Emergence of Reassortment between a New and Reported Types of Betanodavirus in Shellfish
Abstract
:1. Introduction
2. Results
2.1. Identification of Reassortment between the Types of Betanodavirus in Shellfish
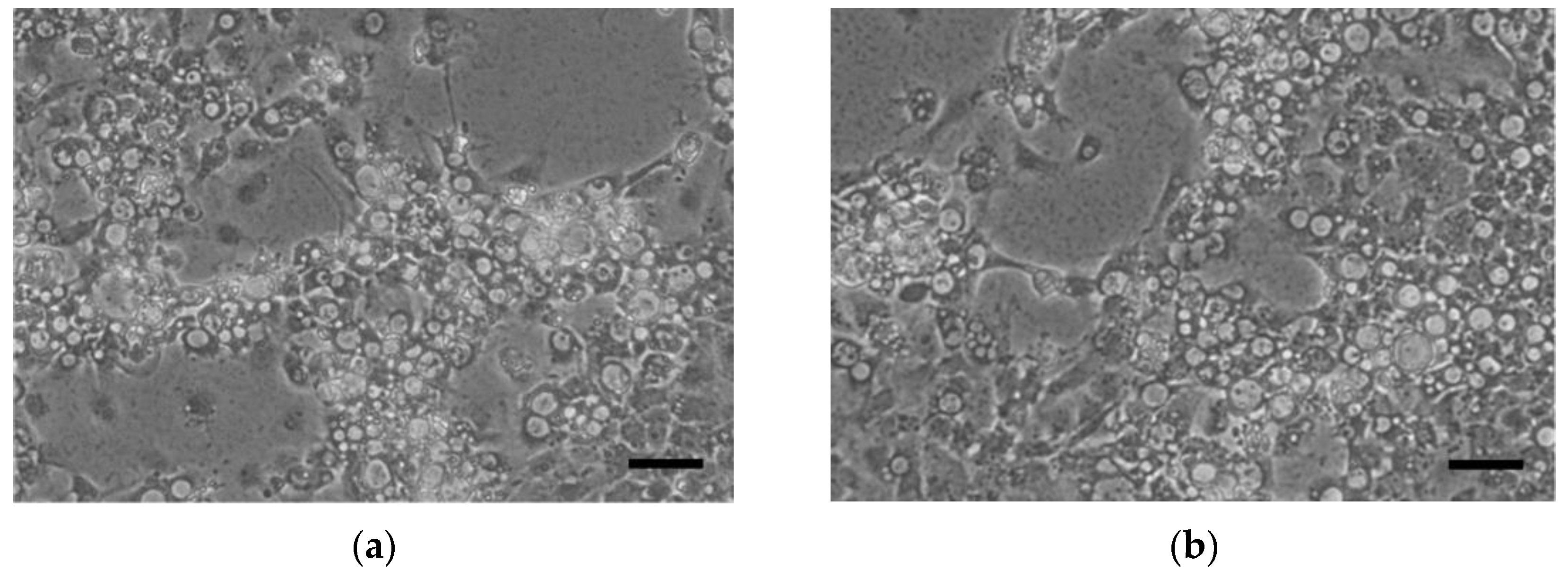
2.2. Isolation of Reassortant Viruses in Shellfish
2.3. Comparision of the Full-Sequence Homology with Other Betanodaviruses
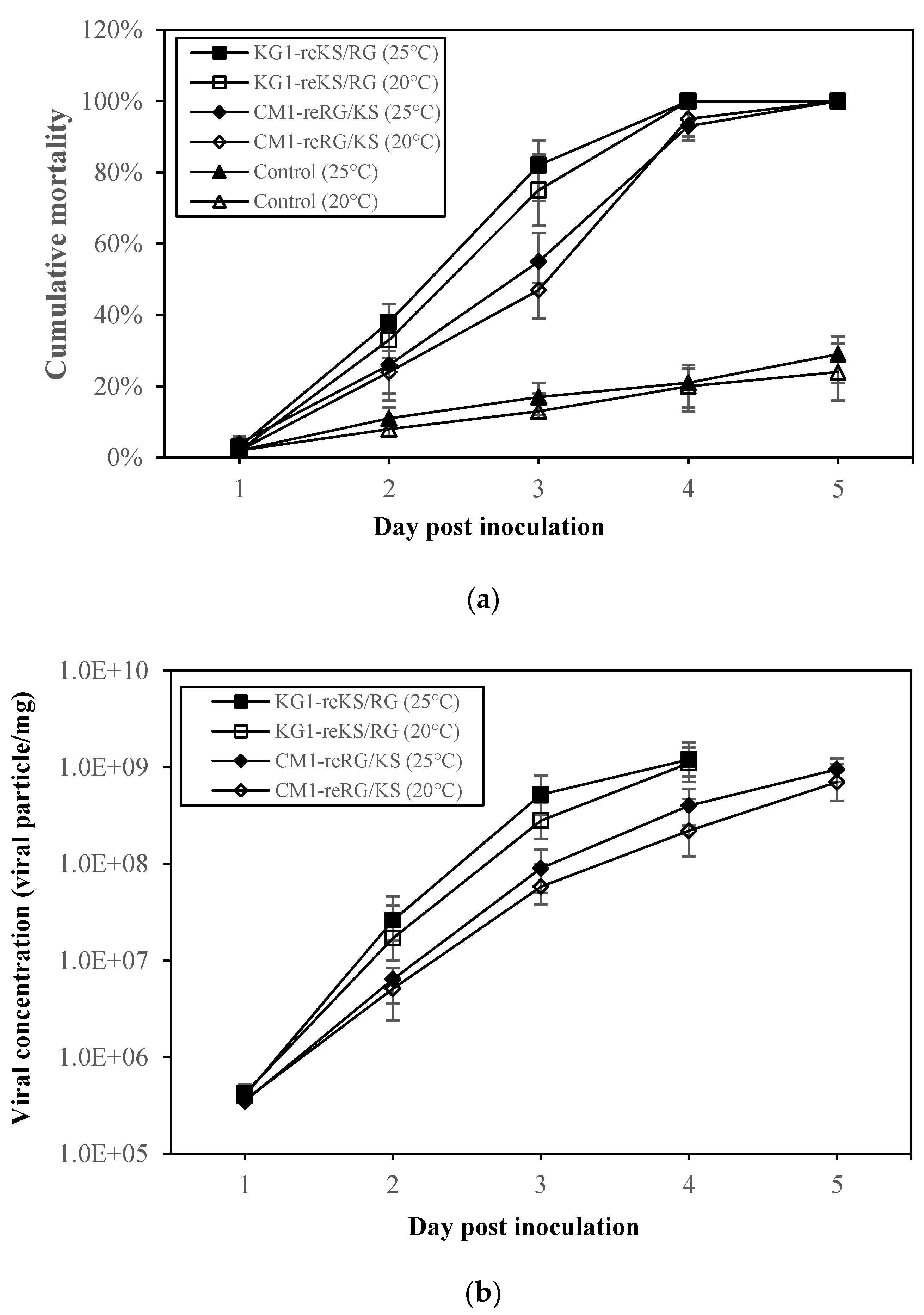
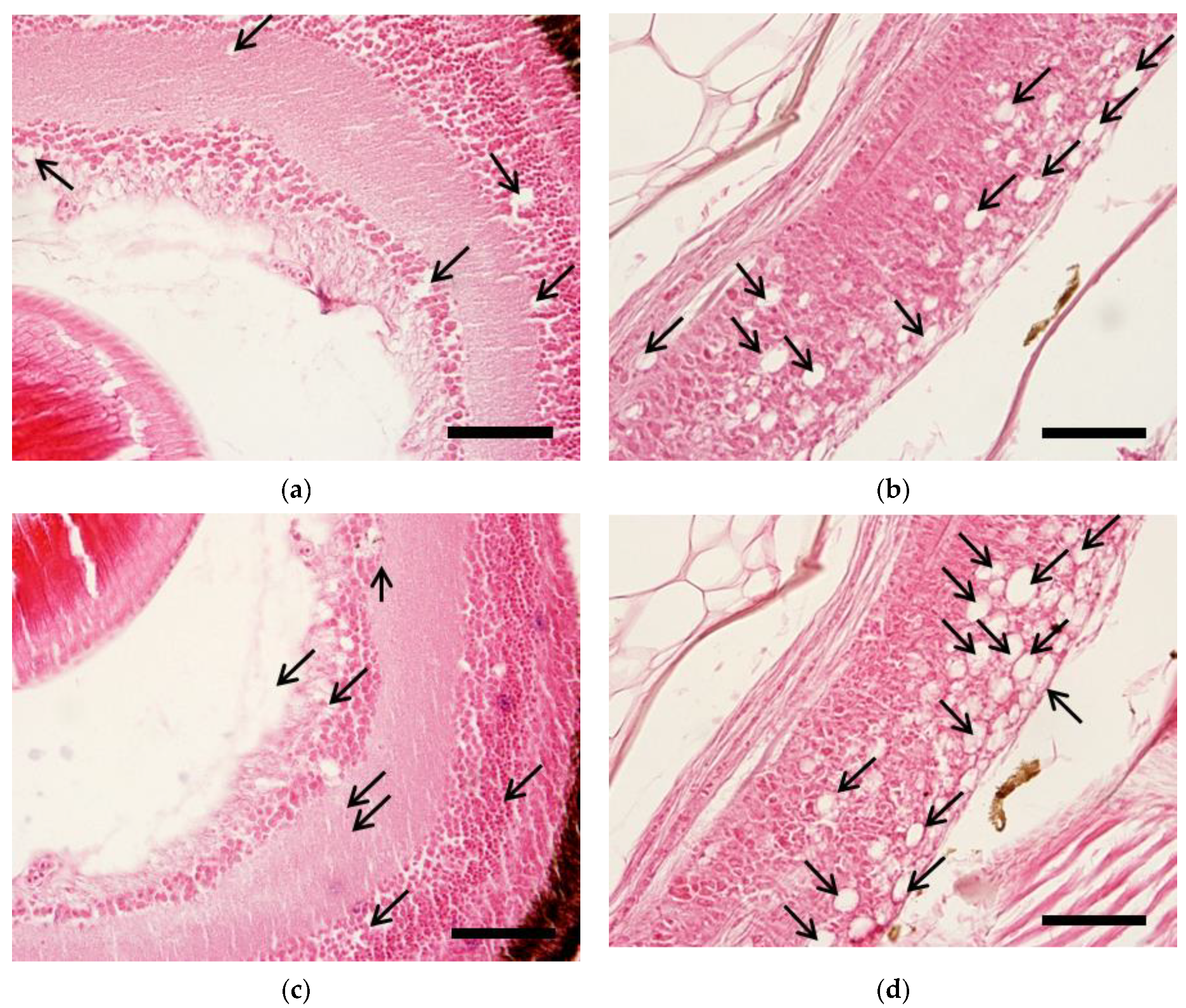
2.4. Pathogenicity of Reassortant Viruses against Sevenband Grouper Larvae
3. Discussion
4. Materials and Methods
4.1. Betanodaviruses in Shellfish and Finfishes
4.2. Primer Design for Genotyping of Betanodaviruses Based on RNA1 Segment
4.3. RT-PCR
4.4. Quantitative RT-PCR
4.5. Determination of Full-Genomic Sequences of Reassortant Virus
4.6. Comparison of Full-Genomic Sequences and Construction of Phylogenetic Tree
4.7. Isolation of Reassortant Virus in Shellfish
4.8. Transmission Electron Microscopy (TEM)
4.9. Pathogenicity of Two Reassortant Viruses to Fish in the Larval Stage
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doan, Q.K.; Vandeputte, M.; Chatain, B.; Morin, T.; Allal, F. Viral encephalopathy and retinopathy in aquaculture: A review. J. Fish Dis. 2017, 40, 717–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K.I.; Nakai, T.; Muroga, K.; Arimoto, M.; Mushiake, M.; Furusawa, I. Properties of a new virus belonging to nodaviridae found in larval striped jack (Pseudocaranx dentex) with nervous necrosis. Virology 1992, 187, 368–371. [Google Scholar] [CrossRef]
- Delsert, C.; Morin, N.; Comps, M.A. A fish encephalitis virus that differs from other nodaviruses by its capsid protein processing. Arch. Virol. 1997, 142, 2359–2371. [Google Scholar] [CrossRef]
