Poxviral Strategies to Overcome Host Cell Apoptosis
Abstract
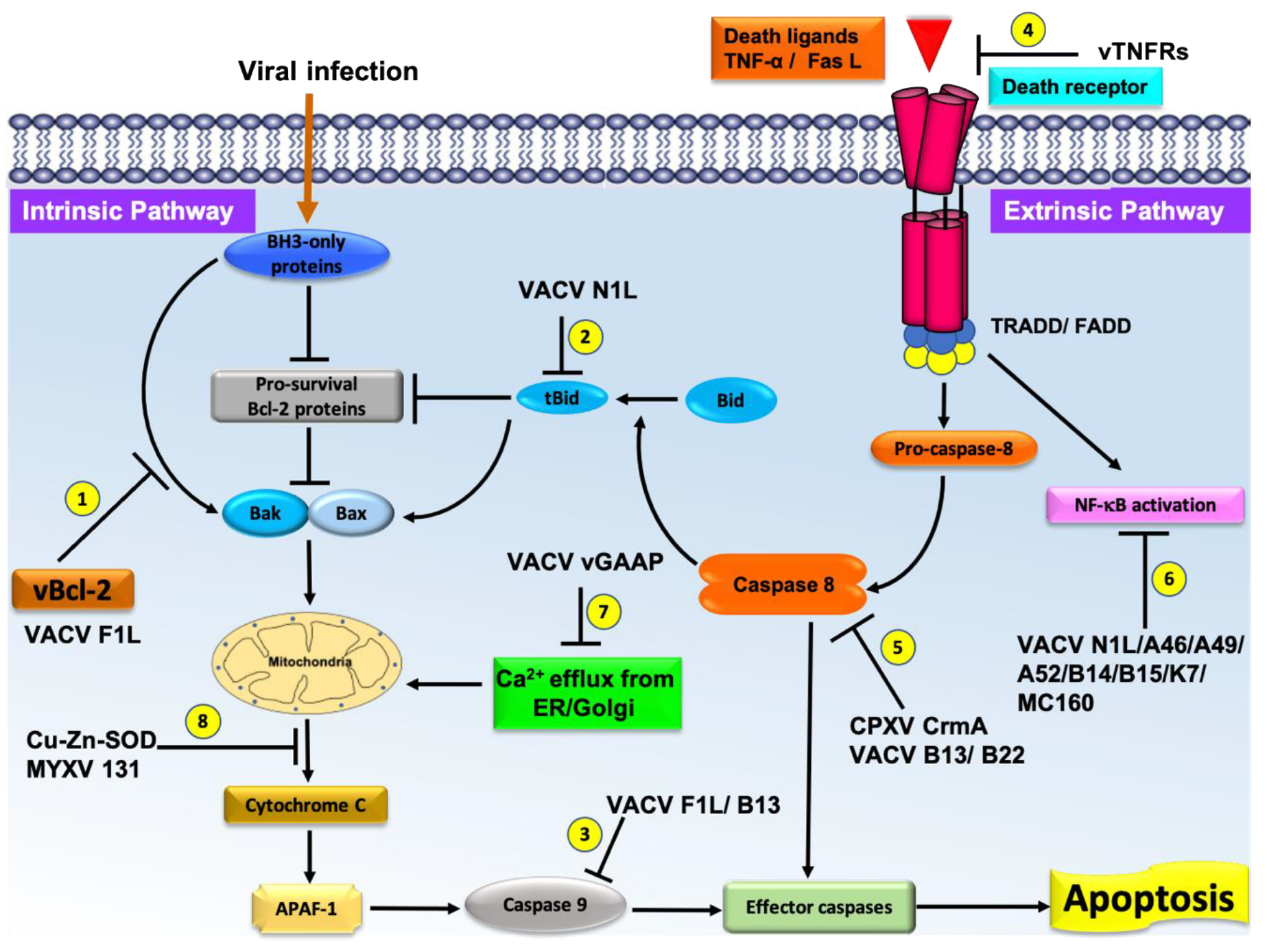
1. Pox Virus Inhibition of Host Intrinsic Activated Apoptosis with Bcl-2 Homologs
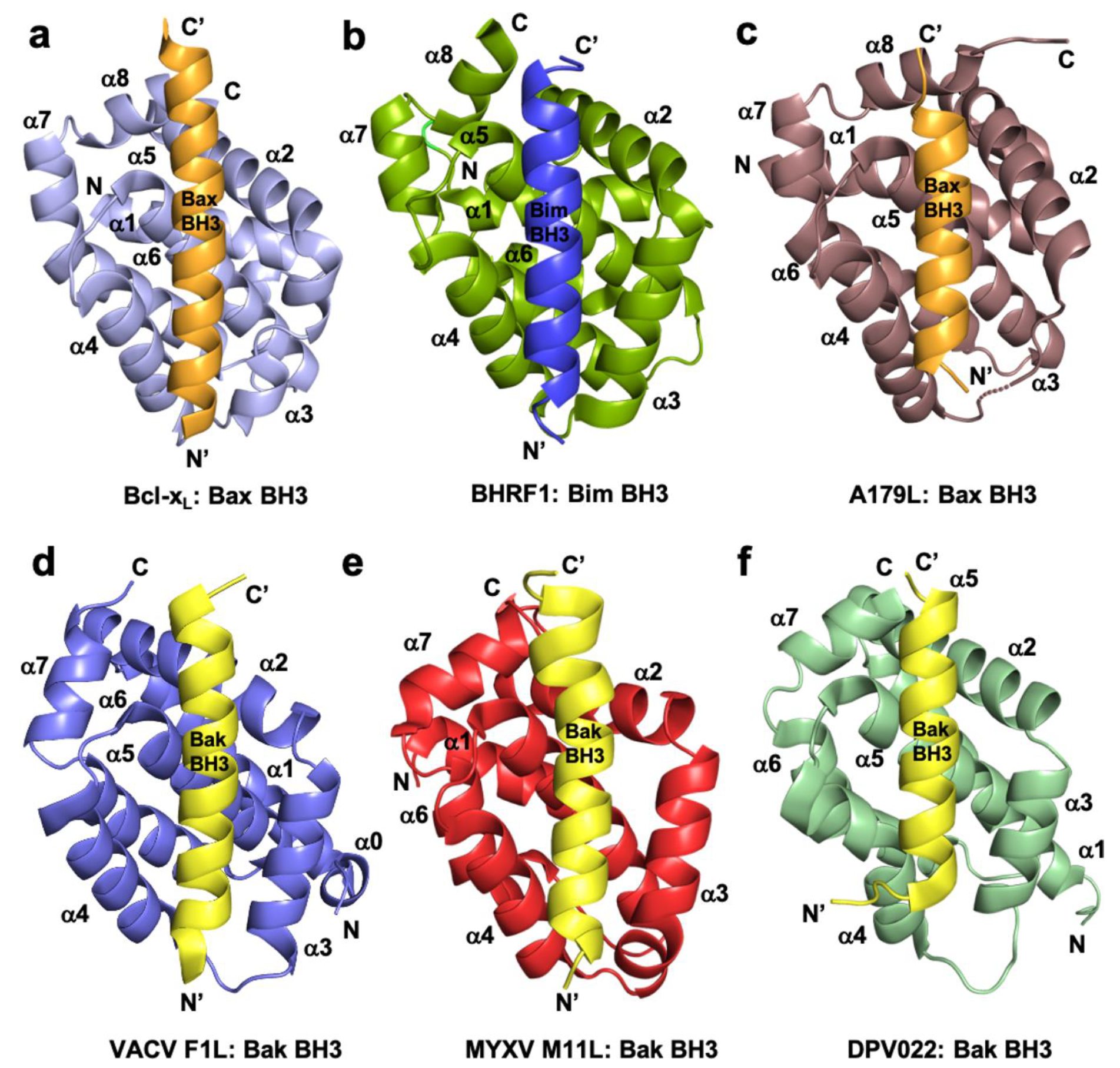
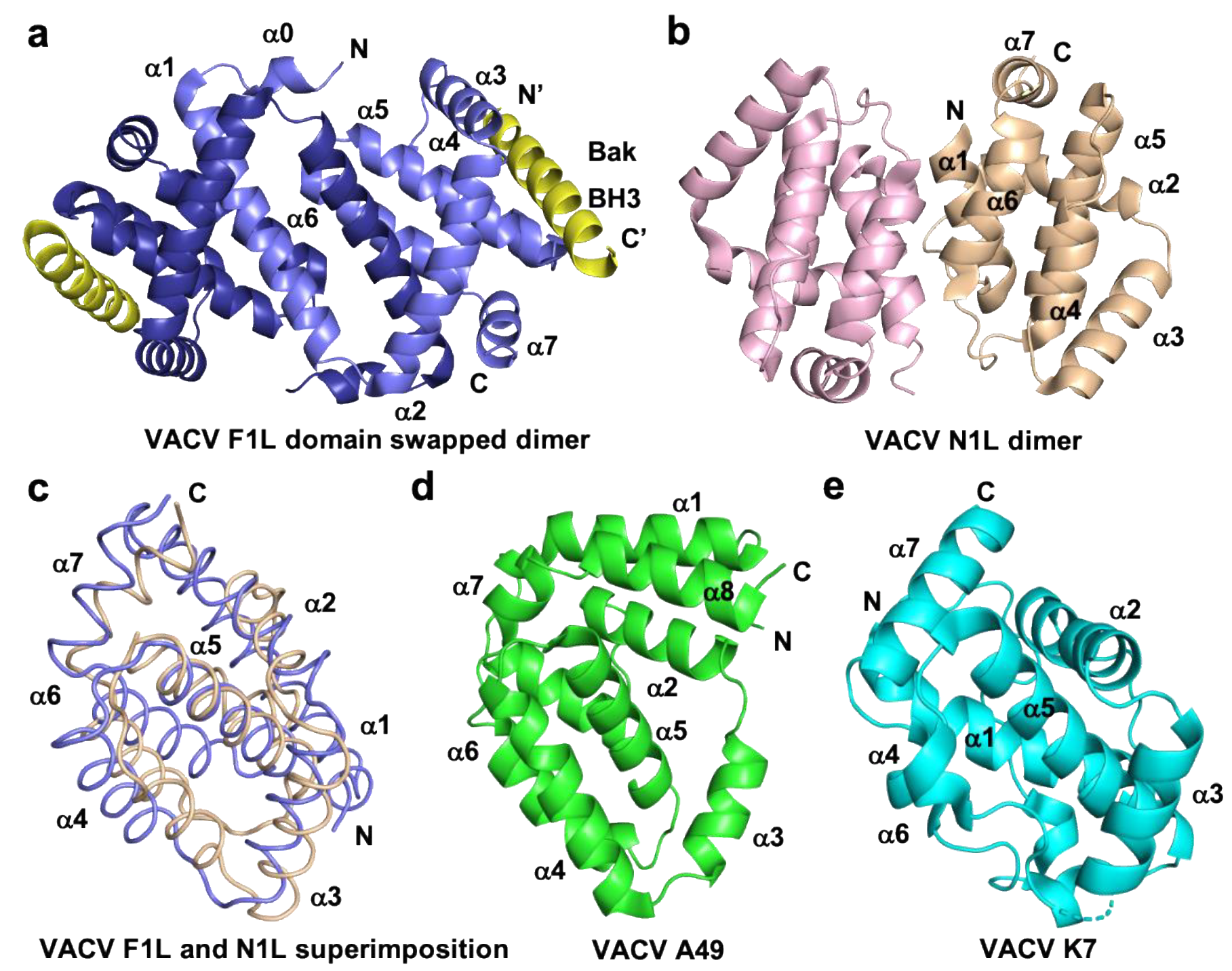
1.1. Orthopoxviruses
1.2. Leporipoxviruses
1.3. Yatapoxviruses
1.4. Parapoxviruses
1.5. Capripoxviruses
| Binding Affinities (nM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pro-apoptotic Protein | VACV F1L [38] | VARV F1L [39] | M11L [37] | TANV16L [110] | SPPV14 [117] | DPV022 [74] | FPV039 [119] | CNP058 [120] | ORFV125 [115] |
| Bak | 4300 | 2640 | 50 | 38 | 48 | 6930 | 76 | 508 | 5802 |
| Bax | 1850 | 960 | 75 | 70 | 26 | 4040 | 76 | 326 | 682 |
| Bok | N/A | N/A | N/A | NB | 7580 | N/A | NB | NB | NB |
| Bad | NB | NB | >1000 | 219 | 5197 | NB | 653 | NB | NB |
| Bid | NB | 3200 | 100 | 719 | 136 | NB | 2 | 50 | NB |
| Bik | NB | NB | >1000 | 1250 | 1766 | NB | 30 | NB | NB |
| Bim | 250 | NB | 5 | 180 | 19 | 340 | 10 | 353 | NB |
| Bmf | NB | NB | 100 | 606 | 44 | NB | 16 | 294 | NB |
| Hrk | NB | NB | >1000 | 3220 | 39 | NB | 24 | 312 | 1912 |
| Noxa | NB | NB | >1000 | NB | NB | NB | 28 | 3284 | NB |
| Puma | NB | NB | >1000 | 468 | 56 | NB | 24 | 2484 | 1753 |
1.6. Avipoxviruses
2. Extrinsic Apoptosis Inhibition
2.1. Poxvirus Encoded Caspase Inhibitors
2.2. Tumor Necrosis Factor (TNF) Homologs Encoded by Poxvirus
2.3. Death Effector Domain (DED) Homologs Encoded by Molluscum Contagious Virus
3. Poxvirus Encoded Indirect Influencers of Apoptotic Signaling
3.1. vGAAP
3.2. Cu-Zn-Superoxide Dismutase Homologs
3.3. Poxvirus Inhibition of Double Stranded RNA (dsRNA) Induced Apoptosis
3.4. Poxvirus Encoded E3-PKR Inhibitor of Apoptosis
| Genus | Virus | Protein Type | Protein | Function |
|---|---|---|---|---|
| Orthopoxviridae | VACV | Bcl-2 like | F1L | Pro-survival [38,54] |
| N1L | NF-κB inhibition [87] | |||
| A46 | NF-κB inhibition [90] | |||
| A49 | NF-κB inhibition [102,104] | |||
| A52 | NF-κB inhibition [95] | |||
| B14 | NF-κB inhibition [95] | |||
| B15 | NF-κB inhibition [187] | |||
| K7 | NF-κB inhibition [187] IFN signaling [100] | |||
| Serpin | B13 (SPI-1) | Caspase inhibition [56,134,136] | ||
| B22 (SPI-2) | Caspase inhibition [136,137] | |||
| SPI-3 | Caspase inhibition [138] | |||
| vTNFR | CrmB | Mimic TNFR1/2 [188] | ||
| CrmC | Mimic TNFR1/2 [188] | |||
| CrmE | Mimic TNFR1/2 [188] | |||
| SOD Homolog | A45 | Inactive SOD like virion [175] | ||
| PKR inhibitor | E3 | Binds to PKR and inhibit activation of PKR [185] | ||
| Decapping enzymes | D9/ D10 | PKR activation inhibitor [189] | ||
| Ankyrin-repeat protein | M1L | Apoptosome inhibitor [105] | ||
| VARV | Bcl-2 like | F1L | Pro-survival [39] | |
| vTNFR | CrmB | Mimic TNFR1/2 [150] | ||
| CPXV | Serpin | CrmA | Caspase inhibition [190,191] | |
| vTNFR | CrmB | Mimic TNFR1/2 [192] | ||
| CrmC | Mimic TNFR1/2 [192] | |||
| CrmD | Mimic TNFR1/2 [192] | |||
| CrmE | Mimic TNFR1/2 [149] | |||
| vCD30 | Mimic TNFR1/2 [147] | |||
| CMLV | vGAAP | 6L | Anti-apoptotic [58] | |
| ECTV | Bcl-2 like | EMV025 | Pro-survival [84] | |
| vTNFR | vCD30 | Mimic TNFR1/2 [146] | ||
| Leporipoxviridae | MYXV | Bcl-2 like | M11L | Pro-survival [37] |
| Serpin | SERP1 | Caspase inhibition [57] | ||
| SERP2 | Caspase inhibition [140] | |||
| SERP3 | Caspase inhibition [141] | |||
| vTNFR | M-T2 | Mimic TNFR1/2 [145] | ||
| SOD homolog | M131 | SOD induced anti-apoptosis [174] | ||
| E3 homolog | M029 | PKR activation inhibitor [186] | ||
| SFV | vTNFR | T2 | Mimic TNFR1/2 [145,193] | |
| SOD homolog | S131 | SOD induced anti-apoptosis [174] | ||
| E3 homolog | SPV032 | PKR activation inhibitor [185] | ||
| Yatapoxviridae | TANV | Bcl-2 like | 16L | Pro-survival [110] |
| vTNFR | 2L | Mimic TNFR1/2 [151] | ||
| Parapoxviridae | ORFV | Bcl-2 like | ORFV125 | Pro-survival [113,114,115] |
| Capripoxviridae | SPPV | Bcl-2 like | SPPV14 | Pro-survival [117,118] |
| Cervidpoxviridae | DPV | Bcl-2 like | DPV022 | Pro-survival [74] |
| Avipoxviridae | FPV | Bcl-2 like | FPV039 | Pro-survival [119,123] |
| CNP | Bcl-2 like | CNP058 | Pro-survival [120] | |
| Molluscipoxviridae | MCV | SOD homolog | MC163 | SOD induced anti-apoptosis [173] |
| Seleno protein | MC066 | Inhibit H2O2 and UV induced apoptosis [194] | ||
| vFLIP | MC159 | Inhibitor TNF-α/FasL induced apoptosis [156,157,159,167] | ||
| MC160 | Inhibitor TNF-α/induced NF-κB inhibition [157,158] |
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Kvansakul, M.; Hinds, M.G. The Bcl-2 family: Structures, interactions and targets for drug discovery. Apoptosis 2015, 20, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Yuan, J. Expansion and evolution of cell death programmes. Nat. Rev. Mol. Cell Biol. 2008, 9, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; Vaux, D.L. Viewing BCL2 and cell death control from an evolutionary perspective. Cell Death Differ. 2017, 25, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; Cory, S.; Adams, J.M. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011, 30, 3667–3683. [Google Scholar] [CrossRef]
- Bugert, J.J.; Darai, G. Poxvirus Homologues of Cellular Genes. In Molecular Evolution of Viruses—Past and Present: Evolution of Viruses by Acquisition of Cellular RNA and DNA; Becker, Y., Darai, G., Eds.; Springer: Boston, MA, USA, 2000; pp. 111–133. [Google Scholar]
- Nichols, D.B.; De Martini, W.; Cottrell, J. Poxviruses Utilize Multiple Strategies to Inhibit Apoptosis. Viruses 2017, 9, 215. [Google Scholar] [CrossRef]
- Smith, G.L.; Benfield, C.T.O.; De Motes, C.M.; Mazzon, M.; Ember, S.W.J.; Ferguson, B.J.; Sumner, R.P. Vaccinia virus immune evasion: Mechanisms, virulence and immunogenicity. J. Gen. Virol. 2013, 94, 2367–2392. [Google Scholar] [CrossRef]
- Smith, G.L.; Talbot-Cooper, C.; Lu, Y. How Does Vaccinia Virus Interfere With Interferon? Adv. Clin. Chem. 2018, 100, 355–378. [Google Scholar] [CrossRef]
- Lawler, C.; Brady, G. Poxviral Targeting of Interferon Regulatory Factor Activation. Viruses 2020, 12, 1191. [Google Scholar] [CrossRef]
