Prevalence and Intensity of Cardicola spp. Infection in Ranched Southern Bluefin Tuna and a Comparison of Diagnostic Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. DNA Analysis
2.3. Statistics
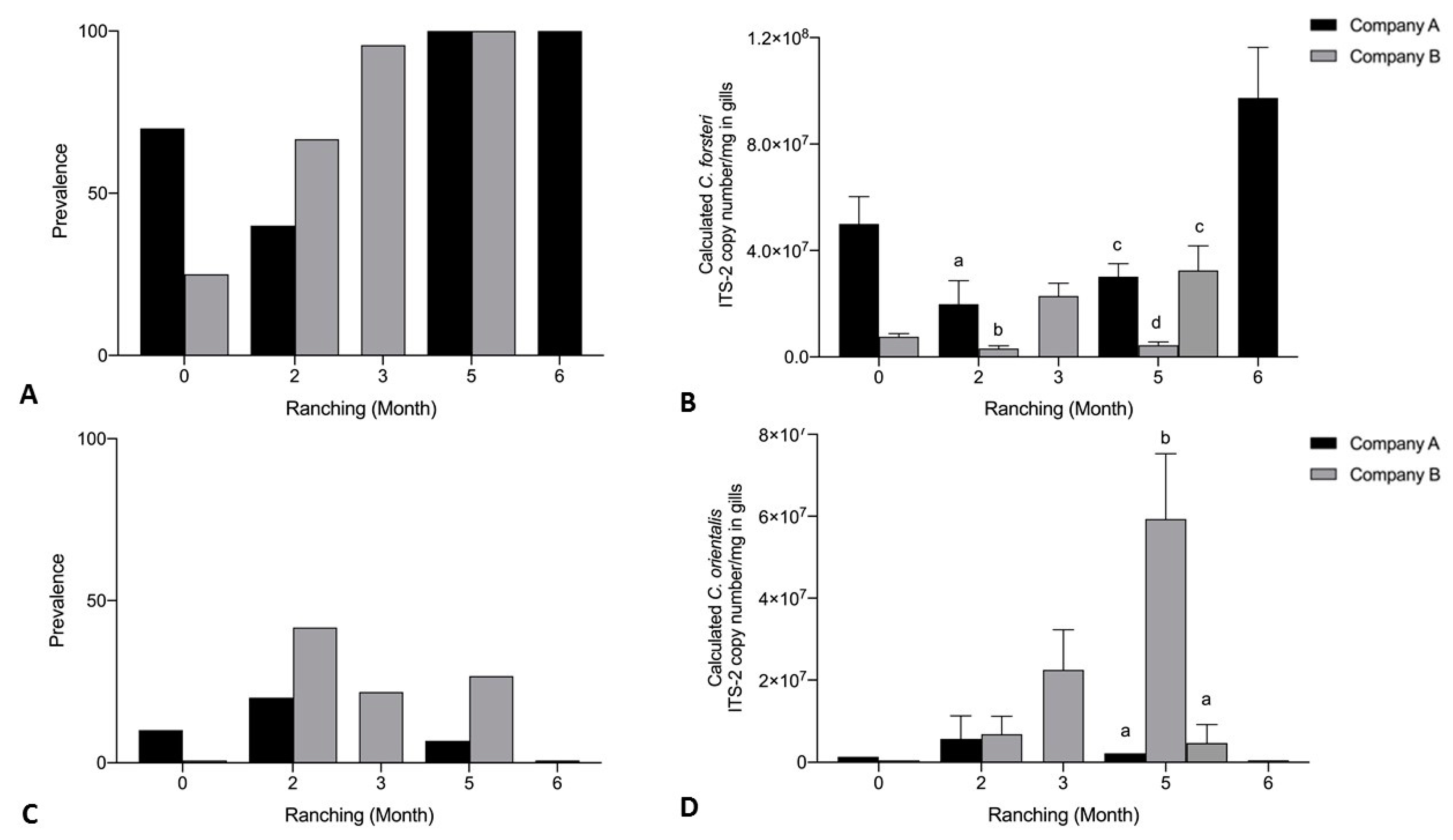
3. Results
3.1. Effect of Treatment
3.2. Effect of Ranching Year
3.3. Effect of Company
3.4. Cumulative Mortality
3.5. Effect of Cardicola spp. Infection Severity on SBT Condition Index
3.6. Comparison between Cardicola spp. Diagnostic Methods
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ellis, D.; Kiessling, I. Ranching of Southern Bluefin Tuna in Australia. In Advances in Tuna Aquaculture: From Hatchery to Market; Elsevier: Oxford, UK, 2016; pp. 217–232. [Google Scholar] [CrossRef]
- Power, C.; Nowak, B.F.; Cribb, T.H.; Bott, N.J. Bloody Flukes: A Review of Aporocotylids as Parasites of Cultured Marine Fishes. Int. J. Parasitol. 2020, 50, 743–753. [Google Scholar] [CrossRef]
- Aiken, H.M.; Hayward, C.J.; Nowak, B.F. An Epizootic and Its Decline of a Blood Fluke, Cardicola forsteri, in farmed Southern Bluefin Tuna, Thunnus maccoyii. Aquaculture 2006, 254, 40–45. [Google Scholar] [CrossRef]
- Aiken, H.M.; Hayward, C.J.; Nowak, B.F. Factors Affecting Abundance and Prevalence of Blood Fluke, Cardicola forsteri, Infection in Commercially Ranched Southern Bluefin Tuna, Thunnus maccoyii, in Australia. Vet. Parasitol. 2015, 210, 106–113. [Google Scholar] [CrossRef]
- Balli, J.; Mladineo, I.; Shirakashi, S.; Nowak, B.F. Diseases in Tuna Aquaculture. In Advances in Tuna Aquaculture: From Hatchery to Market; Elsevier: Oxford, UK, 2016; pp. 253–272. [Google Scholar] [CrossRef]
- Hayward, C.J.; Ellis, D.; Foote, D.; Wilkinson, R.J.; Crosbie, P.B.B.; Bott, N.J.; Nowak, B.F. Concurrent Epizootic Hyper Infections of Sea Lice (Predominantly Caligus chiastos) and Blood Flukes (Cardicola forsteri) in Ranched Southern Bluefin Tuna. Vet. Parasitol. 2010, 173, 107–115. [Google Scholar] [CrossRef]
- Cribb, T.H.; Daintith, M.; Munday, B.L. A New Blood-Luke, Cardicola forsteri (Digenea: Sanguinicolidae) of Southern Bluefin Tuna (Thunnus maccoyii) in Aquaculture. Trans. R. Soc. S. Aust. 2000, 124, 117–120. [Google Scholar] [CrossRef]