- Ball, L.A.; Johnson, K.L. Reverse genetics of nodaviruses. Adv. Virus Res. 1999, 53, 229–244. [Google Scholar] [PubMed]
- Hameed, A.S.; Ninawe, A.S.; Nakai, T.; Chi, S.C.; Johnson, K.L. ICTV virus taxonomy profile: Nodaviridae. J. Gen. Virol. 2019, 100, 3–4. [Google Scholar] [CrossRef]
- Toffolo, V.; Negrisolo, E.; Maltese, C.; Bovo, G.; Belvedere, P.; Colombo, L.; Dalla Valle, L. Phylogeny of betanodaviruses and molecular evolution of their RNA polymerase and coat proteins. Mol. Phylogenet. 2007, 43, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Olveira, J.G.; Souto, S.; Dopazo, C.P.; Thiéry, R.; Barja, J.L.; Bandín, I. Comparative analysis of both genomic segments of betanodaviruses isolated from epizootic outbreaks in farmed fish species provides evidence for genetic reassortment. J. Gen. Virol. 2009, 90, 2940–2951. [Google Scholar] [CrossRef]
- Panzarin, V.; Fusaro, A.; Monne, I.; Cappellozza, E.; Patarnello, P.; Bovo, G.; Capua, I.; Holmes, E.C.; Cattoli, G. Molecular epidemiology and evolutionary dynamics of betanodavirus in southern Europe. Infect. Genet. Evol. 2012, 12, 63–70. [Google Scholar] [CrossRef]
- Toffan, A.; Panzarin, V.; Toson, M.; Cecchettin, K.; Pascoli, F. Water temperature affects pathogenicity of different betanodavirus genotypes in experimentally challenged Dicentrarchus labrax. Dis. Aquat. Org. 2016, 119, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.C.; Kwon, W.J.; Kim, M.S.; Kim, K.I.; Min, J.G.; Jeong, H.D. High prevalence of betanodavirus barfin flounder nervous necrosis virus as well as red-spotted grouper nervous necrosis virus genotype in shellfish. J. Fish Dis. 2018, 41, 233–246. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kwon, W.J.; Min, J.G.; Jeong, H.D. Isolation and initial characterization of new betanodaviruses in shellfish. Transbound. Emerg. Dis. 2018, 65, 1557–1567. [Google Scholar] [CrossRef]