- Garcia-Calvo, M.; Peterson, E.P.; Leiting, B.; Ruel, R.; Nicholson, D.W.; Thornberry, N.A. Inhibition of Human Caspases by Peptide-based and Macromolecular Inhibitors. J. Biol. Chem. 1998, 273, 32608–32613. [Google Scholar] [CrossRef]
- Ashkenazi, A. Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J. Clin. Investig. 2015, 125, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Schütze, S.; Tchikov, V.; Schneider-Brachert, W. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer 2002, 2, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Seyrek, K.; Ivanisenko, N.V.; Richter, M.; Hillert, L.K.; König, C.; Lavrik, I.N. Controlling Cell Death through Post-translational Modifications of DED Proteins. Trends Cell Biol. 2020, 30, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Thome, M.; Schneider, P.; Hofmann, K.; Fickenscher, H.; Meinl, E.; Neipel, F.; Mattmann, C.; Burns, K.; Bodmer, J.-L.; Schröter, M.; et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nat. Cell Biol. 1997, 386, 517–521. [Google Scholar] [CrossRef]
- Schug, Z.T.; Gonzalvez, F.; Houtkooper, R.H.; Vaz, F.M.; Gottlieb, E. BID is cleaved by caspase-8 within a native complex on the mitochondrial membrane. Cell Death Differ. 2010, 18, 538–548. [Google Scholar] [CrossRef]
- Bertrand, M.J.; Milutinovic, S.; Dickson, K.M.; Ho, W.C.; Boudreault, A.; Durkin, J.; Gillard, J.W.; Jaquith, J.B.; Morris, S.J.; Barker, P.A. cIAP1 and cIAP2 Facilitate Cancer Cell Survival by Functioning as E3 Ligases that Promote RIP1 Ubiquitination. Mol. Cell 2008, 30, 689–700. [Google Scholar] [CrossRef]
- Banjara, S.; Suraweera, C.D.; Hinds, M.G.; Kvansakul, M. The Bcl-2 Family: Ancient Origins, Conserved Structures, and Divergent Mechanisms. Biomolecules 2020, 10, 128. [Google Scholar] [CrossRef]
- Caria, S.; Hinds, M.G.; Kvansakul, M. Structural insight into an evolutionarily ancient programmed cell death regulator—The crystal structure of marine sponge BHP2 bound to LB-Bak-2. Cell Death Dis. 2018, 8, e2543. [Google Scholar] [CrossRef]
- Popgeorgiev, N.; Sa, J.D.; Jabbour, L.; Banjara, S.; Nguyen, T.T.M.; Akhavan-E-Sabet, A.; Gadet, R.; Ralchev, N.; Manon, S.; Hinds, M.G.; et al. Ancient and conserved functional interplay between Bcl-2 family proteins in the mitochondrial pathway of apoptosis. Sci. Adv. 2020, 6, eabc4149. [Google Scholar] [CrossRef]
- Banjara, S.; Sa, J.D.; Hinds, M.G.; Kvansakul, M. The structural basis of Bcl-2 mediated cell death regulation in hydra. Biochem. J. 2020, 477, 3287–3297. [Google Scholar] [CrossRef]
- Yan, N.; Chai, J.; Lee, E.S.; Gu, L.; Liu, Q.; He, J.; Wu, J.-W.; Kokel, D.; Li, H.; Hao, Q.; et al. Structure of the CED-4–CED-9 complex provides insights into programmed cell death in Caenorhabditis elegans. Nat. Cell Biol. 2005, 437, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, C.D.; Caria, S.; Järvå, M.; Hinds, M.G.; Kvansakul, M. A structural investigation of NRZ mediated apoptosis regulation in zebrafish. Cell Death Dis. 2018, 9, 967. [Google Scholar] [CrossRef] [PubMed]
- Vaux, D.L.; Haecker, G.; Strasser, A. An evolutionary perspective on apoptosis. Cell 1994, 76, 777–779. [Google Scholar] [CrossRef]
- Kvansakul, M.; Hinds, M.G. Structural biology of the Bcl-2 family and its mimicry by viral proteins. Cell Death Dis. 2013, 4, e909. [Google Scholar] [CrossRef]
- Kvansakul, M.; Hinds, M.G. Chapter Three—The Structural Biology of BH3-Only Proteins. In Methods in Enzymology; Ashkenazi, A., Yuan, J., Wells, J.A., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 544, pp. 49–74. [Google Scholar]
- Luo, X.; O’Neill, K.L.; Huang, K. The third model of Bax/Bak activation: A Bcl-2 family feud finally resolved? F1000Research 2020, 9, 935. [Google Scholar] [CrossRef]
- Strasser, A. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 2005, 5, 189–200. [Google Scholar] [CrossRef]
- Shamas-Din, A.; Brahmbhatt, H.; Leber, B.; Andrews, D.W. BH3-only proteins: Orchestrators of apoptosis. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 508–520. [Google Scholar] [CrossRef]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Vervliet, T.; Parys, J.B.; Bultynck, G. Bcl-2 proteins and calcium signaling: Complexity beneath the surface. Oncogene 2016, 35, 5079–5092. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.M.; Barry, M. Near death experiences: Poxvirus regulation of apoptotic death. Virology 2006, 344, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.A.; Norris, P.S.; Ware, C.F. To kill or be killed: Viral evasion of apoptosis. Nat. Immunol. 2002, 3, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, J.M.; Bellows, D.S. Viral versus cellular BCL-2 proteins. Cell Death Differ. 2003, 10, S68–S76. [Google Scholar] [CrossRef]
- Kvansakul, M.; Van Delft, M.F.; Lee, E.F.; Gulbis, J.M.; Fairlie, W.D.; Huang, D.C.; Colman, P.M. A Structural Viral Mimic of Prosurvival Bcl-2: A Pivotal Role for Sequestering Proapoptotic Bax and Bak. Mol. Cell 2007, 25, 933–942. [Google Scholar] [CrossRef]
- Kvansakul, M.; Yang, H.; Fairlie, W.D.; Czabotar, P.E.; Fischer, S.F.; Perugini, M.A.; Huang, D.C.S.; Colman, P.M. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 2008, 15, 1564–1571. [Google Scholar] [CrossRef]
- Marshall, B.J.; Puthalakath, H.; Caria, S.; Chugh, S.S.; Doerflinger, M.; Colman, P.M.; Kvansakul, M. Variola virus F1L is a Bcl-2-like protein that unlike its vaccinia virus counterpart inhibits apoptosis independent of Bim. Cell Death Dis. 2015, 6, e1680. [Google Scholar] [CrossRef]
- Altmann, M.; Hammerschmidt, W. Epstein-Barr Virus Provides a New Paradigm: A Requirement for the Immediate Inhibition of Apoptosis. PLoS Biol. 2005, 3, e404. [Google Scholar] [CrossRef]
- Kvansakul, M.; Caria, S.; Hinds, M.G. The Bcl-2 Family in Host-Virus Interactions. Viruses 2017, 9, 290. [Google Scholar] [CrossRef]
- Chiou, S.K.; Tseng, C.C.; Rao, L.; White, E. Functional complementation of the adenovirus E1B 19-kilodalton protein with Bcl-2 in the inhibition of apoptosis in infected cells. J. Virol. 1994, 68, 6553–6566. [Google Scholar] [CrossRef]