- Shirakashi, S.; Tsunemoto, K.; Webber, C.; Rough, K.; Ellis, D.; Ogawa, K. Two Species of Cardicola (Trematoda: Aporocotylidae) Found in Southern Bluefin Tuna Thunnus maccoyii Ranched in South Australia. Fish Pathol. 2013, 48, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Polinski, M.; Hamilton, D.B.; Nowak, B.F.; Bridle, A.R. SYBR, TaqMan, or Both: Highly Sensitive, Non-Invasive Detection of Cardicola Blood Fluke Species in Southern Bluefin Tuna (Thunnus maccoyii). Mol. Biochem. Parasitol. 2013, 191, 7–15. [Google Scholar] [CrossRef]
- Colquitt, S.E.; Munday, B.L.; Daintith, M. Pathological Findings in Southern Bluefin Tuna, Thunnus maccoyii (Castelnau). Infected with Cardicola forsteri (Cribb, Daintith & Munday, 2000) (Digenea: Sanguinicolidae), a Blood Fluke. J. Fish Dis. 2001, 24, 225–229. [Google Scholar] [CrossRef]
- Shirakashi, S.; Kishimoto, Y.; Kinami, R.; Katano, H.; Ishimaru, K.; Murata, O.; Itoh, N.; Ogawa, K. Morphology and Distribution of Blood Fluke Eggs and Associated Pathology in the Gills of Cultured Pacific Bluefin Tuna, Thunnus orientalis. Parasitol. Int. 2012, 61, 242–249. [Google Scholar] [CrossRef]
- Dennis, M.M.; Landos, M.; Antignana, D.T. Case-Control Study of Epidemic Mortality and Cardicola forsteri–Associated Disease in farmed Southern Bluefin Tuna (Thunnus maccoyii) of South Australia. Vet. Pathol. 2011, 48, 846–855. [Google Scholar] [CrossRef]
- Hardy-Smith, P.; Ellis, D.; Humphrey, J.; Evans, M.; Evans, D.; Rough, K.; Valdenegro, V.; Nowak, B.F. In Vitro and In Vivo Efficacy of Anthelmintic Compounds against Blood Fluke (Cardicola forsteri). Aquaculture 2012, 334–337, 39–44. [Google Scholar] [CrossRef]
- Power, C.; Webber, C.; Rough, K.; Staunton, R.; Nowak, B.F.; Bott, N.J. The Effect of Different Treatment Strategies on Cardicola spp. (Trematoda: Aporocotylidae) infection in ranched Southern Bluefin Tuna (Thunnus maccoyii) from Port Lincoln, South Australia. Aquaculture 2019, 513, 734401. [Google Scholar] [CrossRef]
- Neumann, L.; Bridle, A.R.; Leef, M.J.; Nowak, B.F. Annual Variability of Infection with Cardicola forsteri and Cardicola orientalis in Ranched and Wild Southern Bluefin Tuna (Thunnus maccoyii). Aquaculture 2018, 487, 1–6. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575. [Google Scholar] [CrossRef]
- Shirakashi, S.; Andrews, M.; Kishimoto, Y.; Ishimaru, K.; Okada, T.; Sawada, Y.; Ogawa, K. Oral Treatment of Praziquantel as an Effective Control Measure against Blood Fluke Infection in Pacific Bluefin Tuna (Thunnus orientalis). Aquaculture 2012, 326–329, 15–19. [Google Scholar] [CrossRef]
- Alsaqabi, S.M.; Lotfy, W.M. Praziquantel: A Review. J. Vet. Sci. Technol. 2014, 5. [Google Scholar] [CrossRef]