- Gomez, D.K.; Baeck, G.W.; Kim, J.H.; Choresca, C.H., Jr.; Park, S.C. Molecular detection of betanodavirus in wild marine fish populations in Korea. J. Vet. Diagn. 2008, 20, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Gomez, D.K.; Baeck, G.W.; Kim, J.H.; Choresca, C.H., Jr.; Park, S.C. Molecular detection of betanodaviruses from apparently healthy wild marine invertebrates. J. Invertebr. Pathol. 2008, 97, 197–202. [Google Scholar] [CrossRef]
- Yoshikoshi, K.; Inoue, K. Viral nervous necrosis in hatchery-reared larvae and juveniles of Japanese parrotfish, Oplegnathus fasciatus (Temminck & Schlegel). J. Fish Dis. 1990, 13, 69–77. [Google Scholar]
- Costa, J.Z.; Thompson, K.D. Understanding the interaction between Betanodavirus and its host for the development of prophylactic measures for viral encephalopathy and retinopathy. Fish Shellfish Immunol. 2016, 53, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Rippey, S.R. Infectious diseases associated with molluscan shellfish consumption. Clin. Microbiol. Rev. 1994, 7, 419–425. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kwon, W.J.; Min, J.G.; Kim, K.I.; Jeong, H.D. Complete genome sequence and pathogenic analysis of a new betanodavirus isolated from shellfish. J. Fish Dis. 2019, 42, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Souto, S.; Lopez-Jimena, B.; Alonso, M.D.C.; Garcia-Rosado, E.; Bandin, I. Experimental susceptibility of European sea bass and Senegalese sole to different betanodavirus isolates. Vet. Microbiol. 