Highly Variable Clinical Pictures in Dogs Naturally Infected with Angiostrongylus vasorum
Abstract
1. Introduction
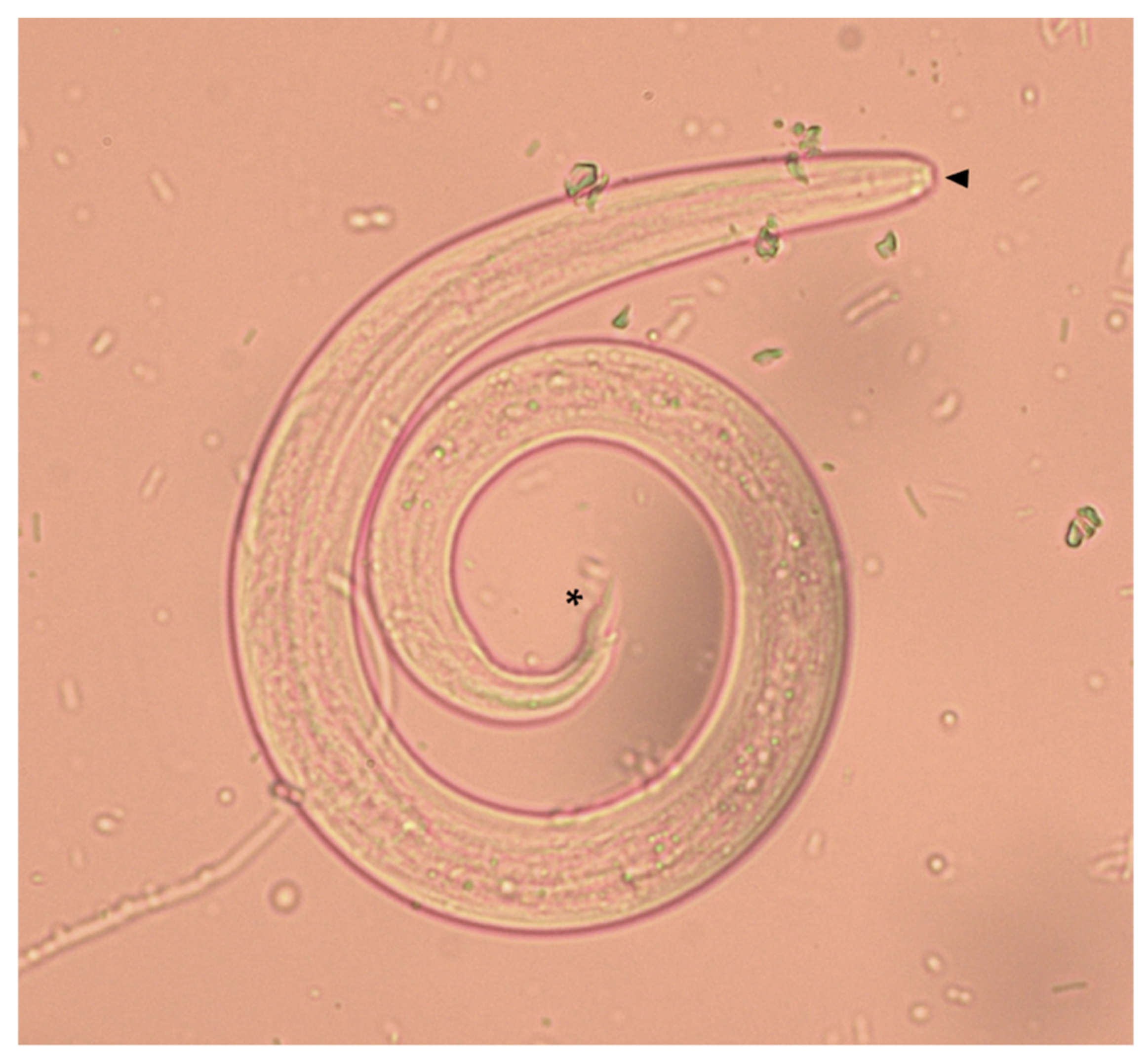
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vieira, F.M.; Muniz-Pereira, L.C.; Lima, S.D.S.; Neto, A.H.; Guimarães, E.V.; Luque, J.L. A new metastrongyloidean species (Nematoda) parasitizing pulmonary arteries of Puma (Herpailurus) yagouaroundi (É. Geoffroy, 1803) (Carnivora: Felidae) from Brazil. J. Parasitol. 2013, 99, 327–331, Erratum in: J. Parasitol. 2013, 99, 409. [Google Scholar] [CrossRef]
- Di Cesare, A.; Morelli, S.; Colombo, M.; Simonato, G.; Veronesi, F.; Marcer, F.; Diakou, A.; D’Angelosante, R.; Pantchev, N.; Psaralexi, E.; et al. Is Angiostrongylosis a Realistic Threat for Domestic Cats? Front. Vet. Sci. 2020, 7, 195. [Google Scholar] [CrossRef]
- Diakou, A.; Dimzas, D.; Astaras, C.; Savvas, I.; Di Cesare, A.; Morelli, S.; Neofitos, Κ.; Migli, D.; Traversa, D. Clinical investigations and treatment outcome in a European wildcat (Felis silvestris silvestris) infected by cardio-pulmonary nematodes. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100357. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.R.; Jefferies, R.; van Otterdijk, L.; McEniry, R.B.; Allen, F.; Bakewell, M.; Shaw, S.E. Angiostrongylus vasorum infection in dogs: Presentation and risk factors. Vet. Parasitol. 2010, 173, 255–261. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Holmes, S.A.; Wright, I.; Morgan, E.R.; Lacher, D.W. Recent advances in the epidemiology, clinical and diagnostic features, and control of canine cardio-pulmonary angiostrongylosis. Vet. Res. 2014, 45, 92. [Google Scholar] [CrossRef] [PubMed]
- Bolt, G.; Monrad, J.; Henriksen, P.; Dietz, H.H.; Koch, J.; Bindseil, E.; Jensen, A.L. The fox (Vulpes vulpes) as a reservoir for canine angiostrongylosis in Denmark. Field survey and experimental infections. Acta Vet. Scand. 1992, 33, 357–362. [Google Scholar] [CrossRef]
- Bolt, G.; Monrad, J.; Koch, J.; Jensen, A.L. Canine angiostrongylosis: A review. Vet. Rec. 1994, 135, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Gillis-Germitsch, N.; Manser, M.B.; Hilbe, M.; Schnyder, M. Meerkats (Suricata suricatta), a new definitive host of the canid nematode Angiostrongylus vasorum. Int. J. Parasitol. Parasites Wildl. 2017, 6, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, A.; Traversa, D. Canine angiostrongylosis: Recent advances in diagnosis, prevention and treatment. Vet. Med. 2014, 5, 181–192. [Google Scholar]