- Van Wyk, J.A. Refugia—Overlooked as Perhaps the Most Potent Factor Concerning the Development of Anthelmintic Resistance. Onderstepoort J. Vet. Res. 2001, 68, 55–67. [Google Scholar] [PubMed]
- Norte dos Santos, C.; Leef, M.J.; Jones, B.; Bott, N.J.; Giblot-Ducray, D.; Nowak, B.F. Distribution of Cardicola forsteri Eggs in the Gills of Southern Bluefin Tuna (Thunnus maccoyii) (Castelnau, 1872). Aquaculture 2012, 344–349, 54–57. [Google Scholar] [CrossRef]
- Kirchhoff, N.T.; Leef, M.J.; Ellis, D.; Purser, J.; Nowak, B.F. Effects of the First Two Months of Ranching on the Health of Southern Bluefin Tuna Thunnus maccoyii. Aquaculture 2011, 315, 207–212. [Google Scholar] [CrossRef]
- Ishimaru, K.; Mine, R.; Shirakashi, S.; Kaneko, E.; Kubono, K.; Okada, T.; Sawada, Y.; Ogawa, K. Praziquantel Treatment against Cardicola Blood Flukes: Determination of the Minimal Effective Dose and Pharmacokinetics in Juvenile Pacific Bluefin Tuna. Aquaculture 2013, 402–403, 24–27. [Google Scholar] [CrossRef]
- Ravindran, V.B.; Khallaf, B.; Surapaneni, A.; Crosbie, N.D.; Soni, S.K.; Ball, A.S. Detection of Helminth Ova in Wastewater Using Recombinase Polymerase Amplification Coupled to Lateral Flow Strips. Water 2020, 12, 691. [Google Scholar] [CrossRef] [Green Version]
- Huston, D.C.; Ogawa, K.; Shirakashi, S.; Nowak, B.F. Metazoan Parasite Life Cycles: Significance for Fish Mariculture. Trends Parasitol. 2020, 36, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Cribb, T.H.; Adlard, R.D.; Hayward, C.J.; Bott, N.J.; Ellis, D.; Evans, D.; Nowak, B.F. The Life Cycle of Cardicola forsteri (Trematoda: Aporocotylidae), a Pathogen of Ranched Southern Bluefin Tuna, Thunnus maccoyii. Int. J. Parasitol. 2011, 41, 861–870. [Google Scholar] [CrossRef]
- Ogawa, K.; Shirakashi, S.; Tani, K.; Shin, S.P.; Ishimaru, K.; Honryo, T.; Sugihara, Y.; Uchida, H. Developmental Stages of Fish Blood Flukes, Cardicola forsteri and Cardicola opisthorchis (Trematoda: Aporocotylidae), in Their Polychaete Intermediate Hosts collected at Pacific Bluefin Tuna Culture Sites in Japan. Parasitol. Int. 2017, 66, 972–977. [Google Scholar] [CrossRef] [Green Version]