2015, 177, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Toffan, A.; Pascoli, F.; Pretto, T.; Panzarin, V.; Abbadi, M.; Buratin, A.; Quartesan, R.; Gijón, D.; Padrós, F. Viral nervous necrosis in gilthead sea bream (Sparus aurata) caused by reassortant betanodavirus RGNNV/SJNNV: An emerging threat for Mediterranean aquaculture. Sci. Rep. 2017, 7, 46755. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Nguyen, H.D.; Furuhashi, M.; Nakai, T. Mass mortality of cultured sevenband grouper, Epinephelus septemfasciatus, associated with viral nervous necrosis. Fish Pathol. 1996, 21, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, T.; Nakai, T.; Mori, K.; Arimoto, M.; Furusawa, I. Cloning of the fish cell line SSN-1 for piscine nodaviruses. Dis. Aquat. Org. 2000, 43, 81–89. [Google Scholar] [CrossRef]
- Chi, S.C.; Hu, W.W.; Lo, B.J. Establishment and characterization of a continuous cell line (GF-1) derived from grouper, Epinephelus coioides (Hamilton): A cell line susceptible to grouper nervous necrosis virus (GNNV). J. Fish Dis. 1999, 22, 173–182. [Google Scholar] [CrossRef]
- Chi, S.C.; Lo, B.J.; Lin, S.C. Characterization of grouper nervous necrosis virus (GNNV). J. Fish Dis. 2001, 24, 3–13. [Google Scholar] [CrossRef]
- Chi, S.C.; Wu, Y.C.; Hong, J.R. Nodaviruses of fish. In Aquaculture Virology; Elisevier: Amsterdam, The Netherlands, 2016; Chapter 25; pp. 371–393. [Google Scholar]
- Munday, B.L.; Kwang, J.; Moody, N. Betanodaviruse infections of teleost fish: A review. J. Fish Dis. 2002, 25, 127–142. [Google Scholar] [CrossRef]
- Marshall, N.; Priyamvada, L.; Ende, Z.; Steel, J.; Lowen, A.C. Influenza virus reassortment occurs with high frequency in the absence of segment mismatch. PLoS Pathog. 2013, 9, e1003421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamoto, T.; Mori, K.I.; Arimoto, M.; Nakai, T. High permissivity of the fish cell line SSN-1 for piscine nodaviruses. Dis. Aquat. Org. 1999, 39, 37–47. [Google Scholar] [CrossRef] [PubMed]