- Morgan, E.R.; Shaw, S.E.; Brennan, S.F.; De Waal, T.D.; Jones, B.R.; Mulcahy, G. Angiostrongylus vasorum: A real heartbreaker. Trends Parasitol. 2005, 21, 49–51. [Google Scholar] [CrossRef]
- Mozzer, L.R.; Lima, W.S. Gallus gallus domesticus: Paratenic host of Angiostrongylus vasorum. Vet. Parasitol. 2015, 207, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Robbins, W.; Conboy, G.; Greenwood, S.; Schaper, R. Infectivity of gastropod-shed third-stage larvae of Angiostrongylus vasorum and Crenosoma vulpis to dogs. Parasites Vectors 2021, 14, 307. [Google Scholar] [CrossRef]
- Koch, J.; Willesen, J.L. Canine pulmonary angiostrongylosis: An update. Vet. J. 2009, 179, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, N.E.; Hofer-Inteeworn, N.; Jud Schefer, R.; Kuemmerle-Fraune, C.; Schnyder, M.; Kutter, A.P.N. Hyperfibrinolysis and hypofibrinogenemia diagnosed with rotational thromboelastometry in dogs naturally infected with Angiostrongylus vasorum. J. Vet. Intern. Med. 2017, 31, 1091–1099. [Google Scholar] [CrossRef]
- Tritten, L.; Gillis-Germitsch, N.; Kockmann, T.; Schnyder, M. Quantitative proteomics analysis of Angiostrongylus vasorum-induced alterations in dog serum sheds light on the pathogenesis of canine angiostrongylosis. Sci. Rep. 2021, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Garosi, L.S.; Platt, S.R.; McConnell, J.F.; Wrayt, J.D.; Smith, K.C. Intracranial haemorrhage associated with Angiostrongylus vasorum infection in three dogs. J. Small Anim. Pract. 2005, 46, 93–99. [Google Scholar] [CrossRef]
- Lepri, E.; Veronesi, F.; Traversa, D.; Conti, M.B.; Marchesi, M.C.; Miglio, A.; Mandara, M.T. Disseminated angiostrongylosis with massive cardiac and cerebral involvement in a dog from Italy. Parasitol. Res. 2011, 109, 505–508. [Google Scholar] [CrossRef]