- White, E.; Cipriani, R.; Sabbatini, P.; Denton, A. Adenovirus E1B 19-kilodalton protein overcomes the cytotoxicity of E1A proteins. J. Virol. 1991, 65, 2968–2978. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Sabbatini, P.; Perez, D.; Rao, L.; Modha, D.; White, E. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 1996, 10, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Kvansakul, M.; Wei, A.H.; Fletcher, J.I.; Willis, S.N.; Chen, L.; Roberts, A.W.; Huang, D.C.S.; Colman, P.M. Structural Basis for Apoptosis Inhibition by Epstein-Barr Virus BHRF1. PLoS Pathog. 2010, 6, e1001236. [Google Scholar] [CrossRef] [PubMed]
- Desbien, A.L.; Kappler, J.W.; Marrack, P. The Epstein-Barr virus Bcl-2 homolog, BHRF1, blocks apoptosis by binding to a limited amount of Bim. Proc. Natl. Acad. Sci. USA 2009, 106, 5663–5668. [Google Scholar] [CrossRef]
- Cheng, E.H.-Y.; Nicholas, J.; Bellows, D.S.; Hayward, G.S.; Guo, H.-G.; Reitz, M.S.; Hardwick, J.M. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc. Natl. Acad. Sci. USA 1997, 94, 690–694. [Google Scholar] [CrossRef]
- Reddy, V.R.A.P.; Sadigh, Y.; Tang, N.; Yao, Y.; Nair, V. Novel Insights into the Roles of Bcl-2 Homolog Nr-13 (vNr-13) Encoded by Herpesvirus of Turkeys in the Virus Replication Cycle, Mitochondrial Networks, and Apoptosis Inhibition. J. Virol. 2020, 94, 2049-19. [Google Scholar] [CrossRef]
- Nava, V.E.; Cheng, E.H.; Veliuona, M.; Zou, S.; Clem, R.J.; Mayer, M.L.; Hardwick, J.M. Herpesvirus saimiri encodes a functional homolog of the human bcl-2 oncogene. J. Virol. 1997, 71, 4118–4122. [Google Scholar] [CrossRef]
- Ku, B.; Woo, J.-S.; Liang, C.; Lee, K.-H.; Hong, H.-S.; Kim, K.-S.; Jung, J.U.; Oh, B.-H. Structural and Biochemical Bases for the Inhibition of Autophagy and Apoptosis by Viral BCL-2 of Murine γ-Herpesvirus 68. PLoS Pathog. 2008, 4, e25. [Google Scholar] [CrossRef]
- Galindo, I.; Hernaez, B.; Díaz-Gil, G.; Escribano, J.M.; Alonso, C. A179L, a viral Bcl-2 homologue, targets the core Bcl-2 apoptotic machinery and its upstream BH3 activators with selective binding restrictions for Bid and Noxa. Virology 2008, 375, 561–572. [Google Scholar] [CrossRef]
- Banjara, S.; Caria, S.; Dixon, L.K.; Hinds, M.G.; Kvansakul, M. Structural Insight into African Swine Fever Virus A179L-Mediated Inhibition of Apoptosis. J. Virol. 2017, 91, e02228-16. [Google Scholar] [CrossRef]
- Banjara, S.; Mao, J.; Ryan, T.M.; Caria, S.; Kvansakul, M. Grouper iridovirus GIV66 is a Bcl-2 protein that inhibits apoptosis by exclusively sequestering Bim. J. Biol. Chem. 2018, 293, 5464–5477. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Thibault, J.; Mehta, N.; Colman, P.M.; Barry, M.; Kvansakul, M. Structural Insight into BH3 Domain Binding of Vaccinia Virus Antiapoptotic F1L. J. Virol. 2014, 88, 8667–8677. [Google Scholar] [CrossRef] [PubMed]
- Loparev, V.N.; Parsons, J.M.; Knight, J.C.; Panus, J.F.; Ray, C.A.; Buller, R.M.L.; Pickup, D.J.; Esposito, J.J. A third distinct tumor necrosis factor receptor of orthopoxviruses. Proc. Natl. Acad. Sci. USA 1998, 95, 3786–3791. [Google Scholar] [CrossRef] [PubMed]
- Kettle, S.; Khanna, A.; Alcam, A.; Jassoy, C.; Ehret, R.; Smith, G.L. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1beta-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1beta-induced fever. J. Gen. Virol. 1997, 78, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Macen, J.L.; Upton, C.; Nation, N.; McFadden, G. SERP1, a Serine Proteinase Inhibitor Encoded by Myxoma Virus, Is a Secreted Glycoprotein That Interferes with Inflammation. Virology 1993, 195, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, N.; Prole, D.L.; Carrara, G.; De Motes, C.M.; Johnson, B.; Byrne, B.; Taylor, C.W.; Smith, G.L. Human and Viral Golgi Anti-apoptotic Proteins (GAAPs) Oligomerize via Different Mechanisms and Monomeric GAAP Inhibits Apoptosis and Modulates Calcium. J. Biol. Chem. 2013, 288, 13057–13067. [Google Scholar] [CrossRef] [PubMed]
- Kibler, K.V.; Shors, T.; Perkins, K.B.; Zeman, C.C.; Banaszak, M.P.; Biesterfeldt, J.; Langland, J.O.; Jacobs, B.L. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J. Virol. 1997, 71, 1992–2003. [Google Scholar] [CrossRef]
- González-Santamaría, J.; Campagna, M.; Garcia, M.A.; Marcos-Villar, L.; González, D.; Gallego, P.; Lopitz-Otsoa, F.; Guerra, S.; Rodríguez, M.S.; Esteban, M.; et al. Regulation of Vaccinia Virus E3 Protein by Small Ubiquitin-Like Modifier Proteins. J. Virol. 2011, 85, 12890–12900. [Google Scholar] [CrossRef]
- Teoh, M.L.T.; Walasek, P.J.; Evans, D.H. Leporipoxvirus Cu,Zn-Superoxide Dismutase (SOD) Homologs Are Catalytically Inert Decoy Proteins That Bind Copper Chaperone for SOD. J. Biol. Chem. 2003, 278, 33175–33184. [Google Scholar] [CrossRef]
- Weaver, J.R.; Isaacs, S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008, 225, 96–113. [Google Scholar] [CrossRef]
- Moss, B. Poxvirus DNA Replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a010199. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.W.; McFadden, G. Chapter 19—Origin and Evolution of Poxviruses. In Origin and Evolution of Viruses, 2nd ed.; Domingo, E., Parrish, C.R., Holland, J.J., Eds.; Academic Press: London, UK, 2008; pp. 431–446. [Google Scholar]
- Mccollum, A.M.; Damon, I.K. Human Monkeypox. Clin. Infect. Dis. 2013, 58, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.L.; Peng, C.; McFadden, G.; Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 2014, 21, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Fenner, F. Risks and benefits of vaccinia vaccine use in the worldwide smallpox eradication campaign. Res. Virol. 1989, 140, 465–466. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Guagliardo, S.A.; Nakazawa, Y.J.; Doty, J.B.; Mauldin, M.R. Understanding orthopoxvirus host range and evolution: From the enigmatic to the usual suspects. Curr. Opin. Virol. 2018, 28, 108–115. [Google Scholar] [CrossRef]
- Barry, M.; Wasilenko, S.T.; Stewart, T.L.; Taylor, J.M. Apoptosis Regulator Genes Encoded by Poxviruses. In Viruses and Apoptosis. Progress in Molecular and Subcellular Biology; Springer: Berlin/Heidelberg, Germany, 2004; Volume 36, pp. 19–37. [Google Scholar]
- Everett, H.; McFadden, G. Poxviruses and apoptosis: A time to die. Curr. Opin. Microbiol. 2002, 5, 395–402. [Google Scholar] [CrossRef]