| Samples | No. of Single Betanodavirus Positive 1 | No. of Homologous Type 2 | No. of Reassortant Virus 3 | ||
|---|---|---|---|---|---|
| RG/KSNNV | KS/RGNNV | SJ/RGNNV | |||
| Domestic shellfish | 306 | 302 | 1 | 1 | 2 |
| Pacific oyster Crassostrea gigas | 179 | 178 | 1 | 0 | 0 |
| Mussel Mytilus edulis | 49 | 49 | 0 | 0 | 0 |
| Manila clam Ruditapes philippinarum | 29 | 29 | 0 | 0 | 0 |
| Common orient clam Meretrix meretrix | 28 | 26 | 0 | 0 | 2 |
| Granular ark Tegillarca granosa | 21 | 20 | 0 | 1 | 0 |
| Domestic finfish | 35 | 35 | 0 | 0 | 0 |
| Olive flounder Paralichthys olivaceus | 23 | 23 | 0 | 0 | 0 |
| Rock bream Oplegnathus fasciatus | 3 | 3 | 0 | 0 | 0 |
| Red sea bream Pagrus major | 2 | 2 | 0 | 0 | 0 |
| Rock fish Sebastes schlegeli | 2 | 2 | 0 | 0 | 0 |
| Stone flounder Platichthys stellatus | 5 | 5 | 0 | 0 | 0 |
| Imported shellfish from China | 48 | 47 | 1 | 0 | 0 |
| Manila clam Ruditapes philippinarum | 14 | 13 | 1 | 0 | 0 |
| Common orient clam Meretrix meretrix | 13 | 13 | 0 | 0 | 0 |
| Adams venus clam Mercenaria mercenaria | 13 | 13 | 0 | 0 | 0 |
| Venus clam Mercenaria stimpsoni | 3 | 3 | 0 | 0 | 0 |
| Wrinkled venus clam Callista brevisiphonata | 3 | 3 | 0 | 0 | 0 |
| Scallop Saxidomus purpurata | 1 | 1 | 0 | 0 | 0 |
| Chinese cyclina Cyclina sinensis | 1 | 1 | 0 | 0 | 0 |
| Imported shellfish from Japan | 31 | 31 | 0 | 0 | 0 |
| Manila clam Ruditapes philippinarum | 5 | 5 | 0 | 0 | 0 |
| Venus clam Mercenaria stimpsoni | 5 | 5 | 0 | 0 | 0 |
| Granular ark Tegillarca granosa | 9 | 9 | 0 | 0 | 0 |
| Scallop Patinopecten yessoensis | 12 | 12 | 0 | 0 | 0 |
