J.P.; Sato, T.; Nakamura, Y.; MacKenzie, J.; Will, R.G.; Ladogana, A.; Pocchiari, M.; Leschek, E.W.; Schonberger, L.B. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg. Infect. Dis. 2012, 18, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Hilton, D.A.; Ghani, A.C.; Conyers, L.; Edwards, P.; McCardle, L.; Ritchie, D.; Penney, M.; Hegazy, D.; Ironside, J.W. Prevalence of lymphoreticular prion protein accumulation in UK tissue samples. J. Pathol. 2004, 203, 733–739. [Google Scholar] [CrossRef]
- Gill, O.N.; Spencer, Y.; Richard-Loendt, A.; Kelly, C.; Dabaghian, R.; Boyes, L.; Linehan, J.; Simmons, M.; Webb, P.; Bellerby, P.; et al. Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: Large scale survey. BMJ 2013, 347, f5675. [Google Scholar] [CrossRef] [PubMed]
- Ironside, J.W.; Bishop, M.T.; Connolly, K.; Hegazy, D.; Lowrie, S.; Le Grice, M.; Ritchie, D.L.; McCardle, L.M.; Hilton, D.A. Variant Creutzfeldt-Jakob disease: Prion protein genotype analysis of positive appendix tissue samples from a retrospective prevalence study. BMJ 2006, 332, 1186–1188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Public Health England. Summary results of the third national survey of abnormal prion prevalence in archived appendix specimens. Health Protection Report 2016; 10 (26):11–12, 12th August 2016. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/546883/hpr2616.pdf (accessed on 1 July 2021).
- Gill, O.N.; Spencer, Y.; Richard-Loendt, A.; Kelly, C.; Brown, D.; Sinka, K.; Andrews, N.; Dabaghian, R.; Simmons, M.; Edwards, P.; et al. Prevalence in Britain of abnormal prion protein in human appendices before and after exposure to the cattle BSE epizootic. Acta Neuropathol. 2020, 139, 965–976. [Google Scholar] [CrossRef]
- Turnbull, A.; Osborn, M.; Nicholas, N. Hospital autopsy: Endangered or extinct? J. Clin. Pathol. 2015, 68, 601–604. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritchie, D.L.; Peden, A.H.; Barria, M.A. Variant CJD: Reflections a Quarter of a Century on. Pathogens 2021, 10, 1413. https://doi.org/10.3390/pathogens10111413
Ritchie DL, Peden AH, Barria MA. Variant CJD: Reflections a Quarter of a Century on. Pathogens. 2021; 10(11):1413. https://doi.org/10.3390/pathogens10111413
Chicago/Turabian StyleRitchie, Diane L., Alexander H. Peden, and Marcelo A. Barria. 2021. "Variant CJD: Reflections a Quarter of a Century on" Pathogens 10, no. 11: 1413. https://doi.org/10.3390/pathogens10111413
APA StyleRitchie, D. L., Peden, A. H., & Barria, M. A. (2021). Variant CJD: Reflections a Quarter of a Century on. Pathogens, 10(11), 1413. https://doi.org/10.3390/pathogens10111413