- Seet, B.T.; Johnston, J.B.; Brunetti, C.R.; Barrett, J.W.; Everett, H.; Cameron, C.; Sypula, J.; Nazarian, S.H.; Lucas, A.; McFadden, G. Poxviruses Andimmuneevasion. Annu. Rev. Immunol. 2003, 21, 377–423. [Google Scholar] [CrossRef]
- Aoyagi, M.; Zhai, D.; Jin, C.; Aleshin, A.E.; Stec, B.; Reed, J.C.; Liddington, R.C. Vaccinia virus N1L protein resembles a B cell lymphoma-2 (Bcl-2) family protein. Protein Sci. 2007, 16, 118–124. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lee, E.F.; Thompson, G.V.; Wardak, A.Z.; Fairlie, W.D.; Colman, P.M. Mutation to Bax beyond the BH3 Domain Disrupts Interactions with Pro-survival Proteins and Promotes Apoptosis. J. Biol. Chem. 2011, 286, 7123–7131. [Google Scholar] [CrossRef]
- Burton, D.R.; Caria, S.; Marshall, B.; Barry, M.; Kvansakul, M. Structural basis of Deerpox virus -mediated inhibition of apoptosis. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 1593–1603. [Google Scholar] [CrossRef]
- Veyer, D.L.; Carrara, G.; De Motes, C.M.; Smith, G.L. Vaccinia virus evasion of regulated cell death. Immunol. Lett. 2017, 186, 68–80. [Google Scholar] [CrossRef]
- Wasilenko, S.T.; Stewart, T.L.; Meyers, A.F.A.; Barry, M. Vaccinia virus encodes a previously uncharacterized mitochondrial-associated inhibitor of apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 14345–14350. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.F.; Ludwig, H.; Holzapfel, J.; Kvansakul, M.; Chen, L.; Huang, D.C.S.; Sutter, G.; Knese, M.; Häcker, G. Modified vaccinia virus Ankara protein F1L is a novel BH3-domain-binding protein and acts together with the early viral protein E3L to block virus-associated apoptosis. Cell Death Differ. 2005, 13, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Hazes, B.; Kvansakul, M.; Colman, P.; Barry, M. Vaccinia Virus F1L Interacts with Bak Using Highly Divergent Bcl-2 Homology Domains and Replaces the Function of Mcl-1. J. Biol. Chem. 2009, 285, 4695–4708. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Yu, E.; Jin, C.; Welsh, K.; Shiau, C.-W.; Chen, L.; Salvesen, G.S.; Liddington, R.; Reed, J.C. Vaccinia Virus Protein F1L Is a Caspase-9 Inhibitor. J. Biol. Chem. 2009, 285, 5569–5580. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Zhai, D.; Jin, C.; Gerlic, M.; Reed, J.C.; Liddington, R. Structural Determinants of Caspase-9 Inhibition by the Vaccinia Virus Protein, F1L. J. Biol. Chem. 2011, 286, 30748–30758. [Google Scholar] [CrossRef] [PubMed]
- Caria, S.; Marshall, B.; Burton, R.-L.; Campbell, S.; Pantaki-Eimany, D.; Hawkins, C.J.; Barry, M.; Kvansakul, M. The N Terminus of the Vaccinia Virus Protein F1L Is an Intrinsically Unstructured Region That Is Not Involved in Apoptosis Regulation. J. Biol. Chem. 2016, 291, 14600–14608. [Google Scholar] [CrossRef] [PubMed]
- Gerlic, M.; Faustin, B.; Postigo, A.; Yu, E.C.-W.; Proell, M.; Gombosuren, N.; Krajewska, M.; Flynn, R.; Croft, M.; Way, M.; et al. Vaccinia virus F1L protein promotes virulence by inhibiting inflammasome activation. Proc. Natl. Acad. Sci. USA 2013, 110, 7808–7813. [Google Scholar] [CrossRef]
- Martinon, F.; Mayor, A.; Tschopp, J. The Inflammasomes: Guardians of the Body. Annu. Rev. Immunol. 2009, 27, 229–265. [Google Scholar] [CrossRef]
- Mehta, N.; Taylor, J.; Quilty, D.; Barry, M. Ectromelia virus encodes an anti-apoptotic protein that regulates cell death. Virology 2015, 475, 74–87. [Google Scholar] [CrossRef]
- Antignani, A.; Youle, R.J. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr. Opin. Cell Biol. 2006, 18, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Cooray, S.; Bahar, M.W.; Abrescia, N.G.A.; McVey, C.E.; Bartlett, N.W.; Chen, R.A.-J.; Stuart, D.I.; Grimes, J.M.; Smith, G.L. Functional and structural studies of the vaccinia virus virulence factor N1 reveal a Bcl-2-like anti-apoptotic protein. J. Gen. Virol. 2007, 88, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- De Motes, C.M.; Cooray, S.; Ren, H.; Almeida, G.M.F.; McGourty, K.; Bahar, M.W.; Stuart, D.I.; Grimes, J.M.; Graham, S.C.; Smith, G.L. Inhibition of Apoptosis and NF-κB Activation by Vaccinia Protein N1 Occur via Distinct Binding Surfaces and Make Different Contributions to Virulence. PLoS Pathog. 2011, 7, e1002430. [Google Scholar] [CrossRef]
- DiPerna, G.; Stack, J.; Bowie, A.G.; Boyd, A.; Kotwal, G.; Zhang, Z.; Arvikar, S.; Latz, E.; Fitzgerald, K.A.; Marshall, W.L. Poxvirus Protein N1L Targets the I-κB Kinase Complex, Inhibits Signaling to NF-κB by the Tumor Necrosis Factor Superfamily of Receptors, and Inhibits NF-κB and IRF3 Signaling by Toll-like Receptors. J. Biol. Chem. 2004, 279, 36570–36578. [Google Scholar] [CrossRef] [PubMed]
- González, J.M.; Esteban, M. A poxvirus Bcl-2-like gene family involved in regulation of host immune response: Sequence similarity and evolutionary history. Virol. J. 2010, 7, 59. [Google Scholar] [CrossRef]
- Fedosyuk, S.; Grishkovskaya, I.; Ribeiro, E.D.A.; Skern, T. Characterization and Structure of the Vaccinia Virus NF-κB Antagonist A46. J. Biol. Chem. 2013, 289, 3749–3762. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, H.; Heo, L.; Seok, C.; Choe, J. Structure of vaccinia virus A46, an inhibitor of TLR4 signaling pathway, shows the conformation of VIPER motif. Protein Sci. 2014, 23, 906–914. [Google Scholar] [CrossRef]
- O’Neill, L.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef]
- Fedosyuk, S.; Bezerra, G.A.; Radakovics, K.; Smith, T.K.; Sammito, M.; Bobik, N.; Round, A.; Eyck, L.F.T.; Djinović-Carugo, K.; Usón, I.; et al. Vaccinia Virus Immunomodulator A46: A Lipid and Protein-Binding Scaffold for Sequestering Host TIR-Domain Proteins. PLoS Pathog. 2016, 12, e1006079. [Google Scholar] [CrossRef]
- Toshchakov, V.Y.; Fenton, M.J.; Vogel, S.N. Cutting Edge: Differential inhibition of TLR signaling pathways by cell-permeable peptides representing BB loops of TLRs. J. Immunol. 2007, 178, 2655–2660. [Google Scholar] [CrossRef]
- Graham, S.C.; Bahar, M.W.; Cooray, S.; Chen, R.A.-J.; Whalen, D.M.; Abrescia, N.G.A.; Alderton, D.; Owens, R.J.; Stuart, D.I.; Smith, G.L.; et al. Vaccinia Virus Proteins A52 and B14 Share a Bcl-2–Like Fold but Have Evolved to Inhibit NF-κB rather than Apoptosis. PLoS Pathog. 2008, 4, e1000128. [Google Scholar] [CrossRef] [PubMed]