| Total | 420 | 415 | 2 | 1 | 2 |
| Isolates | Type | RNA1 | RNA2 | RNA3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | RdRp | 5′ UTR | 3′ UTR | Total | CP | 5′ UTR | 3′ UTR | Protein B2 | ||
| KG1-reKS/RG | KS/RGNNV | 3123 | 2946 | 97 | 77 | 1434 | 1014 | 26 | 391 | 375 |
| CM1-reRG/KS | RG/KSNNV | 3105 | 2946 | 78 | 78 | 1444 | 1020 | 30 | 391 | 375 |
| KSNNV-KOR1 | KSNNV | 3123 | 2946 | 97 | 77 | 1444 | 1020 | 30 | 391 | 375 |
| SGWak97 | RGNNV | 3105 | 2946 | 78 | 78 | 1434 | 1014 | 26 | 391 | 375 |
| Segment | Part | Reassortant Strains | Homology to Corresponding Sequence (%) | ||||
|---|---|---|---|---|---|---|---|
| KSNNV-KOR1 (KSNNV) | SGWak97 (RGNNV) | JFIwa98 (BGNNV) | SJNag93 (SJNNV) | TPKag93 (TPNNV) | |||
| RNA1 | Total | KG1-reKS/RG | 99.8 1 | 82.7 | 82.2 | 81.2 | 82.5 |
| CM1-reRG/KS | 83.2 | 97.2 | 83.1 | 82.2 | 82.8 | ||
| RdRp | KG1-reKS/RG | 99.6 (99.8) 2 | 87.3 (88.6) | 86.5 (87.3) | 86.2 (87.2) | 86.0 (87.1) | |
| CM1-reRG/KS | 87.5 (89.6) | 98.0 (98.7) | 88.2 (89.6) | 87.6 (88.9) | 88.6 (89.5) | ||
| RNA3 | KG1-reKS/RG | 99.5 | 81.0 | 80.6 | 80.9 | 81.5 | |
| CM1-reRG/KS | 82.8 | 94.1 | 83.4 | 81.6 | 84.5 | ||
| Protein B2 | KG1-reKS/RG | 99.6 (99.7) | 83.8 (84.5) | 83.3 (84.8) | 83.3 (84.7) | 82.5 (83.8) | |
| CM1-reRG/KS | 85.5 (87.0) | 95.2 (96.4) | 86.8 (87.8) | 83.3 (85.1) | 87.3 (89.1) | ||
| 5′ UTR | KG1-reKS/RG | 100.0 | 70.1 | 72.4 | 71.1 | 82.7 | |
| CM1-reRG/KS | 87.5 | 98.0 | 88.2 | 87.6 | 88.6 | ||
| 3′ UTR | KG1-reKS/RG | 98.7 | 84.6 | 79.0 | 84.6 | 84.6 | |
| CM1-reRG/KS | 84.6 | 96.2 | 79.7 | 82.3 | 82.3 | ||
| RNA2 | Total | KG1-reKS/RG | 76.8 | 97.4 | 83.0 | 79.6 | 80.3 |
| CM1-reRG/KS | 98.5 | 76.6 | 78.2 | 77.4 | 78.0 | ||