- Chapman, P.S.; Boag, A.K.; Guitian, J.; Boswood, A. Angiostrongylus vasorum infection in 23 dogs (1999–2002). J. Small Anim. Pract. 2004, 45, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Torbidone, A.; Malatesta, D.; Guglielmini, C. Occurrence of fatal canine Angiostrongylus vasorum infection in Italy. Vet. Parasitol. 2008, 152, 162–166. [Google Scholar] [CrossRef]
- Traversa, D.; Di Cesare, A.; Meloni, S.; di Regalbono, A.F.; Milillo, P.; Pampurini, F.; Venco, L. Canine angiostrongylosis in Italy: Occurrence of Angiostrongylus vasorum in dogs with compatible clinical pictures. Parasitol. Res. 2013, 112, 2473–2480. [Google Scholar] [CrossRef]
- Di Cesare, A.; Traversa, D.; Manzocchi, S.; Meloni, S.; Grillotti, E.; Auriemma, E.; Pampurini, F.; Garofani, C.; Ibba, F.; Venco, L. Elusive Angiostrongylus vasorum infections. Parasites Vectors 2015, 8, 438. [Google Scholar] [CrossRef]
- Holmes, S.; Paine, P.; Wright, I.; Morgan, E.R.; Elsheikha, H.M. Risk factors and predictors of angiostrongylosis in naturally infected dogs in the southeast of England. Companion Anim. 2020, 25, 233–240. [Google Scholar] [CrossRef]
- Matos, J.N.; Malbon, A.; Dennler, M.; Glaus, T. Intrapulmonary arteriovenous anastomoses in dogs with severe Angiostrongylus vasorum infection: Clinical, radiographic, and echocardiographic evaluation. J. Vet. Cardiol. 2016, 18, 110–124. [Google Scholar] [CrossRef]
- Szatmári, V. Spontaneous tricuspid valve chordal rupture in a dog with severe, irreversible pulmonary hypertension caused by Angiostrongylus vasorum infection. BMC Vet. Res. 2020, 16, 311. [Google Scholar] [CrossRef]
- Helm, J.R.; Morgan, E.R.; Jackson, M.W.; Wotton, P.; Bell, R. Canine angiostrongylosis: An emerging disease in Europe. J. Vet. Emerg. Crit. Care 2010, 20, 98–109. [Google Scholar] [CrossRef]
- Gallagher, B.; Brennan, S.F.; Zarelli, M.; Mooney, C.T. Geographical, clinical, clinicopathological and radiographic features of canine angiostrongylosis in Irish dogs: A retrospective study. Ir. Vet. J. 2012, 65, 5. [Google Scholar] [CrossRef] [PubMed]
- Tachmazidou, A.; Papaioannou, N.; Diakou, A.; Savvas, I.; Patsikas, M.; Stylianaki, I.; Morelli, S.; Di Cesare, A.; Mylonakis, M.E. First report of fatal autochthonous angiostrongylosis in a dog in Greece. Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100519. [Google Scholar]