- Kalverda, A.P.; Thompson, G.S.; Vogel, A.; Schröder, M.; Bowie, A.G.; Khan, A.R.; Homans, S.W. Poxvirus K7 Protein Adopts a Bcl-2 Fold: Biochemical Mapping of Its Interactions with Human DEAD Box RNA Helicase DDX3. J. Mol. Biol. 2009, 385, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Chakraborty, S.; Xu, G. Mechanism of vaccinia viral protein B14–mediated inhibition of IκB kinase β activation. J. Biol. Chem. 2018, 293, 10344–10352. [Google Scholar] [CrossRef] [PubMed]
- Azar, D.F.; Haas, M.; Fedosyuk, S.; Rahaman, H.; Hedger, A.; Kobe, B.; Skern, T. Vaccinia Virus Immunomodulator A46: Destructive Interactions with MAL and MyD88 Shown by Negative-Stain Electron Microscopy. Structure 2020, 28, 1271–1287.e5. [Google Scholar] [CrossRef]
- Harte, M.T.; Haga, I.R.; Maloney, G.; Gray, P.; Reading, P.C.; Bartlett, N.W.; Smith, G.L.; Bowie, A.G.; O’Neill, L.A.J. The Poxvirus Protein A52R Targets Toll-like Receptor Signaling Complexes to Suppress Host Defense. J. Exp. Med. 2003, 197, 343–351. [Google Scholar] [CrossRef]
- Schröder, M.; Baran, M.; Bowie, A.G. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKε-mediated IRF activation. EMBO J. 2008, 27, 2147–2157. [Google Scholar] [CrossRef]
- Oda, S.-I.; Schröder, M.; Khan, A.R. Structural Basis for Targeting of Human RNA Helicase DDX3 by Poxvirus Protein K7. Structure 2009, 17, 1528–1537. [Google Scholar] [CrossRef]
- Neidel, S.; Ren, H.; Torres, A.A.; Smith, G.L. NF-κB activation is a turn on for vaccinia virus phosphoprotein A49 to turn off NF-κB activation. Proc. Natl. Acad. Sci. USA 2019, 116, 5699–5704. [Google Scholar] [CrossRef]
- Mansur, D.S.; De Motes, C.M.; Unterholzner, L.; Sumner, R.P.; Ferguson, B.J.; Ren, H.; Strnadova, P.; Bowie, A.G.; Smith, G.L. Poxvirus Targeting of E3 Ligase β-TrCP by Molecular Mimicry: A Mechanism to Inhibit NF-κB Activation and Promote Immune Evasion and Virulence. PLoS Pathog. 2013, 9, e1003183. [Google Scholar] [CrossRef]
- Neidel, S.; De Motes, C.M.; Mansur, D.S.; Strnadova, P.; Smith, G.L.; Graham, S.C. Vaccinia Virus Protein A49 Is an Unexpected Member of the B-cell Lymphoma (Bcl)-2 Protein Family. J. Biol. Chem. 2015, 290, 5991–6002. [Google Scholar] [CrossRef]
- Ryerson, M.R.; Richards, M.M.; Kvansakul, M.; Hawkins, C.J.; Shisler, J.L. Vaccinia Virus Encodes a Novel Inhibitor of Apoptosis That Associates with the Apoptosome. J. Virol. 2017, 91, e01385-17. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, G.; Barrett, J.W.; Irvine, T.S.; Gao, X.; McFadden, G. Myxoma Virus M11L Blocks Apoptosis through Inhibition of Conformational Activation of Bax at the Mitochondria. J. Virol. 2006, 80, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E.; Corbett, K.D.; Berger, J.M.; McFadden, G.; Handel, T.M. Structure of M11L: A myxoma virus structural homolog of the apoptosis inhibitor, Bcl-2. Protein Sci. 2007, 16, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Barrett, J.W.; Nazarian, S.H.; Everett, H.; Gao, X.; Bleackley, C.; Colwill, K.; Moran, M.F.; McFadden, G. Myxoma Virus M11L Prevents Apoptosis through Constitutive Interaction with Bak. J. Virol. 2004, 78, 7097–7111. [Google Scholar] [CrossRef]
- Nazarian, S.H.; Barrett, J.W.; Frace, A.M.; Olsen-Rasmussen, M.; Khristova, M.; Shaban, M.; Neering, S.; Li, Y.; Damon, I.K.; Esposito, J.J.; et al. Comparative genetic analysis of genomic DNA sequences of two human isolates of Tanapox virus. Virus Res. 2007, 129, 11–25. [Google Scholar] [CrossRef]
- Suraweera, C.D.; Anasir, M.I.; Chugh, S.; Javorsky, A.; Impey, R.E.; Zadeh, M.H.; Da Costa, T.P.S.; Hinds, M.G.; Kvansakul, M. Structural insight into tanapoxvirus-mediated inhibition of apoptosis. FEBS J. 2020, 287, 3733–3750. [Google Scholar] [CrossRef]
- Haig, D.; Mercer, A.A. Parapoxviruses. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, UK, 2008; pp. 57–63. [Google Scholar]
- Delhon, G.; Tulman, E.R.; Afonso, C.L.; Lu, Z.; De La Concha-Bermejillo, A.; Lehmkuhl, H.D.; Piccone, M.E.; Kutish, G.F.; Rock, D.L. Genomes of the Parapoxviruses Orf Virus and Bovine Papular Stomatitis Virus. J. Virol. 2004, 78, 168–177. [Google Scholar] [CrossRef]
- Westphal, D.; Ledgerwood, E.C.; Hibma, M.H.; Fleming, S.B.; Whelan, E.M.; Mercer, A.A. A Novel Bcl-2-Like Inhibitor of Apoptosis Is Encoded by the Parapoxvirus Orf Virus. J. Virol. 2007, 81, 7178–7188. [Google Scholar] [CrossRef]
- Westphal, D.; Ledgerwood, E.C.; Tyndall, J.D.A.; Hibma, M.H.; Ueda, N.; Fleming, S.B.; Mercer, A.A. The orf virus inhibitor of apoptosis functions in a Bcl-2-like manner, binding and neutralizing a set of BH3-only proteins and active Bax. Apoptosis 2009, 14, 1317–1330. [Google Scholar] [CrossRef]
- Suraweera, C.D.; Hinds, M.G.; Kvansakul, M. Crystal structures of ORFV125 provide insight into orf virus-mediated inhibition of apoptosis. Biochem. J. 2020, 477, 4527–4541. [Google Scholar] [CrossRef]
- Tuppurainen, E.S.M.; Venter, E.H.; Shisler, J.L.; Gari, G.; Mekonnen, G.A.; Juleff, N.; Lyons, N.A.; De Clercq, K.; Upton, C.; Bowden, T.R.; et al. Review: Capripoxvirus Diseases: Current Status and Opportunities for Control. Transbound. Emerg. Dis. 2017, 64, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, C.D.; Burton, D.R.; Hinds, M.G.; Kvansakul, M. Crystal structures of the sheeppox virus encoded inhibitor of apoptosis SPPV14 bound to the proapoptotic BH3 peptides Hrk and Bax. FEBS Lett. 2020, 594, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Campbell, S.; Mehta, N.; Thibault, J.; Colman, P.M.; Barry, M.; Huang, D.C.S.; Kvansakul, M. Sheeppox Virus SPPV14 Encodes a Bcl-2-Like Cell Death Inhibitor That Counters a Distinct Set of Mammalian Proapoptotic Proteins. J. Virol. 2012, 86, 11501–11511. [Google Scholar] [CrossRef] [PubMed]
- Anasir, M.I.; Caria, S.; Skinner, M.A.; Kvansakul, M. Structural basis of apoptosis inhibition by the fowlpox virus protein FPV039. J. Biol. Chem. 2017, 292, 9010–9021. [Google Scholar] [CrossRef]
