Triatomine Feeding Profiles and Trypanosoma cruzi Infection, Implications in Domestic and Sylvatic Transmission Cycles in Ecuador
Abstract
1. Introduction
2. Materials and Methods
2.1. Triatomine Selection
2.2. DNA Extraction and Natural Infection with Trypanosomes
2.3. Comparison of Primer Sets for Detection of Blood Meals
2.4. Sensitivity of the Different Primer Sets
2.5. Amplification and Identification of the Sources of Blood Meals
2.6. Analyses of Trophic Networks of Triatomines and Their Blood Meals
3. Results
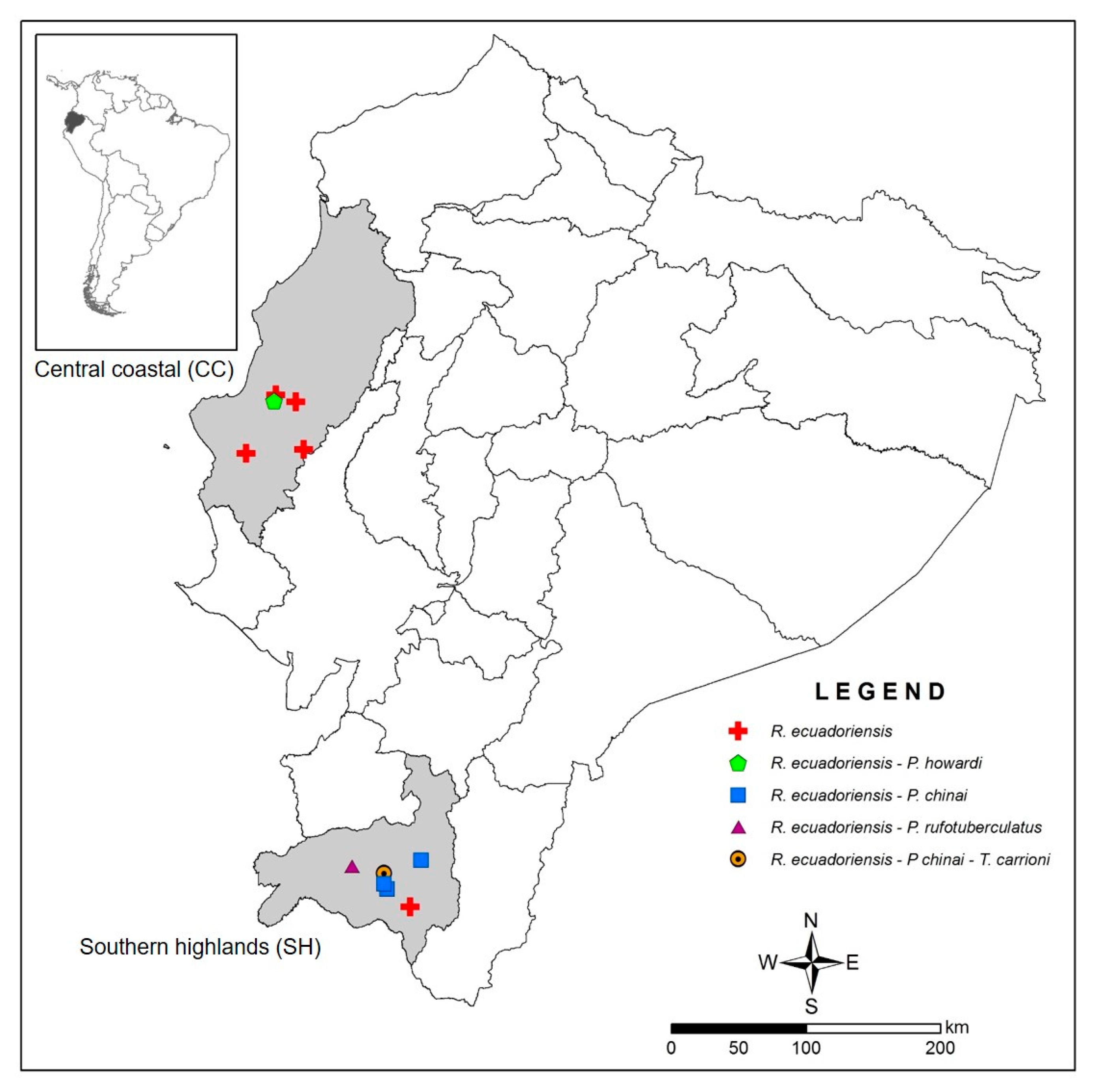
3.1. Vertebrate Diversity as Source of Blood for Triatomines
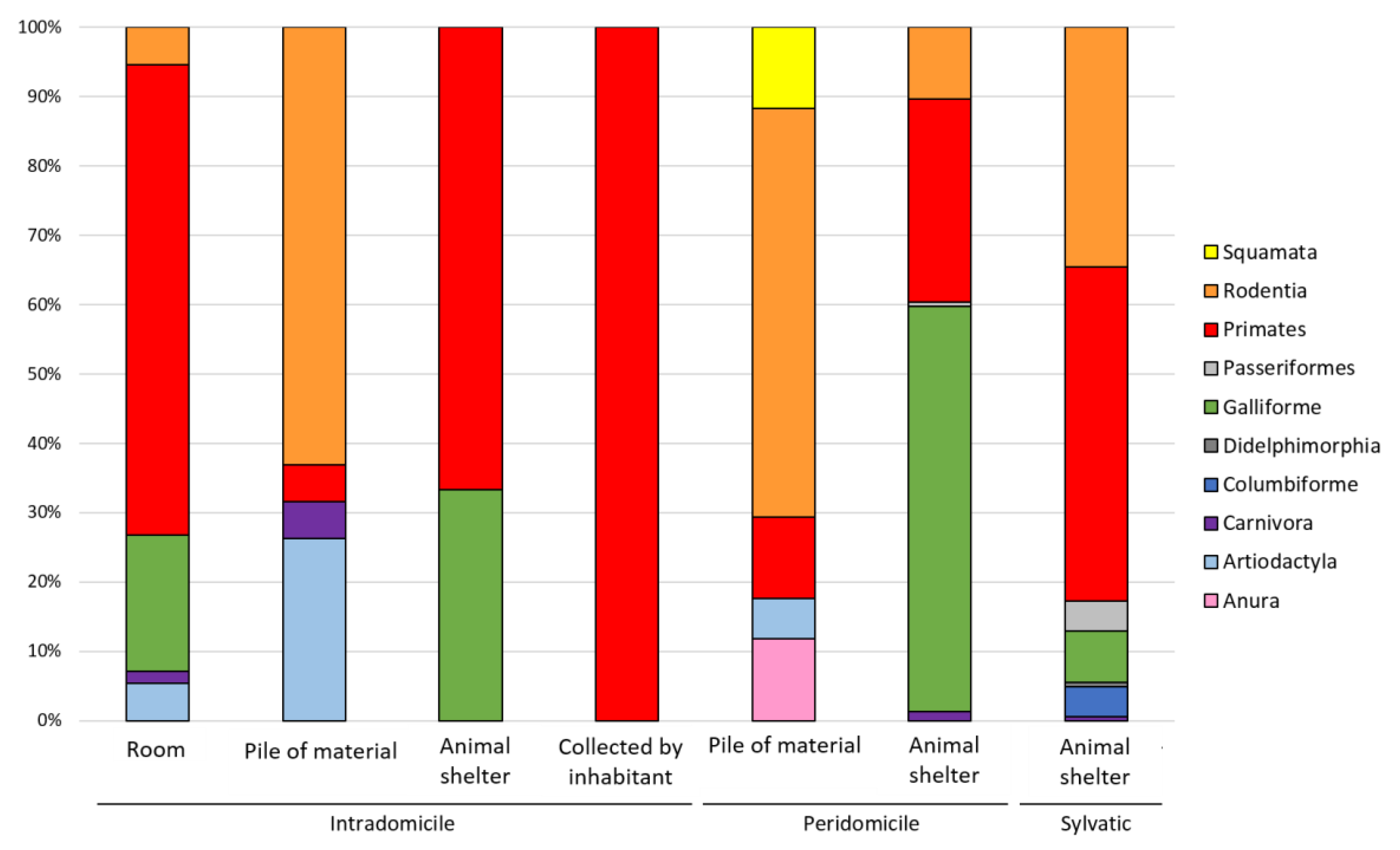
3.2. Vertebrate Blood Meal Source per Geographic Area, Triatomine Species, Developmental Stage and Transmission Cycle
3.3. T. cruzi Infection and Blood Meal Diversity in the Different Transmission Cycles
4. Discussion
4.1. Epidemiological Importance of the Identification of the Vertebrate Sources of Blood for Triatomines
4.2. Diversity of Mammal Hosts and Its Implication in T. cruzi Transmission
4.3. Birds and Their Role in Triatomine Population Maintenance
4.4. Triatomine Species and Blood Meal Preference
4.5. Triatomine Dispersal and Access to Vertebrate Hosts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eastwood, G.; Cunningham, A.A.; Kramer, L.D.; Goodman, S.J. The vector ecology of introduced Culex quinquefasciatus populations, and implications for future risk of West Nile virus emergence in the Galapagos archipelago. Med. Vet. Entomol. 2018, 33, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Goodman, H.; Egizi, A.; Fonseca, D.M.; Leisnham, P.T.; LaDeau, S.L. Primary blood-hosts of mosquitoes are influenced by social and ecological conditions in a complex urban landscape. Parasit. Vectors 2018, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Bennai, K.; Tahir, D.; Lafri, I.; Bendjaballah-Laliam, A.; Bitam, I.; Parola, P. Molecular detection of Leishmania infantum DNA and host blood meal identification in Phlebotomus in a hypoendemic focus of human leishmaniasis in northern Algeria. PLoS Negl. Trop. Dis. 2018, 12, e0006513. [Google Scholar] [CrossRef] [PubMed]