- Hurníková, Z.; Čabanová, V.; Karpjak, P.; Kasenčák, M.; Miterpáková, M. Rare Case of Angiostrongylus vasorum intraocular infestation in an asymptomatic dog. Helminthologia 2019, 56, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Alho, A.M.; Schnyder, M.; Schaper, R.; Meireles, J.; Belo, S.; Deplazes, P.; de Carvalho, L.M. Seroprevalence of circulating Angiostrongylus vasorum antigen and parasite-specific antibodies in dogs from Portugal. Parasitol. Res. 2016, 115, 2567–2572. [Google Scholar] [CrossRef]
- Morchón, R.; Montoya-Alonso, J.A.; Sánchez-Agudo, J.Á.; de Vicente-Bengochea, J.; Murcia-Martínez, X.; Carretón, E. Angiostrongylus vasorum in domestic dogs in Castilla y León, Iberian Peninsula, Spain. Animals 2021, 11, 1513. [Google Scholar] [CrossRef] [PubMed]
- Lempereur, L.; Martinelle, L.; Marechal, F.; Bayrou, C.; Dalemans, A.C.; Schnyder, M.; Losson, B. Prevalence of Angiostrongylus vasorum in southern Belgium, a coprological and serological survey. Parasites Vectors 2016, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Grandi, G.; Lind, E.O.; Schaper, R.; Ågren, E.; Schnyder, M. Canine angiostrongylosis in Sweden: A nationwide seroepidemiological survey by enzyme-linked immunosorbent assays and a summary of five-year diagnostic activity (2011–2015). Acta Vet. Scan. 2017, 59, 85. [Google Scholar] [CrossRef] [PubMed]
- Tiškina, V.; Lindqvist, E.L.; Blomqvist, A.C.; Orav, M.; Stensvold, C.R.; Jokelainen, P. Autochthonous Angiostrongylus vasorum in Finland. Vet. Rec. Open 2019, 6, e000314. [Google Scholar] [CrossRef]
- Traversa, D.; Morelli, S.; Cassini, R.; Crisi, P.E.; Russi, I.; Grillotti, E.; Manzocchi, S.; Simonato, G.; Beraldo, P.; Viglietti, A.; et al. Occurrence of canine and feline extra-intestinal nematodes in key endemic regions of Italy. Acta Trop. 2019, 193, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Benda, T.; Csivincsik, Á.; Nemes, C.; Turbók, J.; Zsolnai, A.; Simonyai, E.; Majoros, G.; Nagy, G. Lethal Angiostrongylus vasorum infection in a Hungarian dog. Acta Parasitol. 2017, 62, 221–224. [Google Scholar] [CrossRef]
