Genetic Diversity and Distribution of Virulence-Associated Genes in Y. enterocolitica and Y. enterocolitica-Like Isolates from Humans and Animals in Poland
Abstract
1. Introduction
2. Results
2.1. Virulotyping
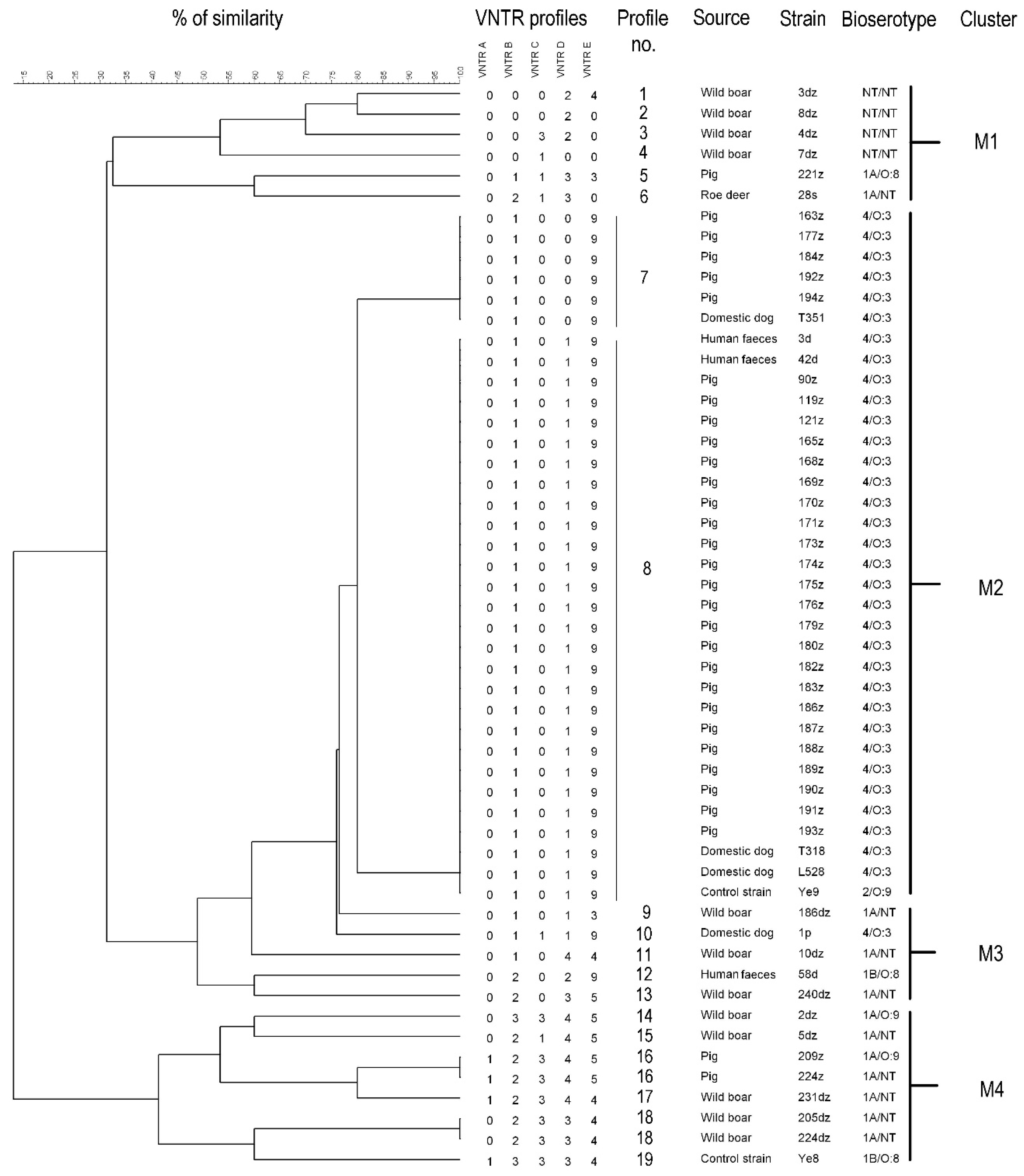
2.2. Tracking Potential Variable-Number Tandem Repeats—VNTRs and Cluster Analysis
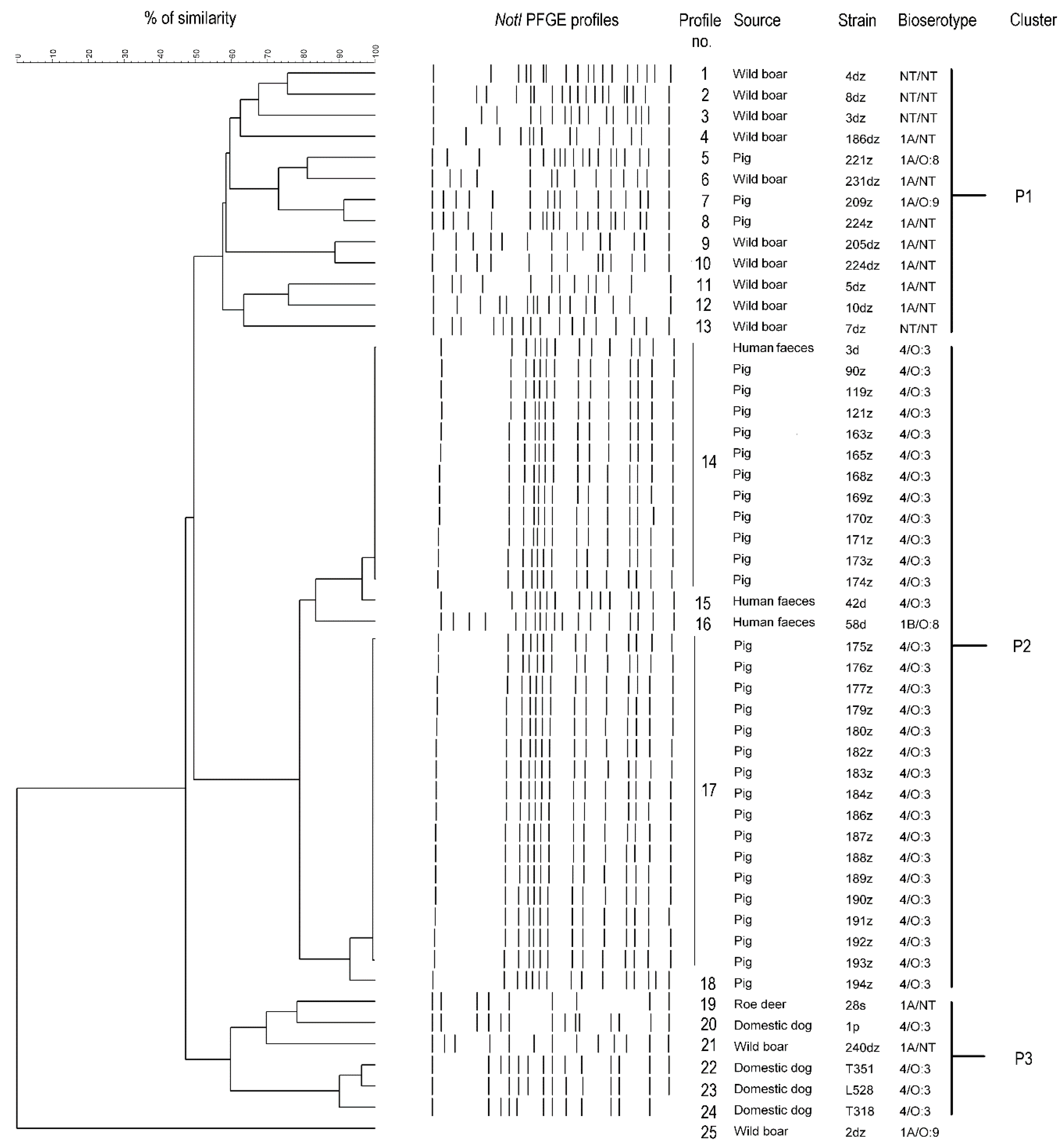
2.3. PFGE-CHEF (Contour-Clamped Homogeneous Electric Field)
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. DNA Isolation
4.3. Detection of Virulence-Associated Genes of Yersinia sp.
4.4. Multiple Locus Variable-Number Tandem Repeat (VNTR) Analysis—MLVA
4.5. Pulsed-Field Gel Electrophoresis—Contour-Clamped Homogenous Electric Field—PFGE-CHEF
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-based phylogeny and taxonomy of the “Enterobacteriales”: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar]
- LPSN—List of Prokaryotic Names with Standing in Nomenclature—Genus Yersinia. Available online: http://www.bacterio.net/yersinia.html#r (accessed on 5 March 2018).