- De Avila, M.M.; Brilhante, A.F.; de Souza, C.F.; Bevilacqua, P.D.; Galati, E.A.B.; Brazil, R.P. Ecology, feeding and natural infection by Leishmania spp. of phlebotomine sand flies in an area of high incidence of American tegumentary leishmaniasis in the municipality of Rio Branco, Acre, Brazil. Parasit. Vectors 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Chepkorir, E.; Venter, M.; Lutomiah, J.; Mulwa, F.; Arum, S.; Tchouassi, D.P.; Sang, R. The occurrence, diversity and blood feeding patterns of potential vectors of dengue and yellow fever in Kacheliba, West Pokot County, Kenya. Acta Trop. 2018, 186, 50–57. [Google Scholar] [CrossRef]
- Dumonteil, E.; Ramirez-Sierra, M.J.; Perez-Carrillo, S.; Teh-Poot, C.; Herrera, C.; Gourbiere, S.; Waleckx, E. Detailed ecological associations of triatomines revealed by metabarcoding and next-generation sequencing: Implications for triatomine behavior and Trypanosoma cruzi transmission cycles. Sci. Rep. 2018, 8, 4140. [Google Scholar] [CrossRef]
- Orantes, L.C.; Monroy, C.; Dorn, P.L.; Stevens, L.; Rizzo, D.M.; Morrissey, L.; Hanley, J.P.; Rodas, A.G.; Richards, B.; Wallin, K.F.; et al. Uncovering vector, parasite, blood meal and microbiome patterns from mixed-DNA specimens of the Chagas disease vector Triatoma dimidiata. PLoS Negl. Trop. Dis. 2018, 12, e0006730. [Google Scholar] [CrossRef]
- Guhl, F. Chagas disease in pre-Colombian civilizations. In American Trypanosomiasis. Chagas Disease. One Hundred Years of Research, 2nd ed.; Telleria, J., Tibayrenc, M., Eds.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Jansen, A.M.; Xavier, S.C.; Roque, A.L. The multiple and complex and changeable scenarios of the Trypanosoma cruzi transmission cycle in the sylvatic environment. Acta Trop. 2015, 151, 1–15. [Google Scholar] [CrossRef]
- Jansen, A.M.; Xavier, S.C.C.; Roque, A.L.R. Ecological aspects on Trypanosoma cruzi: Wild hosts and reservoirs. In American Trypanosomiasis. Chagas Disease. One Hundred Years of Research, 2nd ed.; Telleria, J., Tibayrenc, M., Eds.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Noireau, F.; Diosque, P.; Jansen, A.M. Trypanosoma cruzi: Adaptation to its vectors and its hosts. Vet. Res. 2009, 40, 26. [Google Scholar] [CrossRef]