- Deak, G.; Gillis-Germitsch, N.; Ionică, A.M.; Mara, A.; Păstrav, I.R.; Cazan, C.D.; Ioniță, M.; Mitrea, I.L.; Răileanu, C.; Bărburaș, D.; et al. The first seroepidemiological survey for Angiostrongylus vasorum in domestic dogs from Romania. Parasites Vectors 2019, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Tieri, E.; Pomilio, F.; Di Francesco, G.; Saletti, M.A.; Totaro, P.; Troilo, M.; Menna, S.; Tampieri, M.P.; Morelli, D. Angiostrongylus vasorum in 20 dogs in the province of Chieti, Italy. Vet. Ital. 2011, 47, 77–88. [Google Scholar] [PubMed]
- Glaus, T.; Sigrist, N.; Hofer-Inteeworn, N.; Kuemmerle-Fraune, C.; Mueller, C.; Geissweid, K.; Beckmann, K.; Wenger, M.; Matos, J.N. Unexplained bleeding as primary clinical complaint in dogs infected with Angiostrongylus vasorum. Schweiz. Arch. Tierheilkd. 2016, 158, 701–709. [Google Scholar] [CrossRef]
- Schnyder, M.; Fahrion, A.; Riond, B.; Ossent, P.; Webster, P.; Kranjc, A.; Glaus, T.; Deplazes, P. Clinical, laboratory and pathological findings in dogs experimentally infected with Angiostrongylus vasorum. Parasitol. Res. 2010, 107, 1471–1480. [Google Scholar] [CrossRef]
- Brennan, S.F.; McCarthy, G.; McAllister, H.; Bassett, H.; Jones, B.R. Clinical signs, diagnosis and treatment of three dogs with angiostrongylosis in Ireland. Ir. Vet. J. 2004, 57, 103–109. [Google Scholar] [CrossRef]
- Willesen, J.; Bjornvad, C.; Koch, J. Acute haemoabdomen associated with Angiostrongylus vasorum infection in a dog: A case report. Ir. Vet. J. 2008, 61, 591–593. [Google Scholar] [CrossRef]
- Sasanelli, M.; Paradies, P.; Otranto, D.; Lia, R.P.; de Caprariis, D. Haemothorax associated with Angiostrongylus vasorum infection in a dog. J. Small Anim. Pract. 2008, 49, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Paradies, P.; Schnyder, M.; Capogna, A.; Lia, R.P.; Sasanelli, M. Canine angiostrongylosis in naturally infected dogs: Clinical approach and monitoring of infection after treatment. Sci. World J. 2013, 2013, 702056. [Google Scholar] [CrossRef] [PubMed]
- Miterpáková, M.; Hurníková, Z.; Zalewski, A.P. The first clinically manifested case of angiostrongylosis in a dog in Slovakia. Acta Parasitol. 2014, 59, 661–665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olivieri, E.; Zanzani, S.A.; Gazzonis, A.L.; Giudice, C.; Brambilla, P.; Alberti, I.; Romussi, S.; Lombardo, R.; Mortellaro, C.M.; Banco, B.; et al. Angiostrongylus vasorum infection in dogs from a cardiopulmonary dirofilariosis endemic area of Northwestern Italy: A case study and a retrospective data analysis. BMC Vet. Res. 2017, 13, 165. [Google Scholar] [CrossRef]