- Shirakashi, S.; Tani, K.; Ishimaru, K.; Shin, S.P.; Honryo, T.; Uchida, H.; Ogawa, K. Discovery of Intermediate Hosts for Two Species of Blood Flukes Cardicola orientalis and Cardicola forsteri (Trematoda: Aporocotylidae) Infecting Pacific Bluefin Tuna in Japan. Parasitol. Int. 2016, 65, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Sugihara, Y.; Yamada, T.; Tamaki, A.; Yamanishi, R.; Kanai, K. Larval Stages of the Bluefin Tuna Blood Fluke Cardicola opisthorchis (Trematoda: Aporocotylidae) Found from Terebella sp. (Polychaeta: Terebellidae). Parasitol. Int. 2014, 63, 295–299. [Google Scholar] [CrossRef]
- Sugihara, Y.; Yamada, T.; Ogawa, K.; Yokoyama, F.; Matsukura, K.; Kanai, K. Occurrence of the Bluefin Tuna Blood Fluke Cardicola opisthorchis in the Intermediate Host Terebella sp. Fish Pathol. 2015, 50, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Shirakashi, S.; Tani, K.; Ishimuru, K.; Honryo, T.; Shin, S.P.; Uchida, H.; Ogawa, K. Spatial and Temporal Changes in the Distribution of blood fluke infection in Nicolea gracilibranchis (Polychaeta: Terebellidae), the Intermediate Host for Cardicola orientalis (Digenea: Aporocotylidae), at a Tuna Farming Site in Japan. J. Parasitol. 2017, 103, 541–546. [Google Scholar] [CrossRef]
- Shirakashi, S.; Matsuda, T.; Asai, N.; Honryo, T.; Ogawa, K. In Vivo Cultivation of Tuna Blood Fluke Cardicola orientalis in Terebellid Intermediate Hosts. Int. J. Parasitol. 2020, 50, 851–857. [Google Scholar] [CrossRef]
- Sugihara, Y.; Yamada, T.; Iwanaga, S.; Kanai, K. Transplantation of Cardicola opisthorchis (Trematoda: Aporocotylidae) Sporocysts into the Intermediate Host, Terebella sp. (Polychaeta: Terebellidae). Parasitol. Int. 2017, 66, 839–842. [Google Scholar] [CrossRef]








| Transfer Date | Treatment Date | Treatment Dose (mg/kg) | Sampling Size | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 3 | Week 8 | Week 12 | Week 17 | Week 21 | Week 24 | ||||
| COMPANY A 2018 | ||||||||||
| Transfer | 12 | - | - | - | - | - | - | |||
| Pontoon 1 | 28 Feb | 9 Mar (Week 2) | 15 | - | 12 | 12 | 12 | - | - | - |
| Pontoon 2 | 28 Feb | 3 Apr (Week 6) | 15 | - | 12 | 12 | 12 | - | - | - |
| Pontoon 3 | 28 Feb | NA | NA | - | 12 | 12 | 12 | - | - | - |
| Pontoon 4 | 28 Feb | 3 Apr (Week 6) | 15 | - | - | - | - | 15 | - | - |
| COMPANY A 2019 | ||||||||||
| Transfer | 12 | - | - | - | - | - | - | |||
| Pontoon 1 | 8 Mar | NA | NA | - | 10 | - | - | - | 15 | 15 |
| Pontoon 2 | 9 Mar | 9 Apr (Week 5) | 15-22 | - | - | - | 12 | 12 | - | - |
| Pontoon 3 | 7 Mar | 11 Apr (Week 5) | 15-22 | - | - | - | - | 15 | - | - |
| COMPANY B 2019 | ||||||||||
| Transfer | 12 | - | - | - | - | - | - | |||
| Pontoon 1 | 28 Mar | NA | NA | - | - | 12 | 12 | - | - | - |