- Jansen, A.M.; Roque, A.L.R.; Xavier, S.C.C. Trypanosoma cruzi enzootic cycle: General aspects, domestic and synanthropic hosts and reservoirs. In American Trypanosomiasis. Chagas Disease. One Hundred Years of Research, 2nd ed.; Telleria, J., Tibayrenc, M., Eds.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Miles, M.A.; Feliciangeli, M.D.; de Arias, A.R. American trypanosomiasis (Chagas’ disease) and the role of molecular epidemiology in guiding control strategies. BMJ 2003, 326, 1444–1448. [Google Scholar] [CrossRef]
- Walter, A.; Lozano-Kasten, F.; Bosseno, M.F.; Ruvalcaba, E.G.; Gutierrez, M.S.; Luna, C.E.; Baunaure, F.; Phelinas, P.; Magallon-Gastelum, E.; Breniere, S.F. Peridomicilary habitat and risk factors for Triatoma infestation in a rural community of the Mexican occident. Am. J. Trop. Med. Hyg. 2007, 76, 508–515. [Google Scholar] [CrossRef]
- Gottdenker, N.L.; Chaves, L.F.; Calzada, J.E.; Saldana, A.; Carroll, C.R. Host life history strategy, species diversity, and habitat influence Trypanosoma cruzi vector infection in Changing landscapes. PLoS Negl. Trop. Dis. 2012, 6, e1884. [Google Scholar] [CrossRef] [PubMed]
- Grijalva, M.J.; Villacis, A.G.; Moncayo, A.L.; Ocana-Mayorga, S.; Yumiseva, C.A.; Baus, E.G. Distribution of triatomine species in domestic and peridomestic environments in central coastal Ecuador. PLoS Negl. Trop. Dis. 2017, 11, e0005970. [Google Scholar] [CrossRef] [PubMed]
- Grijalva, M.J.; Villacis, A.G.; Ocana-Mayorga, S.; Yumiseva, C.A.; Moncayo, A.L.; Baus, E.G. Comprehensive Survey of Domiciliary Triatomine Species Capable of Transmitting Chagas Disease in Southern Ecuador. PLoS Negl. Trop. Dis. 2015, 9, e0004142. [Google Scholar] [CrossRef] [PubMed]
- Grijalva, M.J.; Teran, D.; Dangles, O. Dynamics of sylvatic Chagas disease vectors in coastal Ecuador is driven by changes in land cover. PLoS Negl. Trop. Dis. 2014, 8, e2960. [Google Scholar] [CrossRef] [PubMed]
- Grijalva, M.J.; Villacis, A.G. Presence of Rhodnius ecuadoriensis in sylvatic habitats in the southern highlands (Loja Province) of Ecuador. J. Med. Entomol. 2009, 46, 708–711. [Google Scholar] [CrossRef]