- Stenkova, A.M.; Bystritskaya, E.P.; Guzev, K.V.; Rakin, A.V.; Isaeva, M.P. Molecular Evolution of the Yersinia Major Outer Membrane Protein C (OmpC). Evol. Bioinform. 2016, 12, 185–191. [Google Scholar]
- Galindo, C.L.; Rosenzweig, J.A.; Kirtley, M.L.; Chopra, A.K. Pathogenesis of Y. enterocolitica and Y. pseudotuberculosis in Human Yersiniosis. J. Pathog. 2011, 2011, 182051. [Google Scholar] [PubMed]
- Skorek, K.; Raczkowska, A.; Dudek, B.; Miętka, K.; Guz-Regner, K.; Pawlak, A.; Klausa, E.; Bugla-Płoskońska, G.; Brzostek, K. Regulatory Protein OmpR Influences the Serum Resistance of Yersinia enterocolitica O:9 by Modifying the Structure of the Outer Membrane. PLoS ONE 2013, 8, e79525. [Google Scholar]
- European Food Safety Authority (EFSA). Monitoring and identification of human enteropathogenic Yersinia spp.—Scientific Opinion of the Panel on Biological Hazards. EFSA J. 2007, 595, 1–30. [Google Scholar]
- Huovinen, E.; Sihvonen, L.M.; Virtanen, M.J.; Haukka, K.; Siitonen, A.; Kuusi, M. Symptoms and sources of Yersinia enterocolitica-infection: A case-control study. BMC Infect. Dis. 2010, 10, 122. [Google Scholar]
- Batzilla, J.; Heesemann, J.; Rakin, A. The pathogenic potential of Yersinia enterocolitica 1A. Int. J. Med. Microbiol. 2011, 301, 556–561. [Google Scholar]
- Bhagat, N.; Virdi, J.S. Distribution of virulence-associated genes in Yersinia enterocolitica biovar 1A correlates with clonal groups and not the source of isolation. FEMS Microbiol. Lett. 2007, 266, 177–183. [Google Scholar]
- Bhagat, N.; Virdi, J.S. The enigma of Yersinia enterocolitica biovar 1A. Crit. Rev. Microbiol. 2011, 37, 25–39. [Google Scholar]
- Platt-Samoraj, A.; Syczyło, K.; Szczerba-Turek, A.; Bancerz-Kisiel, A.; Jabłoński, A.; Łabuć, S.; Pajdak, J.; Oshakbaeva, N.; Szweda, W. Presence of ail and ystB genes in Yersinia enterocolitica biotype 1A isolates from game animals in Poland. Vet. J. 2017, 221, 11–13. [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2013. EFSA J. 2015, 13, 3991. [Google Scholar]
- Latha, C.; Anu, C.J.; Ajaykumar, V.J.; Sunil, B. Prevalence of Listeria monocytogenes, Yersinia enterocolitica, Staphylococcus aureus, and Salmonella enterica Typhimurium in meat and meat products using multiplex polymerase chain reaction. Vet. World 2017, 10, 927–931. [Google Scholar] [PubMed]
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: A review. Int. J. Environ. Res. Public. Health 2018, 15, 863–892. [Google Scholar]
- Fosse, J.; Seegers, H.; Magras, C. Prevalence and risk factors for bacterial food-borne zoonotic hazards in slaughter pigs: A review. Zoonoses Public Health 2009, 56, 429–454. [Google Scholar] [PubMed]
- Felin, E.; Hälli, O.; Heinonen, M.; Jukola, E.; Fredriksson-Ahomaa, M. Assessment of the feasibility of serological monitoring and on-farm information about health status for the future meat inspection of fattening pigs. Prev. Vet. Med. 2019, 162, 76–82. [Google Scholar]
- Murros-Kontiainen, A.; Johansson, P.; Niskanen, T.; Fredriksson-Ahomaa, M.; Korkeala, H.; Björkroth, J. Yersinia pekkanenii sp. nov. Int. J. Syst. Evol. Microbiol. 2011, 61, 2363–2367. [Google Scholar]
- Sulakvelidze, A. Yersiniae other than Y. enterocolitica, Y. pseudotuberculosis, and Y. pestis: The ignored species. Microbes Infect. 2000, 2, 497–513. [Google Scholar] [PubMed]
- Gierczyński, R.; Jagielski, M.; Rastawicki, W. Evaluation of usefulness for selected virulence markers for identifying pathogenic Yersinia enterocolitica strains. IV. Genes myfA and ureC. Med. Dośw. Mikrobiol. 2002, 54, 347–355. [Google Scholar]
- Brzostek, K. Mechanizmy regulacji czynników wirulencji Yersinia enterocolitica. Postępy Mikrobiol. 2009, 43, 7–38. [Google Scholar]
- von Tils, D.; Blädel, I.; Schmidt, M.A.; Heusipp, G. Type II secretion in Yersinia-a secretion system for pathogenicity and environmental fitness. Front. Cell. Infect. Microbiol. 2012, 2, 1–11. [Google Scholar]
- Bancerz-Kisiel, A.; Pieczywek, M.; Łada, P.; Szweda, W. The most important virulence markers of Yersinia enterocolitica and their role during infection. Genes 2018, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.; Williams, P. Yersinia virulence factors—A sophisticated arsenal for combating host defence. F1000Research 2016, 5, 1370. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Raza, H.; Niazi, S.; Khan, I.M.; Akhtar, W.; Safdar, W.; Wang, Z. A comprehensive review on the prevalence, pathogenesis and detection of Yersinia enterocolitica. RSC Adv. 2019, 9, 41010–44102. [Google Scholar] [CrossRef]