| Pontoon 2 | 28 Mar | NA | NA | - | - | - | 12 | 15 | - | - |
| Pontoon 3 | 26 Mar | NA | NA | - | - | - | - | 15 | - | - |
| Pontoon 4 | 27 Mar | 29 Apr (Week 5) | 15-24 | - | - | - | 12 | 15 | - | - |
| Cumulative Mortality (%) | Weeks in Ranching | Total Stocked Fish | |
|---|---|---|---|
| COMPANY A 2018 | |||
| Pontoon 1 | 0.16 | 16 | 3128 |
| Pontoon 2 | 0.31 | 16 | 3205 |
| Pontoon 3 | 0.20 | 12 | 2545 |
| Pontoon 4 | 0.43 | 17 | 3108 |
| COMPANY A 2019 | |||
| Pontoon 1 | 0.10 | 24 | 2003 |
| Pontoon 2 | 0.43 | 17 | 2082 |
| Pontoon 3 | 0.43 | 17 | 3239 |
| COMPANY B 2019 | |||
| Pontoon 1 | 0.46 | 15 | 1525 |
| Pontoon 2 | 1.82 | 17 | 2808 |
| Pontoon 3 | 0.54 | 17 | 2771 |
| Pontoon 4 | 0.49 | 17 | 2849 |
| Cardicola forsteri (ITS-2) in Heart Samples | ||||
|---|---|---|---|---|
| COMPANY A 2018 | ||||
| Adult Cardicola forsteri in heart | + | − | Total | |
| + | 78 | 7 | 85 | |
| − | 94 | 59 | 153 | |
| Total | 172 | 66 | 238 | |
| Cardicola spp. (ITS-2) in Gill Samples | ||||
|---|---|---|---|---|
| COMPANY A 2018 | ||||
| Cardicola spp. eggs in gill filament | + | − | Total | |
| + | 218 | 24 | 242 | |
| − | 31 | 35 | 66 | |
| Total | 249 | 59 | 308 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Power, C.; Evenden, S.; Rough, K.; Webber, C.; Widdicombe, M.; Nowak, B.F.; Bott, N.J. Prevalence and Intensity of Cardicola spp. Infection in Ranched Southern Bluefin Tuna and a Comparison of Diagnostic Methods. Pathogens 2021, 10, 1248. https://doi.org/10.3390/pathogens10101248
Power C, Evenden S, Rough K, Webber C, Widdicombe M, Nowak BF, Bott NJ. Prevalence and Intensity of Cardicola spp. Infection in Ranched Southern Bluefin Tuna and a Comparison of Diagnostic Methods. Pathogens. 2021; 10(10):1248. https://doi.org/10.3390/pathogens10101248
Chicago/Turabian StylePower, Cecilia, Shannon Evenden, Kirsten Rough, Claire Webber, Maree Widdicombe, Barbara F. Nowak, and Nathan J. Bott. 2021. "Prevalence and Intensity of Cardicola spp. Infection in Ranched Southern Bluefin Tuna and a Comparison of Diagnostic Methods" Pathogens 10, no. 10: 1248. https://doi.org/10.3390/pathogens10101248
APA StylePower, C., Evenden, S., Rough, K., Webber, C., Widdicombe, M., Nowak, B. F., & Bott, N. J. (2021). Prevalence and Intensity of Cardicola spp. Infection in Ranched Southern Bluefin Tuna and a Comparison of Diagnostic Methods. Pathogens, 10(10), 1248. https://doi.org/10.3390/pathogens10101248