- Barutzki, D.; Schaper, R. Endoparasites in dogs and cats in Germany 1999–2002. Parasitol. Res. 2003, 90, S148–S150. [Google Scholar] [CrossRef]
- Barutzki, D.; Schaper, R. Natural infections of Angiostrongylus vasorum and Crenosoma vulpis in dogs in Germany (2007–2009). Parasitol. Res. 2009, 105, S39–S48. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.T.; DeBess, E. Detection of parasites in canine feces at three off-leash dog parks in Portland, Oregon 2014. Vet. Parasitol. Reg. Stud. 2020, 22, 100494. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Morelli, S.; Di Cesare, A.; Diakou, A. Felid cardiopulmonary nematodes: Dilemmas solved and new questions posed. Pathogens 2021, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Júnior, S.D.; Barçante, J.M.; Barçante, T.A.; Ribeiro, V.M.; Lima, W.S. Ectopic location of adult worms and first-stage larvae of Angiostrongylus vasorum in an infected dog. Vet. Parasitol. 2004, 121, 293–296. [Google Scholar] [CrossRef]
- Cury, M.C.; Lima, W.S. Ocorrência de Angiostrongylus vasorum no rim de cäo experimentalmente infectado. Arq. Bras. Med. Vet. Zootec. 1995, 47, 593–595. [Google Scholar]
- Cury, M.C.; Lima, W.S. Rupture of femoral artery in a dog infected with Angiostrongylus vasorum. Vet. Parasitol. 1996, 65, 313–315. [Google Scholar] [CrossRef]
- Mozzer, L.R.; Lima, W.S. Rupture of the thoracic aorta associated with experimental Angiostrongylus vasorum infection in a dog. Parasite 2012, 19, 189–191. [Google Scholar] [CrossRef] [PubMed]
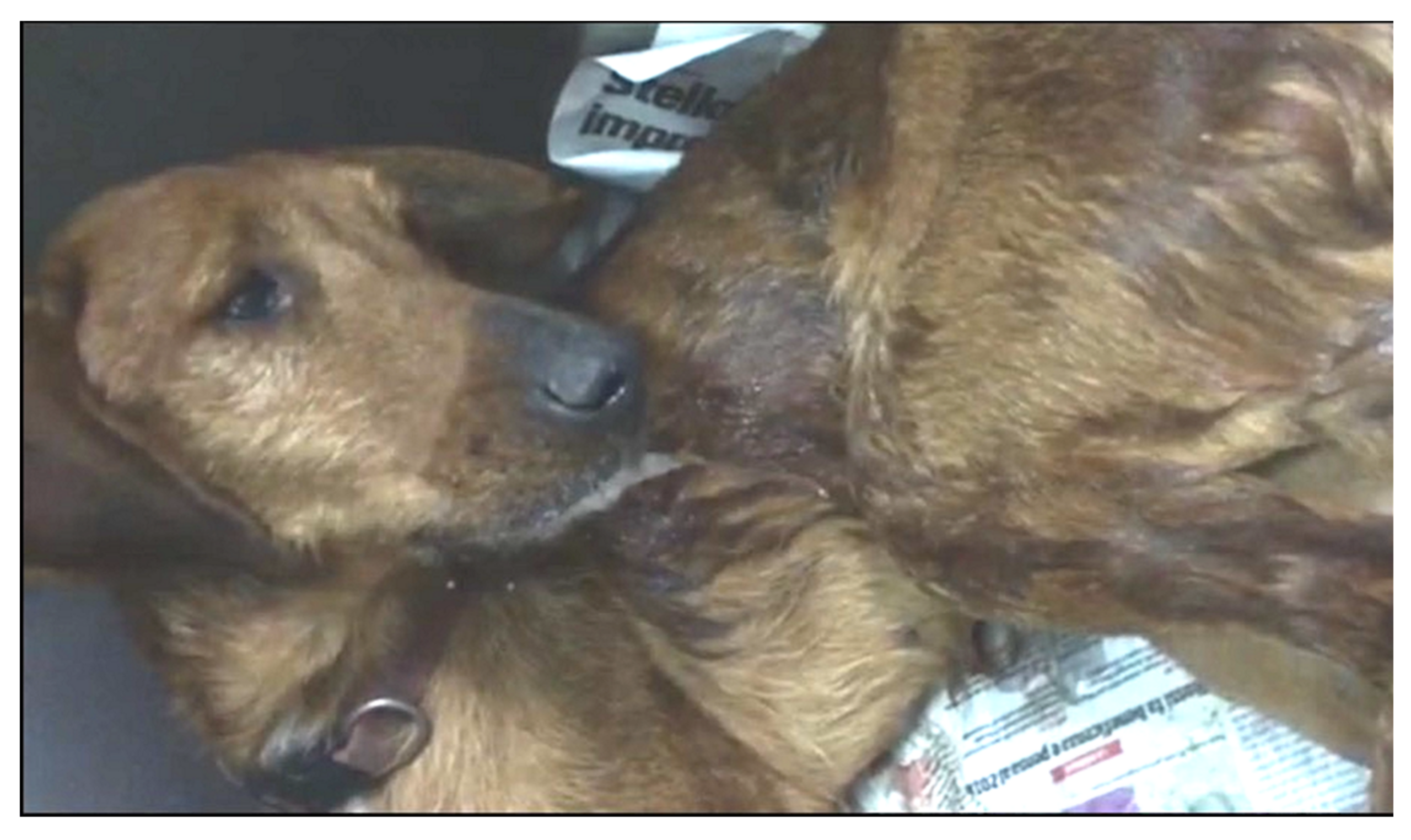
- Cavana, P.; Bensignor, E.; Blot, S.; Carlus, M.; Chermette, R.; Crosaz, O.; Grimm, F.; Hurion, M.; Jeandel, A.; Polack, B. Nematode dermatitis due to Angiostrongylus vasorum infection in a dog. Vet. Dermatol. 2015, 26, 293-e65. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Priestnall, S.L.; Blake, D.; Meeson, R.L. Angiostrongylus vasorum causing severe granulomatous hepatitis with concurrent multiple acquired PSS. J. Am. Anim. Hosp. Assoc. 2015, 51, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Cortese, L.; Meomartino, L.; Pagano, T.B.; Pepe, P.; Cringoli, G.; Papparella, S. Angiostrongylus vasorum: Epidemiological, clinical and histopathological insights. BMC Vet. Res. 2014, 10, 236. [Google Scholar] [CrossRef]