- Fàbrega, A.; Vila, J. Yersinia enterocolitica: Pathogenesis, virulence and antimicrobial resistance. Enferm. Infecc. Microbiol. Clin. 2012, 30, 24–32. [Google Scholar] [CrossRef]
- Bancerz-Kisiel, A.; Szweda, W. Yersiniosis—A zoonotic foodborne disease of relevance to public health. Ann. Agric. Environ. Med. 2015, 22, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Morka, K.; Bystroń, J.; Bania, J.; Korzeniowska-Kowal, A.; Korzekwa, K.; Guz-Regner, K.; Bugla-Płoskońska, G. Identification of Yersinia enterocolitica isolates from humans, pigs and wild boars by MALDI TOF MS. BMC Microbiol. 2018, 18, 86. [Google Scholar] [CrossRef] [PubMed]
- Syczyło, K.; Platt-Samoraj, A.; Bancerz-Kisiel, A.; Szczerba-Turek, A.; Lipczyńska, K.; Jabłoński, A.; Procajło, Z.; Szweda, W. Monitoring of Yersinia enterocolitica strains from free-living animals using different methods. Pol. J. Vet. Sci. 2016, 19, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Syczyło, K.; Platt-Samoraj, A.; Bancerz-Kisiel, A.; Szczerba-Turek, A.; Pajdak-Czaus, J.; Łabuć, S.; Procajło, Z.; Socha, P.; Chuzhebayeva, G.; Szweda, W. The prevalence of Yersinia enterocolitica in game animals in Poland. PLoS ONE 2018, 13, e0195136. [Google Scholar] [CrossRef]
- Bancerz-Kisiel, A.; Socha, P.; Szweda, W. Detection and characterisation of Yersinia enterocolitica strains in cold-stored carcasses of large game animals in Poland. Vet. J. 2016, 208, 102–103. [Google Scholar] [CrossRef]
- Bancerz-Kisiel, A.; Platt-Samoraj, A.; Szczerba-Turek, A.; Syczyło, K.; Szweda, W. The first pathogenic Yersinia enterocolitica bioserotype 4/O:3 strain isolated from a hunted wild boar (Sus scrofa) in Poland. Epidemiol. Infect. 2015, 143, 2758–2765. [Google Scholar] [CrossRef]
- Gierczyński, R. Genetyczna Struktura Populacji i Determinanty Chorobotwórczości Pałeczek Yersinia Enterocolitica Izolowanych z Materiału Klinicznego od Ludzi w Polsce w Latach 1996–2008. Habilitation Thesis, Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny, Warszawa, Poland, 2010. [Google Scholar]
- European Centre for Disease Prevention and Control. Yersiniosis. Annual Epidemiological Report for 2018. ECDC: Stockholm, Sweden, 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2018-yersiniosis-corrected.pdf (accessed on 29 December 2020).
- Furman, S.; Napiórkowska, A.; Sadkowska-Todys, M. Yersiniosis in Poland in 2009. Przegl. Epidemiol. 2011, 65, 235–238. [Google Scholar] [PubMed]
- Bancerz-Kisiel, A.; Szczerba-Turek, A.; Platt-Samoraj, A.; Socha, P.; Szweda, W. Bioserotypes and virulence markers of Y. enterocolitica strains isolated from roe deer (Capreolus capreolus) and red deer (Cervus elaphus). Pol. J. Vet. Sci. 2014, 17, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.L.; Hossain, M.A.; Isshiki, K.; Ukuku, D. Behavior of Yersinia enterocolitica in foods. J. Pathog. 2011, 2011, 420732. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Virdi, J.S. Production of Yersinia stable toxin (YST) and distribution of yst genes in biotype 1A strains of Yersinia enterocolitica. J. Med. Microbiol. 2004, 53, 1065–1068. [Google Scholar] [CrossRef]
- Fanelli, A.; Battisti, E.; Zanet, S.; Trisciuoglio, A.; Ferroglio, E. A systematic review and meta-analysis of Toxoplasma gondii in roe deer (Capreolus capreolus) and red deer (Cervus elaphus) in Europe. Zoonoses Public Health 2020, 8. [Google Scholar] [CrossRef]
- Platt-Samoraj, A.; Ugorski, M.; Szweda, W.; Szczerba-Turek, A.; Wojciech, K.; Procajło, Z. Analysis of the presence of ail, ystA and ystB genes in Yersinia enterocolitica strains isolated from aborting sows and aborted fetuses. J. Vet. Med. B Infect. Dis. Vet. Public Health 2006, 53, 341–346. [Google Scholar] [CrossRef]
- Bancerz-Kisiel, A.; Szczerba-Turek, A.; Platt-Samoraj, A.; Szweda, W. Distribution of the ymoA and ystA genes and enterotoxins Yst production by Yersinia enterocolitica strains isolated from humans and pigs. Pol. J. Vet. Sci. 2012, 15, 609–614. [Google Scholar] [CrossRef]
- Paixão, R.; Moreno, L.Z.; Sena de Gobbi, D.D.; Raimundo, D.C.; Ferreira, T.S.; Spindola, M.G.; Hofer, E.; Falavina Dos Reis, C.M.; Matté, M.H.; Micke Moreno, A. Genotypic Characterization of Yersinia enterocolitica Biotype 4/O:3 Isolates from Pigs and Slaughterhouses Using SE-AFLP, ERIC-PCR, and PFGE. J. Pathog. 2013, 2013, 521510. [Google Scholar] [CrossRef]
- Li, H.; Bhaduri, S.; Magee, W.E. Maximizing plasmid stability and production of released proteins in Yersinia enterocolitica. Appl. Environ. Microbiol. 1998, 64, 1812–1815. [Google Scholar] [CrossRef]