| CP | KG1-reKS/RG | 76.0 (77.4) | 99.7 (99.8) | 86.4 (87.6) | 81.5 (82.5) | 81.5 (82.8) | |
| CM1-reRG/KS | 98.8 (99.1) | 76.3 (77.5) | 78.9 (80.2) | 77.5 (78.8) | 79.2 (80.4) | ||
| 5′ UTR | KG1-reKS/RG | 70.0 | 100.0 | 88.5 | 85.2 | 77.8 | |
| CM1-reRG/KS | 100.0 | 70.0 | 63.3 | 70.0 | 70.0 | ||
| 3′ UTR | KG1-reKS/RG | 78.3 | 98.0 | 83.2 | 80.2 | 81.4 | |
| CM1-reRG/KS | 98.7 | 77.7 | 82.2 | 80.2 | 79.8 | ||
| Primer | Sequence (5′ to 3′) | Object | Reference |
|---|---|---|---|
| c1VNNR | CCGTCTAATGCGACAGACATC | 1st & 2nd round R1-DSN-2 RT-PCR | This study |
| c1VNNF1 | GCGTTCCAAAAGAAAGAAGCATAC | 1st round R1-DSN-2 RT-PCR | This study |
| c1VNNF2 | GTTCCGTGGTACATGCCAAC | 2nd round R1-DSN-2 RT-PCR R1-DMT RT-PCR | This study |
| s1VNNKSR | AAGCTCGTCAGCCACGATG | R1-DMT RT-PCR (for KSNNV) | This study |
| s1VNNRGR | TCTCATTAGCCAATAAAGTTGTTA | R1-DMT RT-PCR (for RGNNV) | This study |
| s1VNNBFR | CAGTATCAGTGAGGAGGGTGTC | R1-DMT RT-PCR (for BFNNV) | This study |
| s1VNNSJR | CATCATCCATGCCAGCTTG | R1-DMT RT-PCR (for SJNNV) | This study |
| s1VNNTPR | CAGCCAATATCCTCAATTTCG | R1-DMT RT-PCR (for TPNNV) | This study |
| c2VNNR | TGGTCATCAACGATACGC | 1st & 2nd round R2-DSN-2 RT-PCR | [10] |
| c2VNNF1 | GCTTCCTGCCTGATCCAAC | 1st round R2-DSN-2 RT-PCR | [10] |
| c2VNNF2 | TGCCAAATGGTGGGAAAG | 2nd round R2-DSN-2 RT-PCR qRT-PCR | [10] |
| q2VNNR | TTGTTGCCGACACACAGG | qRT-PCR | [10] |
| s2VNNKSR | CGCTTCTGCGTTGTTTGG | R2-DMT RT-PCR (for KSNNV) | [11] |
| s2VNNRGR | TTGAAGTTGTCCCAGATGC | R2-DMT RT-PCR (for RGNNV) | [10] |
| s2VNNBFR | GGTAGAGCCAAGAAGTATTGATTTG | R2-DMT RT-PCR (for BFNNV) | [10] |
| s2VNNSJR | GGCAACGGTTTGTCAGTGAC | R2-DMT RT-PCR (for SJNNV) | [10] |
| s2VNNTPR | AAACCCAGAAGTGGCAGTTG | R2-DMT RT-PCR (for TPNNV) | [10] |
| R1KS-5 GSPR1 | GGGTCACCAAATGGGGCCATCC | 5′ RACE for KSNNV RNA1 | [17] |