- Anasir, M.I.; Baxter, A.A.; Poon, I.K.H.; Hulett, M.D.; Kvansakul, M. Structural and Functional Insight into Canarypox Virus CNP058 Mediated Regulation of Apoptosis. Viruses 2017, 9, 305. [Google Scholar] [CrossRef]
- Banadyga, L.; Lam, S.-C.; Okamoto, T.; Kvansakul, M.; Huang, D.C.S.; Barry, M. Deerpox Virus Encodes an Inhibitor of Apoptosis That Regulates Bak and Bax. J. Virol. 2010, 85, 1922–1934. [Google Scholar] [CrossRef]
- Khan, J.S.; Provencher, J.F.; Forbes, M.R.; Mallory, M.L.; Lebarbenchon, C.; McCoy, K.D. Chapter One—Parasites of Seabirds: A Survey of Effects and Ecological Implications. In Advances in Marine Biology; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 82, pp. 1–50. [Google Scholar]
- Banadyga, L.; Veugelers, K.; Campbell, S.; Barry, M. The Fowlpox Virus BCL-2 Homologue, FPV039, Interacts with Activated Bax and a Discrete Subset of BH3-Only Proteins To Inhibit Apoptosis. J. Virol. 2009, 83, 7085–7098. [Google Scholar] [CrossRef]
- Gyurkovska, V.; Ivanovska, N. Distinct roles of TNF-related apoptosis-inducing ligand (TRAIL) in viral and bacterial infections: From pathogenesis to pathogen clearance. Inflamm. Res. 2016, 65, 427–437. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Luo, X.; Budihardjo, I.; Zou, H.; Slaughter, C.; Wang, X. Bid, a Bcl2 Interacting Protein, Mediates Cytochrome c Release from Mitochondria in Response to Activation of Cell Surface Death Receptors. Cell 1998, 94, 481–490. [Google Scholar] [CrossRef]
- Pickup, D.J.; Ink, B.S.; Hu, W.; Ray, C.A.; Joklik, W.K. Hemorrhage in lesions caused by cowpox virus is induced by a viral protein that is related to plasma protein inhibitors of serine proteases. Proc. Natl. Acad. Sci. USA 1986, 83, 7698–7702. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, D.T.; Kitevska-Ilioski, T.; Pantaki-Eimany, D.; Ji, Y.; Miles, M.A.; Heras, B.; Hawkins, C.J. CrmA orthologs from diverse poxviruses potently inhibit caspases-1 and -8, yet cleavage site mutagenesis frequently produces caspase-1-specific variants. Biochem. J. 2019, 476, 1335–1357. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, T.; Ray, C.A.; Pickup, D.J.; Howard, A.D.; Thornberry, N.A.; Peterson, E.P.; Salvesen, G. Inhibition of interleukin-1 beta converting enzyme by the cowpox virus serpin CrmA. An example of cross-class inhibition. J. Biol. Chem. 1994, 269, 19331–19337. [Google Scholar] [PubMed]
- Quan, L.T.; Caputo, A.; Bleackley, R.C.; Pickup, D.J.; Salvesen, G.S. Granzyme B Is Inhibited by the Cowpox Virus Serpin Cytokine Response Modifier A. J. Biol. Chem. 1995, 270, 10377–10379. [Google Scholar] [CrossRef] [PubMed]
- Ekert, P.G.; Silke, J.; Vaux, D.L. Caspase inhibitors. Cell Death Differ. 1999, 6, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Spriggs, M.K.; Hruby, D.E.; Maliszewski, C.R.; Pickup, D.J.; Sims, J.E.; Buller, R.L.; Vanslyke, J. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell 1992, 71, 145–152. [Google Scholar] [CrossRef]
- Palumbo, G.J.; Buller, R.M.; Glasgow, W.C. Multigenic evasion of inflammation by poxviruses. J. Virol. 1994, 68, 1737–1749. [Google Scholar] [CrossRef]
- Dobbelstein, M.; Shenk, T. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. J. Virol. 1996, 70, 6479–6485. [Google Scholar] [CrossRef]
- Veyer, D.L.; De Motes, C.M.; Sumner, R.P.; Ludwig, L.; Johnson, B.F.; Smith, G.L. Analysis of the anti-apoptotic activity of four vaccinia virus proteins demonstrates that B13 is the most potent inhibitor in isolation and during viral infection. J. Gen. Virol. 2014, 95, 2757–2768. [Google Scholar] [CrossRef]
- Kettle, S.; Blake, N.W.; Law, K.M.; Smith, G.L. Vaccinia virus serpins B13R (SPI-2) and B22R (SPI-1) encode Mr 38.5 and 40K, intracellular polypeptides that do not affect virus virulence in a murine intranasal model. Virology 1995, 206, 136–147. [Google Scholar] [CrossRef]
- Shisler, J.L.; Isaacs, S.N.; Moss, B. Vaccinia Virus Serpin-1 Deletion Mutant Exhibits a Host Range Defect Characterized by Low Levels of Intermediate and Late mRNAs. Virology 1999, 262, 298–311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turner, P.C.; Moyer, R.W. Orthopoxvirus fusion inhibitor glycoprotein SPI-3 (open reading frame K2L) contains motifs characteristic of serine proteinase inhibitors that are not required for control of cell fusion. J. Virol. 1995, 69, 5978–5987. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.; Hota-Mitchell, S.; Chenb, L.; Barrettb, J.; Caob, J.-X.; Macaulayc, C.; Willerd, D.; Evansd, D.; McFadden, G. The Complete DNA Sequence of Myxoma Virus. Virology 1999, 264, 298–318. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.C.; Sancho, M.C.; Thoennes, S.R.; Caputo, A.; Bleackley, R.C.; Moyer, R.W. Myxoma Virus Serp2 Is a Weak Inhibitor of Granzyme B and Interleukin-1β-Converting Enzyme In Vitro and Unlike CrmA Cannot Block Apoptosis in Cowpox Virus-Infected Cells. J. Virol. 1999, 73, 6394–6404. [Google Scholar] [CrossRef] [PubMed]
- Guérin, J.-L.; Gelfi, J.; Camus, C.; Delverdier, M.; Whisstock, J.C.; Amardeihl, M.-F.; Py, R.; Bertagnoli, S.; Messud-Petit, F. Characterization and functional analysis of Serp3: A novel myxoma virus-encoded serpin involved in virulence The GenBank accession number of the sequence reported in this paper is U79714. J. Gen. Virol. 2001, 82, 1407–1417. [Google Scholar] [CrossRef]
- Nathaniel, R.; MacNeill, A.L.; Wang, Y.-X.; Turner, P.C.; Moyer, R.W. Cowpox virus CrmA, Myxoma virus SERP2 and baculovirus P35 are not functionally interchangeable caspase inhibitors in poxvirus infections. J. Gen. Virol. 2004, 85, 1267–1278. [Google Scholar] [CrossRef]
- Seet, B.T.; McFadden, G. Viral chemokine-binding proteins. J. Leukoc. Biol. 2002, 72, 24–34. [Google Scholar]
- Sedger, L.M.; Osvath, S.R.; Xu, X.-M.; Li, G.; Chan, F.K.-M.; Barrett, J.W.; McFadden, G. Poxvirus Tumor Necrosis Factor Receptor (TNFR)-Like T2 Proteins Contain a Conserved Preligand Assembly Domain That Inhibits Cellular TNFR1-Induced Cell Death. J. Virol. 2006, 80, 9300–9309. [Google Scholar] [CrossRef]
- Schreiber, M.; Rajarathnam, K.; McFadden, G. Myxoma Virus T2 Protein, a Tumor Necrosis Factor (TNF) Receptor Homolog, Is Secreted as a Monomer and Dimer That Each Bind Rabbit TNFα, but the Dimer Is a More Potent TNF Inhibitor. J. Biol. Chem. 1996, 271, 13333–13341. [Google Scholar] [CrossRef]