- Wang, H.; Palmer, J.; Flint, S. Function of pYV plasmid on biofilm formation of Yersinia enterocolitica ERL032123 in the Presence of Ca2+. J. Food Prot. 2019, 82, 1683–1687. [Google Scholar] [CrossRef]
- Falcão, J.P.; Falcão, D.P.; Pitondo-Silva, A.; Malaspina, A.C.; Brocchi, M. Molecular typing and virulence markers of Yersinia enterocolitica strains from human, animal and food origins isolated between 1968 and 2000 in Brazil. J. Med. Microbiol. 2006, 55, 1539–1548. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ratnam, S.; Mercer, E.; Picco, B.; Parsons, S.; Butler, R. A nosocomial outbreak of diarrheal disease due to Yersinia enterocolitica serotype 0:5, biotype 1. J. Infect. Dis. 1982, 145, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M.H.; Hooper, W.L. Excretion of Yersinia spp. associated with consumption of pasteurized milk. Epidemiol. Infect. 1990, 104, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Stephan, R.; Joutsen, S.; Hofer, E.; Säde, E.; Björkroth, J.; Ziegler, D.; Fredriksson-Ahomaa, M. Characteristics of Yersinia enterocolitica biotype 1A strains isolated from patients and asymptomatic carriers. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, L.M.; Hallanvuo, S.; Haukka, K.; Skurnik, M.; Siitonen, A. The ail gene is present in some Yersinia enterocolitica biotype 1A strains. Foodborne Pathog. Dis. 2011, 8, 455–457. [Google Scholar] [CrossRef]
- Tennant, S.M.; Grant, T.H.; Robins-Browne, R.M. Pathogenicity of Yersinia enterocolitica biotype 1A. FEMS Immunol. Med. Microbiol. 2003, 38, 127–137. [Google Scholar] [CrossRef]
- Singh, I.; Virdi, J.S. Interaction of Yersinia enterocolitica biotype 1A strains of diverse origin with cultured cells in vitro. Jpn. J. Infect. Dis. 2005, 58, 31–33. [Google Scholar]
- Cheyne, B.M.; Van Dyke, M.I.; Anderson, W.B.; Huck, P.M. The detection of Yersinia enterocolitica in surface water by quantitative PCR amplification of the ail and yadA genes. J. Water Health 2010, 8, 487–499. [Google Scholar] [CrossRef]
- Bancerz-Kisiel, A.; Szczerba-Turek, A.; Platt-Samoraj, A.; Michalczyk, M.; Szweda, W. Characterisation of ail-positive Yersinia enterocolitica of different biotypes using HRMA. Int. J. Food Microbiol. 2018, 269, 46–51. [Google Scholar] [CrossRef]
- Stachelska, M.A. Identification of Pathogenicity of Yersinia enterocolitica in Pig Tonsils Using the Real-Time PCR. Pol. J. Microbiol. 2018, 67, 219–222. [Google Scholar] [CrossRef]
- Gilpin, B.J.; Robson, B.; Lin, S.; Hudson, J.A.; Weaver, L.; Dufour, M.; Strydom, H. The limitations of pulsed-field gel electrophoresis for analysis of Yersinia enterocolitica isolates. Zoonoses Public Health 2014, 61, 405–410. [Google Scholar] [CrossRef]
- Koskinen, J.; Keto-Timonen, R.; Virtanen, S.; Vilar, M.J.; Korkeala, H. Prevalence and dynamics of pathogenic Yersinia enterocolitica 4/O:3 among finnish piglets, fattening pigs, and sows. Foodborne Pathog. Dis. 2019, 16, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson-Ahomaa, M.; Hallanvuo, S.; Korte, T.; Siitonen, A.; Korkeala, H. Correspondence of genotypes of sporadic Yersinia enterocolitica bioserotype 4/O:3 strains from human and porcine sources. Epidemiol. Infect. 2001, 127, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson-Ahomaa, M.; Stolle, A.; Siitonen, A.; Korkeala, H. Sporadic human Yersinia enterocolitica infections caused by bioserotype 4/O:3 originate mainly from pigs. J. Med. Microbiol. 2006, 55, 747–749. [Google Scholar] [CrossRef]
- Fredriksson-Ahomaa, M.; Wacheck, S.; Bonke, R.; Stephan, R. Different Enteropathogenic Yersinia Strains Found in Wild Boars and Domestic Pigs. Foodborne Pathog. Dis. 2011, 8, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, H.; Hensel, A.; Aleksic, S.; Meyer, H. Identification of Yersinia enterocolitica within the genus Yersinia. Syst. Appl. Microbiol. 2000, 23, 58–62. [Google Scholar] [CrossRef]
- Wannet, W.J.B.; Reessink, M.; Brunings, H.A.; Maas, H.M.E. Detection of Pathogenic Yersinia enterocolitica by a Rapid and Sensitive Duplex PCR Assay. J. Clin. Microbiol. 2001, 39, 4483–4486. [Google Scholar] [CrossRef]
- In Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar]
- Hunter, P.R.; Gaston, M.A. Numerical index of the discriminatory ability of typing systems: An application of Simpson’s index of diversity. J. Clin. Microbiol. 1988, 26, 2465–2466. [Google Scholar] [CrossRef]
- CDC—PulseNet. Available online: https://www.cdc.gov/pulsenet/pathogens/index.html (accessed on 30 July 2018).