- Traversa, D.; Guglielmini, C. Feline aelurostrongylosis and canine angiostrongylosis: A challenging diagnosis for two emerging verminous pneumonia infections. Vet. Parasitol. 2008, 157, 163–174. [Google Scholar] [CrossRef]
- Traversa, D.; Di Cesare, A.; Conboy, G. Canine and feline cardiopulmonary parasitic nematodes in Europe: Emerging and underestimated. Parasites Vectors 2010, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.D. Georgi’s Parasitology for Veterinarians, 6th ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1995; pp. 295–296. [Google Scholar]



| Site | Dog ID | Age (Months) | Sex | Breed | Clinical Signs |
|---|---|---|---|---|---|
| Site A | 16 | 120 | Female | English Setter | Y |
| 58 | 96 | Female | Mixed breed | N | |
| 69 | 12 | Female | Segugio italiano | Y | |
| 70 | 24 | Female | Mixed breed | Y | |
| 74 | 4 | Female | Segugio italiano | N | |
| 75 | 5 | Female | Labrador retriever | N | |
| 82 | 96 | Male | Mixed breed | N | |
| 120 | 141 | Male | Mixed breed | Y | |
| 216 | 13 | Female | Segugio italiano | Y | |
| Site B | 353 | 60 | Female | Mixed breed | Y |
| 367 | 36 | Female | Mixed breed | Y | |
| 368 | 168 | Female | Épagneul Breton | Y | |
| 389 | 120 | Male | Mixed breed | N | |
| 413 | 48 | Male | Segugio Maremmano | Y | |
| 448 | 72 | Female | Lagotto Romagnolo | Y | |
| 455 | 12 | Female | Cocker Spaniel | Y | |
| Site C | 694 | 24 | Female | English Setter | Y |
| 710 | 24 | Male | Mixed breed | Y | |
| 723 | 36 | Male | Labrador retriever | N | |
| 746 | 24 | Male | German Shepherd | Y | |
| Site D | 830 | 156 | Male | Mixed breed | Y |
| 831 | 72 | Female | German Shepherd | N | |
| 832 | 120 | Male | Mixed breed | N | |
| 833 | 132 | Male | Mixed breed | N | |
| 834 | 24 | Male | Mixed breed | N | |
| 835 | 24 | Male | Mixed breed | Y | |
| 836 | 96 | Male | Mixed breed | Y | |
| 837 | 60 | Male | Mixed breed | Y | |
| 838 | 72 | Female | English Setter | N | |
| 839 | 36 | Female | Mixed breed | Y | |
| 840 | 24 | Female | Mixed breed | Y | |
| 841 | 24 | Female | Epagneul Breton | Y | |