- Ocana-Mayorga, S.; Aguirre-Villacis, F.; Pinto, C.M.; Vallejo, G.A.; Grijalva, M.J. Prevalence, Genetic Characterization, and 18S Small Subunit Ribosomal RNA Diversity of Trypanosoma rangeli in Triatomine and Mammal Hosts in Endemic Areas for Chagas Disease in Ecuador. Vector Borne Zoonotic Dis. 2015, 15, 732–742. [Google Scholar] [CrossRef]
- Pinto, C.M.; Ocana-Mayorga, S.; Lascano, M.S.; Grijalva, M.J. Infection by trypanosomes in marsupials and rodents associated with human dwellings in Ecuador. J. Parasitol. 2006, 92, 1251–1255. [Google Scholar] [CrossRef]
- Pinto, C.M.; Ocana-Mayorga, S.; Tapia, E.E.; Lobos, S.E.; Zurita, A.P.; Aguirre-Villacis, F.; MacDonald, A.; Villacis, A.G.; Lima, L.; Teixeira, M.M.; et al. Bats, Trypanosomes, and Triatomines in Ecuador: New Insights into the Diversity, Transmission, and Origins of Trypanosoma cruzi and Chagas Disease. PLoS ONE 2015, 10, e0139999. [Google Scholar] [CrossRef]
- Vallejo, G.A.; Guhl, F.; Chiari, E.; Macedo, A.M. Species specific detection of Trypanosoma cruzi and Trypanosoma rangeli in vector and mammalian hosts by polymerase chain reaction amplification of kinetoplast minicircle DNA. Acta Trop. 1999, 72, 203–212. [Google Scholar] [CrossRef]
- Virreira, M.; Alonso-Vega, C.; Solano, M.; Jijena, J.; Brutus, L.; Bustamante, Z.; Truyens, C.; Schneider, D.; Torrico, F.; Carlier, Y.; et al. Congenital Chagas disease in Bolivia is not associated with DNA polymorphism of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2006, 75, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Buitrago, R.; Depickere, S.; Bosseno, M.F.; Patzi, E.S.; Waleckx, E.; Salas, R.; Aliaga, C.; Breniere, S.F. Combination of cytochrome b heteroduplex-assay and sequencing for identification of triatomine blood meals. Infect. Genet. Evol. 2012, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Paabo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef] [PubMed]
- Dormann, C.F.; Gruber, B.; Fründ, J. Introducing the bipartite package: Analysing ecological networks. R News 2008, 8, 8–11. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 14 August 2020).