- Gierczyński, R. Evaluation of the usefulness of selected virulence markers for identification of virulent Yersinia enterocolitica strains. II. Genotypic markers associated with the pYV plasmid. Med. Dośw. Mikrobiol. 2000, 52, 35–49. [Google Scholar]
- Zacharczuk, K. Investigation of molecular virulence factors of Yersinia enterocolitica 1B/08 human clinical isolates collected in Poland in 2009. Med. Dośw. Mikrobiol. 2013, 65, 233–243. [Google Scholar] [PubMed]
- Sharma, S.; Mittal, S.; Mallik, S.; Virdi, J.S. Molecular characterization of β-lactamase genes blaA and blaB of Yersinia enterocolitica biovar 1A. FEMS Microbiol. Lett. 2006, 257, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, H.; Rahimian, M.D.; Dehkordi, F.S. Identification and characterization of Yersinia enterocolitica isolated from raw chicken meat based on molecular and biological techniques. J. Appl. Poult. Res. 2013, 22, 137–145. [Google Scholar] [CrossRef]
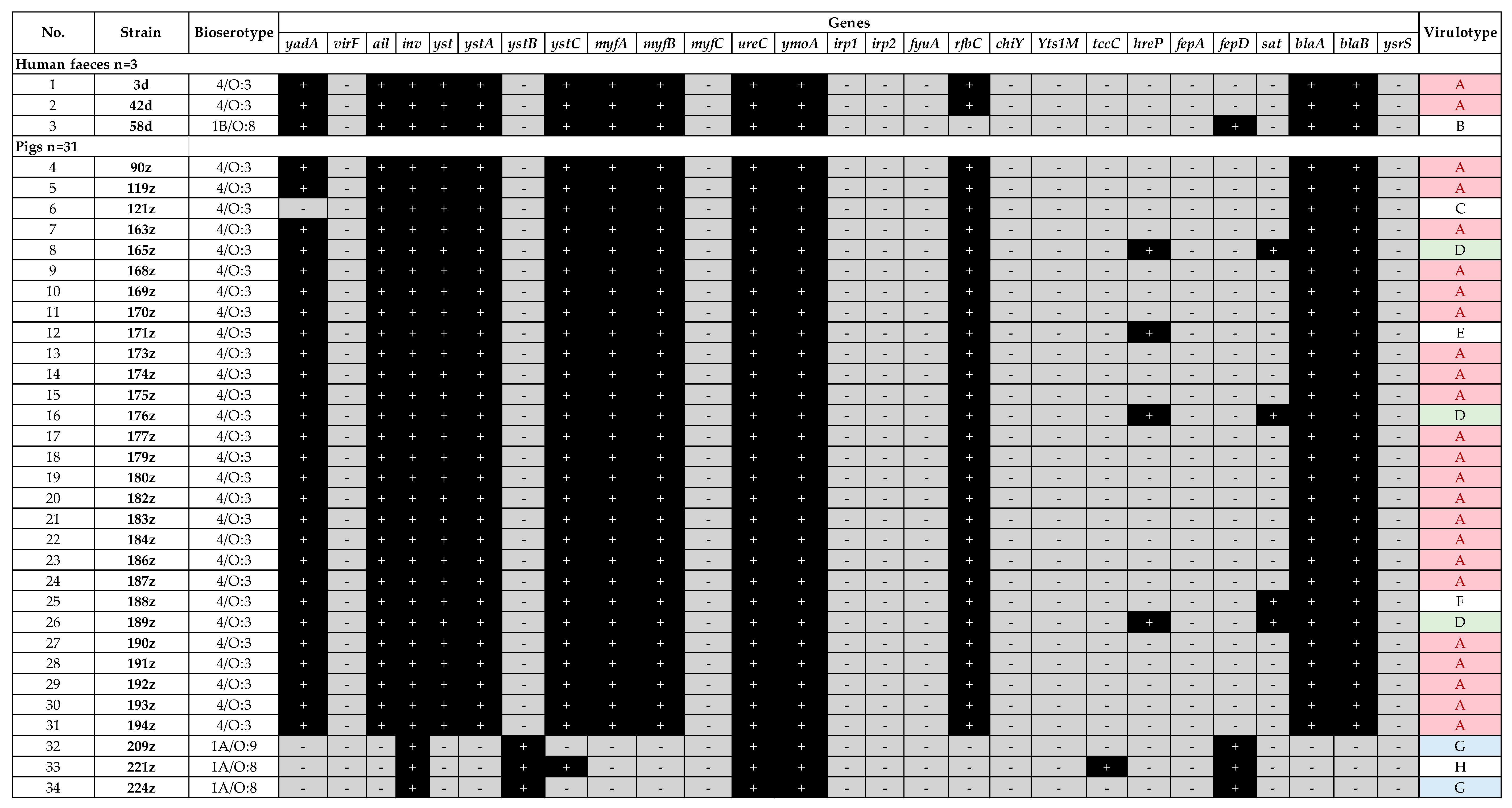
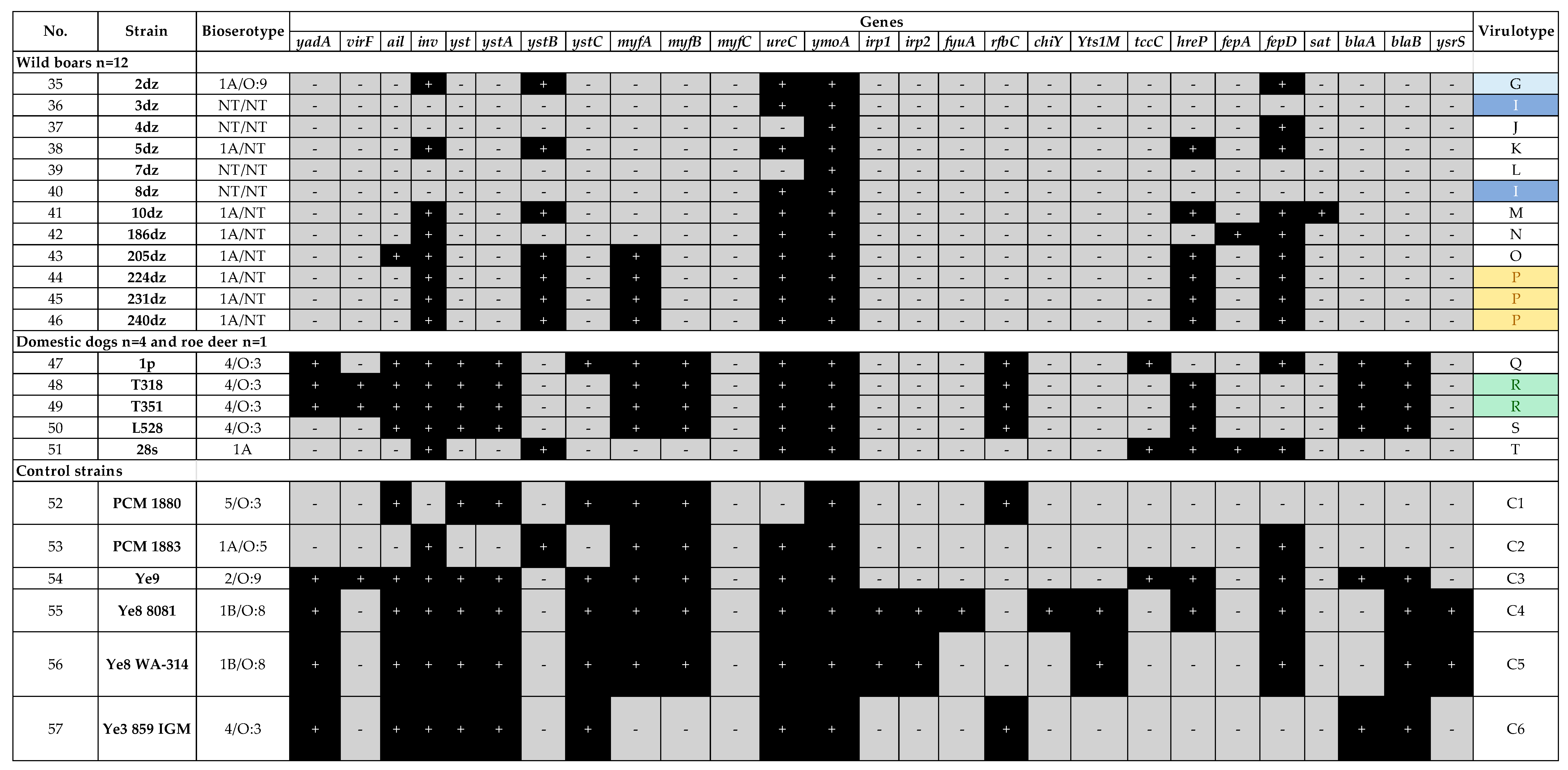
| Species | Origin (Number of Isolates) | Bioserotype—Strain Name |
|---|---|---|
| Y. enterocolitica | Human feces (n = 3) | 4/O:3—3d, 42d 1B/O:8—58d |
| Fattening pig feces (n = 28) | 4/O:3—163z, 165z, 168z, 169z, 170z, 171z, 173z, 174z, 175z, 176z, 177z, 179z, 180z, 182z, 183z, 184z, 186z, 187z, 188z, 189z, 190z, 191z, 192z, 193z, 194z 1A/O:9—209z 1A/O:8—221z, 224z | |
| Fattening pig tonsil (n = 3) | 4/O:3—90z, 119z, 121z | |