| 842 | 36 | Male | Lagotto Romagnolo | Y | |
| 843 | 144 | Female | Mixed breed | Y | |
| 844 | 120 | Male | Mixed breed | N | |
| 845 | 120 | Female | Mixed breed | N |
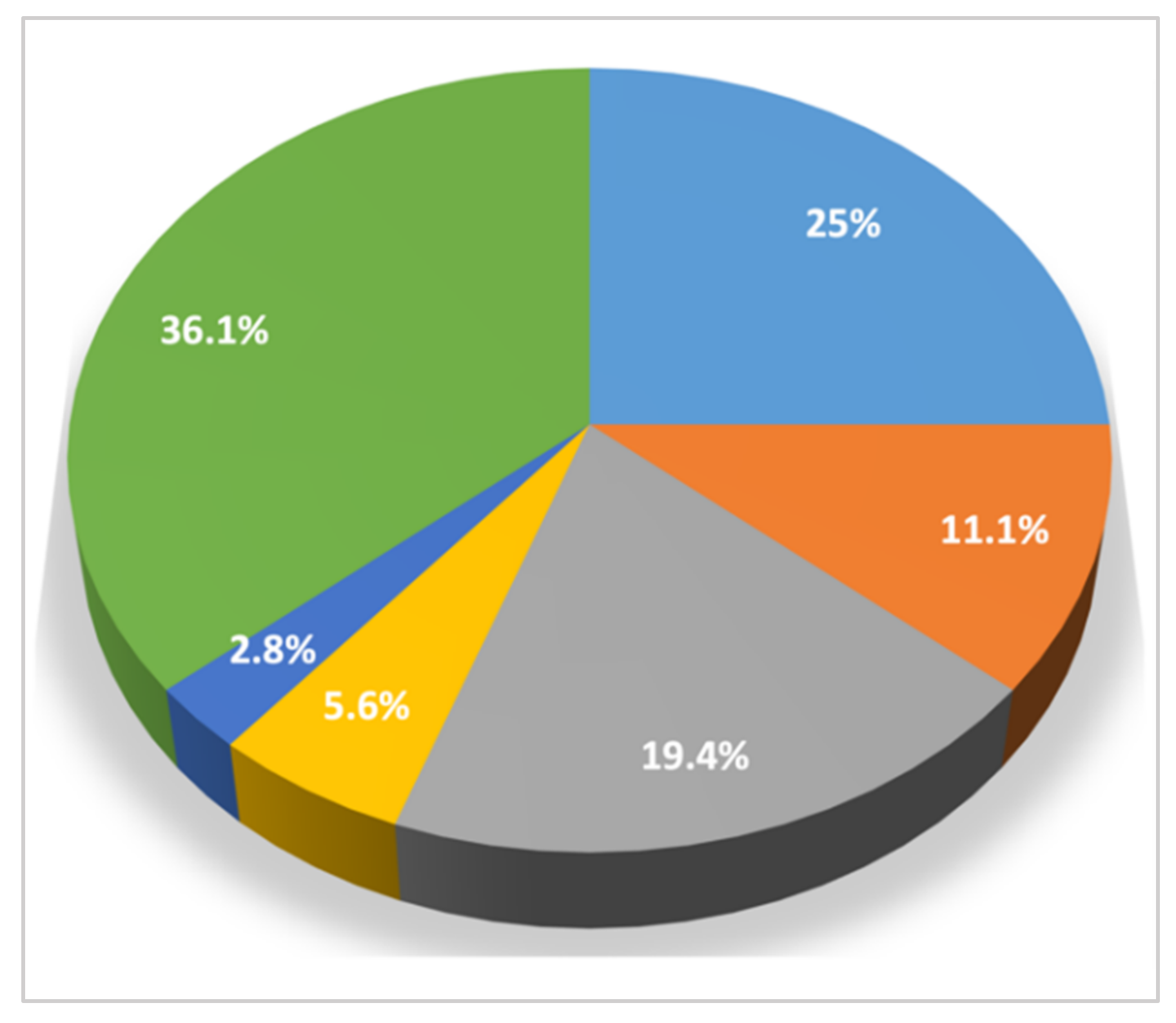
| Category of Clinical Signs n/tot (%) | Clinical Sign | n/tot (%) |
|---|---|---|
| Cardiorespiratory signs 19/36 (52.8) | Cough | 11 (30.5) |
| Dyspnea | 8 (22.2) | |
| Syncope | 3 (8.3) | |
| Cyanosis | 2 (5.6) | |
| Tachypnea | 1 (2.8) | |
| Non-specific signs 14/36 (38.9) | Weight loss | 6 (16.7) |
| Diarrhea | 4 (11.1) | |
| Anorexia | 4 (11.1) | |
| Lethargy | 3 (8.3) | |
| Exercise intolerance | 2 (5.6) | |
| Fever | 1 (2.8) | |
| Signs related to coagulation disorders 2/36 (5.6) | Hematochezia | 1 (2.8) |
| Hemorrhages | 1 (2.8) | |
| Neurological signs 1/36 (2.8) | Shivering | 1 (2.8) |
| Hypersalivation | 1 (2.8) | |
| No clinical signs | 13 (36.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, M.; Traversa, D.; Grillotti, E.; Pezzuto, C.; De Tommaso, C.; Pampurini, F.; Schaper, R.; Drake, J.; Crisi, P.E.; Russi, I.; et al. Highly Variable Clinical Pictures in Dogs Naturally Infected with Angiostrongylus vasorum. Pathogens 2021, 10, 1372. https://doi.org/10.3390/pathogens10111372
Colombo M, Traversa D, Grillotti E, Pezzuto C, De Tommaso C, Pampurini F, Schaper R, Drake J, Crisi PE, Russi I, et al. Highly Variable Clinical Pictures in Dogs Naturally Infected with Angiostrongylus vasorum. Pathogens. 2021; 10(11):1372. https://doi.org/10.3390/pathogens10111372
Chicago/Turabian StyleColombo, Mariasole, Donato Traversa, Eleonora Grillotti, Carlo Pezzuto, Cesare De Tommaso, Fabrizio Pampurini, Roland Schaper, Jason Drake, Paolo Emidio Crisi, Ilaria Russi, and et al. 2021. "Highly Variable Clinical Pictures in Dogs Naturally Infected with Angiostrongylus vasorum" Pathogens 10, no. 11: 1372. https://doi.org/10.3390/pathogens10111372
APA StyleColombo, M., Traversa, D., Grillotti, E., Pezzuto, C., De Tommaso, C., Pampurini, F., Schaper, R., Drake, J., Crisi, P. E., Russi, I., Ripamonti, M., & Di Cesare, A. (2021). Highly Variable Clinical Pictures in Dogs Naturally Infected with Angiostrongylus vasorum. Pathogens, 10(11), 1372. https://doi.org/10.3390/pathogens10111372