| R1KS-5 GSPR2 | CACACAGAGCGGTGATGCCTGTGATGTC | [17] | |
| R1KS-3 GSPF1 | GCGCAACAGCACTGCACGAACTGTCC | 3′ RACE for KSNNV RNA1 | [17] |
| R1KS-3 GSPF2 | GAATGCCATCGTGGCTGACGAGCTTGG | [17] | |
| R2KS-5 GSPR1 | GTTGCCAACGAGGATGGTCCGAAACG | 5′ RACE for KSNNV RNA2 | [17] |
| R2KS-5 GSPR2 | CCGACCTGGAGACGTCAGTCGCTGCTC | [17] | |
| R2KS-3 GSPF1 | CATGTGGAGAAGGCCGCAGGAGATGC | 3′ RACE for KSNNV RNA2 | [17] |
| R2KS-3 GSPF2 | CAGCCGCGGCAGATACTGCTTCC | [17] | |
| R1RG-5 GSPR1 | TCGCCGAAGGGCGCAATACCG | 5′ RACE for RGNNV RNA1 | This study |
| R1RG-5 GSPR2 | CCACGAGGTCGGGAGCACACATACATGG | This study | |
| R1RG-3 GSPF1 | TGGTGCCGGGCTTATCAGAGGAATTG | 3′ RACE for RGNNV RNA1 | This study |
| R1RG-3 GSPF2 | GACTGGAATGACGTTGTAGCCAACGAG | This study | |
| R2RG-5 GSPR1 | CGTGTTTGCGGGGCACATTGG | 5′ RACE for RGNNV RNA2 | This study |
| R2RG-5 GSPR2 | GGCAGGAGGTCGGGGACGATGGTTG | This study | |
| R2RG-3 GSPF1 | CAGCCCCGTCAAATCCTGCTGCCTGT | 3′ RACE for RGNNV RNA2 | This study |
| R2RG-3 GSPF2 | CCGGTTCCCTAGTGCGTATCGTTGA | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.C.; Min, J.G.; Kim, K.I.; Jeong, H.D. Emergence of Reassortment between a New and Reported Types of Betanodavirus in Shellfish. Pathogens 2021, 10, 1232. https://doi.org/10.3390/pathogens10101232
Kim YC, Min JG, Kim KI, Jeong HD. Emergence of Reassortment between a New and Reported Types of Betanodavirus in Shellfish. Pathogens. 2021; 10(10):1232. https://doi.org/10.3390/pathogens10101232
Chicago/Turabian StyleKim, Young Chul, Joon Gyu Min, Kwang Il Kim, and Hyun Do Jeong. 2021. "Emergence of Reassortment between a New and Reported Types of Betanodavirus in Shellfish" Pathogens 10, no. 10: 1232. https://doi.org/10.3390/pathogens10101232