| Wild boar tonsil (n = 8) | 1A/NT—2dz, 5dz, 10dz, 186dz, 205dz, 224dz, 231dz, 240dz | |
| Domestic dog feces * (n = 4) | 4/O:3—1p, T318, T351, L528 | |
| Roe deer tonsil (n = 1) | 1A/NT—28s | |
| Y. kristensenii | Wild boar tonsil (n = 4) | 3dz, 4dz |
| Y. frederiksenii | 7dz | |
| Y. pekkanenii | 8dz |
| VNTR | Repeat Sequence | Primer Sequence (5′→3′) | Size of Repeat [bp] | Size of Offsets [bp] |
|---|---|---|---|---|
| A | GAGCCGCAGCGGTGTTAGCGGCTCTCACTTACCCGAATCACTTACCGTTGTAAGCTCATCGGGATGCATTCGCATGCTGCCTTGCTGCAACACCAATTACTTTGAGTAACATCTTTTTTAATAA | CATTGTATTACCCAGCAGATCC GGTGATACAGAGCGGTAGAC | 124 | 177 |
| B | CAGTAAGTGATTCGGGTAAGTGAGTGCAGTCAACACCGCTGCAACTTGAAAGATGACGGCATACCTCTATCCAATAGATTTCATGTTGCAGCAAGGCGGCAAACGAGATTATCCCGATGAGCTTACAT | ATTGACGGTCGGTATTATCTCG ACTAAGGTTTGGGGTGACATAC | 128 | 167 |
| C | TATTGGCTACATAAATAGATTAGCTACATAAATA | ATTATAGTGGCGGTCATTATCG AATAGCTTTCATCAAGCCTGTC | 34 | 203 |
| D | ATCTTCCTGGGTGCACCATCTTAAGAAACCTCGCTACGGCGGGGTTTTTCGTTTTTAGATTCTACAAATACGCAGAACCGAGCGGTCGGAAGTTCGAGCCGAGCGTAGCGAGACAACGTTGCTTTAGCAACGGCCCGCAGGGCGAGGCGTTAGCCGAGTC | GTCAAATGAACATCAGGTAGTG TCATTCACAATAGGAAATAGCG | 160 | 204 |
| E | TTGCGCGGCCTTTTTAGCTTTCACGCGAGCAATCGCTGCGGCCACGGCAGCTTTGCGCGGATCTTCCTCTGGCGCTTCACTTTCGCTTTTTACCGGTTCTGACTCAAGTTGAGC | GGAAGTGATTGAGGTTGATAGC GTTATTGCTGCTCGTGAGG | 114 | 260 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morka, K.; Wałecka-Zacharska, E.; Schubert, J.; Dudek, B.; Woźniak-Biel, A.; Kuczkowski, M.; Wieliczko, A.; Bystroń, J.; Bania, J.; Bugla-Płoskońska, G. Genetic