- Saraiva, M.; Smith, P.; Fallon, P.G.; Alcami, A. Inhibition of Type 1 Cytokine–mediated Inflammation by a Soluble CD30 Homologue Encoded by Ectromelia (Mousepox) Virus. J. Exp. Med. 2002, 196, 829–839. [Google Scholar] [CrossRef]
- Panus, J.F.; Smith, C.A.; Ray, C.A.; Smith, T.D.; Patel, D.D.; Pickup, D.J. Cowpox virus encodes a fifth member of the tumor necrosis factor receptor family: A soluble, secreted CD30 homologue. Proc. Natl. Acad. Sci. USA 2002, 99, 8348–8353. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Stein, H.; Gerdes, J.; Lemke, H.; Kirchner, H.; Schaadt, M.; Diehl, V. Production of a monoclonal antibody specific for Hodgkin and Sternberg–Reed cells of Hodgkin’s disease and a subset of normal lymphoid cells. Nat. Cell Biol. 1982, 299, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.C.; Bahar, M.W.; Abrescia, N.G.A.; Smith, G.L.; Stuart, D.I.; Grimes, J.M. Structure of CrmE, a Virus-encoded Tumour Necrosis Factor Receptor. J. Mol. Biol. 2007, 372, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Pontejo, S.M.; Alejo, A.; Alcamí, A. Comparative Biochemical and Functional Analysis of Viral and Human Secreted Tumor Necrosis Factor (TNF) Decoy Receptors. J. Biol. Chem. 2015, 290, 15973–15984. [Google Scholar] [CrossRef]
- Yang, Z.; West, A.P., Jr.; Bjorkman, P.J. Crystal structure of TNFα complexed with a poxvirus MHC-related TNF binding protein. Nat. Struct. Mol. Biol. 2009, 16, 1189–1191. [Google Scholar] [CrossRef]
- Mendez-Rios, J.D.; Yang, Z.; Erlandson, K.J.; Cohen, J.I.; Martens, C.A.; Bruno, D.P.; Porcella, S.F.; Moss, B. Molluscum Contagiosum Virus Transcriptome in Abortively Infected Cultured Cells and a Human Skin Lesion. J. Virol. 2016, 90, 4469–4480. [Google Scholar] [CrossRef]
- Beaury, M.; Velagapudi, U.K.; Weber, S.; Soto, C.; Talele, T.T.; Nichols, D.B. The molluscum contagiosum virus death effector domain containing protein MC160 RxDL motifs are not required for its known viral immune evasion functions. Virus Genes 2017, 53, 522–531. [Google Scholar] [CrossRef]
- Bertin, J.; Armstrong, R.C.; Ottilie, S.; Martin, D.A.; Wang, Y.; Banks, S.; Wang, G.-H.; Senkevich, T.G.; Alnemri, E.S.; Moss, B.; et al. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 1172–1176. [Google Scholar] [CrossRef]
- Biswas, S.; Smith, G.L.; Roy, E.J.; Ward, B.; Shisler, J.L. A comparison of the effect of molluscum contagiosum virus MC159 and MC160 proteins on vaccinia virus virulence in intranasal and intradermal infection routes. J. Gen. Virol. 2018, 99, 246–252. [Google Scholar] [CrossRef]
- Shisler, J.L. Immune Evasion Strategies of Molluscum Contagiosum Virus. Adv. Clin. Chem. 2015, 92, 201–252. [Google Scholar] [CrossRef]
- Murao, L.E.; Shisler, J.L. The MCV MC159 protein inhibits late, but not early, events of TNF-α-induced NF-κB activation. Virology 2005, 340, 255–264. [Google Scholar] [CrossRef]
- Nichols, D.B.; Shisler, J.L. Poxvirus MC160 Protein Utilizes Multiple Mechanisms To Inhibit NF-κB Activation Mediated via Components of the Tumor Necrosis Factor Receptor 1 Signal Transduction Pathway. J. Virol. 2009, 83, 3162–3174. [Google Scholar] [CrossRef] [PubMed]
- Shisler, J.L.; Moss, B. Molluscum Contagiosum Virus Inhibitors of Apoptosis: The MC159 v-FLIP Protein Blocks Fas-Induced Activation of Procaspases and Degradation of the Related MC160 Protein. Virology 2001, 282, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Wang, L.; Zheng, L.; Wan, F.; Ahmed, M.; Lenardo, M.J.; Wu, H. Crystal Structure of MC159 Reveals Molecular Mechanism of DISC Assembly and FLIP Inhibition. Mol. Cell 2005, 20, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-Y.; Jeffrey, P.D.; Yu, J.W.; Shi, Y. Crystal Structure of a Viral FLIP. J. Biol. Chem. 2006, 281, 2960–2968. [Google Scholar] [CrossRef]
- Garvey, T.L.; Bertin, J.; Siegel, R.M.; Wang, G.-H.; Lenardo, M.J.; Cohen, J. Binding of FADD and Caspase-8 to Molluscum Contagiosum Virus MC159 v-FLIP Is Not Sufficient for Its Antiapoptotic Function. J. Virol. 2002, 76, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.M.; Martin, D.A.; Zheng, L.; Ng, S.Y.; Bertin, J.; Cohen, J.; Lenardo, M.J. Death-effector Filaments: Novel Cytoplasmic Structures that Recruit Caspases and Trigger Apoptosis. J. Cell Biol. 1998, 141, 1243–1253. [Google Scholar] [CrossRef]
- Fu, T.-M.; Li, Y.; Lu, A.; Li, Z.; Vajjhala, P.R.; Cruz, A.C.; Srivastava, D.B.; DiMaio, F.; Penczek, P.A.; Siegel, R.M.; et al. Cryo-EM Structure of Caspase-8 Tandem DED Filament Reveals Assembly and Regulation Mechanisms of the Death-Inducing Signaling Complex. Mol. Cell 2016, 64, 236–250. [Google Scholar] [CrossRef]
- Thurau, M.; Everett, H.; Tapernoux, M.; Tschopp, J.; Thome, M. The TRAF3-binding site of human molluscipox virus FLIP molecule MC159 is critical for its capacity to inhibit Fas-induced apoptosis. Cell Death Differ. 2006, 13, 1577–1585. [Google Scholar] [CrossRef]
- Hüttmann, J.; Krause, E.; Schommartz, T.; Brune, W. Functional Comparison of Molluscum Contagiosum Virus vFLIP MC159 with Murine Cytomegalovirus M36/vICA and M45/vIRA Proteins. J. Virol. 2015, 90, 2895–2905. [Google Scholar] [CrossRef]
- Gil, J.; Rullas, J.; Alcamí, J.; Esteban, M. MC159L protein from the poxvirus molluscum contagiosum virus inhibits NF-κB activation and apoptosis induced by PKR. J. Gen. Virol. 2001, 82, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Gubser, C.; Bergamaschi, D.; Hollinshead, M.; Lu, X.; Van Kuppeveld, F.J.M.; Smith, G.L. A New Inhibitor of Apoptosis from Vaccinia Virus and Eukaryotes. PLoS Pathog. 2007, 3, e17. [Google Scholar] [CrossRef] [PubMed]
- Carrara, G.; Saraiva, N.; Gubser, C.; Johnson, B.F.; Smith, G.L. Six-transmembrane Topology for Golgi Anti-apoptotic Protein (GAAP) and Bax Inhibitor 1 (BI-1) Provides Model for the Transmembrane Bax Inhibitor-containing Motif (TMBIM) Family. J. Biol. Chem. 2012, 287, 15896–15905. [Google Scholar] [CrossRef]
- Carrara, G.; Saraiva, N.; Parsons, M.; Byrne, B.; Prole, D.L.; Taylor, C.W.; Smith, G.L. Golgi Anti-apoptotic Proteins Are Highly Conserved Ion Channels That Affect Apoptosis and Cell Migration. J. Biol. Chem. 2015, 290, 11785–11801. [Google Scholar] [CrossRef] [PubMed]