Diversity and Distribution of Virulence-Associated Genes in Y. enterocolitica and Y. enterocolitica-Like Isolates from Humans and Animals in Poland. Pathogens 2021, 10, 65. https://doi.org/10.3390/pathogens10010065
Morka K, Wałecka-Zacharska E, Schubert J, Dudek B, Woźniak-Biel A, Kuczkowski M, Wieliczko A, Bystroń J, Bania J, Bugla-Płoskońska G. Genetic Diversity and Distribution of Virulence-Associated Genes in Y. enterocolitica and Y. enterocolitica-Like Isolates from Humans and Animals in Poland. Pathogens. 2021; 10(1):65. https://doi.org/10.3390/pathogens10010065
Chicago/Turabian StyleMorka, Katarzyna, Ewa Wałecka-Zacharska, Justyna Schubert, Bartłomiej Dudek, Anna Woźniak-Biel, Maciej Kuczkowski, Alina Wieliczko, Jarosław Bystroń, Jacek Bania, and Gabriela Bugla-Płoskońska. 2021. "Genetic Diversity and Distribution of Virulence-Associated Genes in Y. enterocolitica and Y. enterocolitica-Like Isolates from Humans and Animals in Poland" Pathogens 10, no. 1: 65. https://doi.org/10.3390/pathogens10010065
APA StyleMorka, K., Wałecka-Zacharska, E., Schubert, J., Dudek, B., Woźniak-Biel, A., Kuczkowski, M., Wieliczko, A., Bystroń, J., Bania, J., & Bugla-Płoskońska, G. (2021). Genetic Diversity and Distribution of Virulence-Associated Genes in Y. enterocolitica and Y. enterocolitica-Like Isolates from Humans and Animals in Poland. Pathogens, 10(1), 65. https://doi.org/10.3390/pathogens10010065