- Mccord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [PubMed]
- Sawhney, N. Some strategic interventions in family welfare programme in India. POPCEN News Lett. Popul. Cent. 1978, 4, 1–6. [Google Scholar]
- Coutu, J.; Ryerson, M.R.; Bugert, J.; Nichols, D.B. The Molluscum Contagiosum Virus protein MC163 localizes to the mitochondria and dampens mitochondrial mediated apoptotic responses. Virology 2017, 505, 91–101. [Google Scholar] [CrossRef]
- Cao, J.X.; Teoh, M.L.; Moon, M.; McFadden, G.; Evans, D.H. Leporipoxvirus Cu-Zn Superoxide Dismutase Homologs Inhibit Cellular Superoxide Dismutase, but Are Not Essential for Virus Replication or Virulence. Virology 2002, 296, 125–135. [Google Scholar] [CrossRef]
- Almazán, F.; Tscharke, D.C.; Smith, G.L. The Vaccinia Virus Superoxide Dismutase-Like Protein (A45R) Is a Virion Component That Is Nonessential for Virus Replication. J. Virol. 2001, 75, 7018–7029. [Google Scholar] [CrossRef]
- Becker, M.N.; Greenleaf, W.B.; Ostrov, D.A.; Moyer, R.W. Amsacta moorei Entomopoxvirus Expresses an Active Superoxide Dismutase. J. Virol. 2004, 78, 10265–10275. [Google Scholar] [CrossRef]
- Teoh, M.L.T.; Turner, P.V.; Evans, D.H. Tumorigenic Poxviruses Up-Regulate Intracellular Superoxide To Inhibit Apoptosis and Promote Cell Proliferation. J. Virol. 2005, 79, 5799–5811. [Google Scholar] [CrossRef] [PubMed]
- Wolferstätter, M.; Schweneker, M.; Späth, M.; Lukassen, S.; Klingenberg, M.; Brinkmann, K.; Wielert, U.; Lauterbach, H.; Hochrein, H.; Chaplin, P.; et al. Recombinant Modified Vaccinia Virus Ankara Generating Excess Early Double-Stranded RNA Transiently Activates Protein Kinase R and Triggers Enhanced Innate Immune Responses. J. Virol. 2014, 88, 14396–14411. [Google Scholar] [CrossRef]
- Ding, S.-W. RNA-based antiviral immunity. Nat. Rev. Immunol. 2010, 10, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Tang, D. PKR-Dependent Inflammatory Signals. Sci. Signal. 2012, 5, pe47. [Google Scholar] [CrossRef] [PubMed]
- Gal-Ben-Ari, S.; Barrera, I.; Ehrlich, M.; Rosenblum, K. PKR: A Kinase to Remember. Front. Mol. Neurosci. 2019, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Watson, J.C.; Jacobs, B.L. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci.USA 1992, 89, 4825–4829. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Muralinath, M.; Brandt, T.; Pearcy, M.; Hauns, K.; Lowenhaupt, K.; Jacobs, B.L.; Rich, A. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 6974–6979. [Google Scholar] [CrossRef]
- Garcia, M.A.; Guerra, S.; Gil, J.; Jiménez, V.; Esteban, M. Anti-apoptotic and oncogenic properties of the dsRNA-binding protein of vaccinia virus, E3L. Oncogene 2002, 21, 8379–8387. [Google Scholar] [CrossRef]
- Myskiw, C.; Arsenio, J.; Hammett, C.; Van Bruggen, R.; Deschambault, Y.; Beausoleil, N.; Babiuk, S.; Cao, J. Comparative Analysis of Poxvirus Orthologues of the Vaccinia Virus E3 Protein: Modulation of Protein Kinase R Activity, Cytokine Responses, and Virus Pathogenicity. J. Virol. 2011, 85, 12280–12291. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liu, J.; Chan, W.M.; Rothenburg, S.; McFadden, G. Myxoma Virus Protein M029 Is a Dual Function Immunomodulator that Inhibits PKR and Also Conscripts RHA/DHX9 to Promote Expanded Host Tropism and Viral Replication. PLoS Pathog. 2013, 9, e1003465. [Google Scholar] [CrossRef]
- Di Pilato, M.; Mejías-Pérez, E.; Sorzano, C.Ó.S.; Esteban, M. Distinct Roles of Vaccinia Virus NF-κB Inhibitor Proteins A52, B15, and K7 in the Immune Response. J. Virol. 2017, 91, e00575-17. [Google Scholar] [CrossRef] [PubMed]
- Reading, P.C.; Khanna, A.; Smith, G.L. Vaccinia Virus CrmE Encodes a Soluble and Cell Surface Tumor Necrosis Factor Receptor That Contributes to Virus Virulence. Virology 2002, 292, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-W.; Katsafanas, G.C.; Liu, R.; Wyatt, L.S.; Moss, B. Poxvirus Decapping Enzymes Enhance Virulence by Preventing the Accumulation of dsRNA and the Induction of Innate Antiviral Responses. Cell Host Microbe 2015, 17, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.A.; Black, R.A.; Kronheim, S.R.; Greenstreet, T.A.; Sleath, P.R.; Salvesen, G.S.; Pickup, D.J. Viral inhibition of inflammation: Cowpox virus encodes an inhibitor of the interleukin-1β converting enzyme. Cell 1992, 69, 597–604. [Google Scholar] [CrossRef]
- Zhou, Q.; Snipas, S.; Orth, K.; Muzio, M.; Dixit, V.M.; Salvesen, G. Target Protease Specificity of the Viral Serpin CrmA. J. Biol. Chem. 1997, 272, 7797–7800. [Google Scholar] [CrossRef]
- Gileva, I.P.; Nepomnyashchikh, T.; Antonets, D.V.; Lebedev, L.R.; Kochneva, G.V.; Grazhdantseva, A.V.; Shchelkunov, S.N. Properties of the recombinant TNF-binding proteins from variola, monkeypox, and cowpox viruses are different. Biochim. Biophys. Acta Proteins Proteom. 2006, 1764, 1710–1718. [Google Scholar] [CrossRef]
- Smith, C.A.; Davis, T.; Anderson, D.; Solam, L.; Beckmann, M.P.; Jerzy, R.; Dower, S.K.; Cosman, D.; Goodwin, R.G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science 1990, 248, 1019. [Google Scholar] [CrossRef]
- Shisler, J.L.; Senkevich, T.G.; Berry, M.J.; Moss, B. Ultraviolet-Induced Cell Death Blocked by a Selenoprotein from a Human Dermatotropic Poxvirus. Science 1998, 279, 102–105. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suraweera, C.D.; Hinds, M.G.; Kvansakul, M. Poxviral Strategies to Overcome Host Cell Apoptosis. Pathogens 2021, 10, 6. https://doi.org/10.3390/pathogens10010006
Suraweera CD, Hinds MG, Kvansakul M. Poxviral Strategies to Overcome Host Cell Apoptosis. Pathogens. 2021; 10(1):6. https://doi.org/10.3390/pathogens10010006
Chicago/Turabian StyleSuraweera, Chathura D., Mark G. Hinds, and Marc Kvansakul. 2021. "Poxviral Strategies to Overcome Host Cell Apoptosis" Pathogens 10, no. 1: 6. https://doi.org/10.3390/pathogens10010006
APA StyleSuraweera, C. D., Hinds, M. G., & Kvansakul, M. (2021). Poxviral Strategies to Overcome Host Cell Apoptosis. Pathogens, 10(1), 6. https://doi.org/10.3390/pathogens10010006