- Ocana-Mayorga, S.; Llewellyn, M.S.; Costales, J.A.; Miles, M.A.; Grijalva, M.J. Sex, subdivision, and domestic dispersal of Trypanosoma cruzi lineage I in southern Ecuador. PLoS Negl. Trop. Dis. 2010, 4, e915. [Google Scholar] [CrossRef]
- Costales, J.A.; Jara-Palacios, M.A.; Llewellyn, M.S.; Messenger, L.A.; Ocana-Mayorga, S.; Villacis, A.G.; Tibayrenc, M.; Grijalva, M.J. Trypanosoma cruzi population dynamics in the Central Ecuadorian Coast. Acta Trop. 2015, 151, 88–93. [Google Scholar] [CrossRef]
- Grijalva, M.J.; Villacis, A.G.; Ocana-Mayorga, S.; Yumiseva, C.A.; Baus, E.G. Limitations of selective deltamethrin application for triatomine control in central coastal Ecuador. Parasit. Vectors 2011, 4, 20. [Google Scholar] [CrossRef]
- Christensen, H.A.; de Vasquez, A.M. Host feeding profiles of Rhodnius pallescens (Hemiptera: Reduviidae) in rural villages of Central Panama. Am. J. Trop. Med. Hyg. 1981, 30, 278–283. [Google Scholar] [CrossRef]
- Cecere, M.C.; Leporace, M.; Fernandez, M.P.; Zarate, J.E.; Moreno, C.; Gurtler, R.E.; Cardinal, M.V. Host-Feeding Sources and Infection With Trypanosoma cruzi of Triatoma infestans and Triatoma eratyrusiformis (Hemiptera: Reduviidae) From the Calchaqui Valleys in Northwestern Argentina. J. Med. Entomol. 2016, 53, 666–673. [Google Scholar] [CrossRef]
- Carrasco, H.J.; Torrellas, A.; Garcia, C.; Segovia, M.; Feliciangeli, M.D. Risk of Trypanosoma cruzi I (Kinetoplastida: Trypanosomatidae) transmission by Panstrongylus geniculatus (Hemiptera: Reduviidae) in Caracas (Metropolitan District) and neighboring States, Venezuela. Int. J. Parasitol. 2005, 35, 1379–1384. [Google Scholar] [CrossRef]
- Cantillo-Barraza, O.; Garces, E.; Gomez-Palacio, A.; Cortes, L.A.; Pereira, A.; Marcet, P.L.; Jansen, A.M.; Triana-Chavez, O. Eco-epidemiological study of an endemic Chagas disease region in northern Colombia reveals the importance of Triatoma maculata (Hemiptera: Reduviidae), dogs and Didelphis marsupialis in Trypanosoma cruzi maintenance. Parasit. Vectors 2015, 8, 482. [Google Scholar] [CrossRef]
- Buitrago, R.; Bosseno, M.F.; Depickere, S.; Waleckx, E.; Salas, R.; Aliaga, C.; Barnabe, C.; Breniere, S.F. Blood meal sources of wild and domestic Triatoma infestans (Hemiptera: Reduviidae) in Bolivia: Connectivity between cycles of transmission of Trypanosoma cruzi. Parasit. Vectors 2016, 9, 214. [Google Scholar] [CrossRef]
- Waleckx, E.; Suarez, J.; Richards, B.; Dorn, P.L. Triatoma sanguisuga blood meals and potential for Chagas disease, Louisiana, USA. Emerg. Infect. Dis. 2014, 20, 2141–2143. [Google Scholar] [CrossRef]
- Buitrago, N.L.; Bosseno, M.F.; Waleckx, E.; Bremond, P.; Vidaurre, P.; Zoveda, F.; Breniere, S.F. Risk of transmission of Trypanosoma cruzi by wild Triatoma infestans (Hemiptera: Reduviidae) in Bolivia supported by the detection of human blood meals. Infect. Genet. Evol. 2013, 19, 141–144. [Google Scholar] [CrossRef]
- Chacon, F.; Bacigalupo, A.; Quiroga, J.F.; Ferreira, A.; Cattan, P.E.; Ramirez-Toloza, G. Feeding profile of Mepraia spinolai, a sylvatic vector of Chagas disease in Chile. Acta Trop. 2016, 162, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.E.; Faucher, L.; Lavina, M.; Costa, J.; Harry, M. Molecular Individual-Based Approach on Triatoma brasiliensis: Inferences on Triatomine Foci, Trypanosoma cruzi Natural Infection Prevalence, Parasite Diversity and Feeding Sources. PLoS Negl. Trop. Dis. 2016, 10, e0004447. [Google Scholar] [CrossRef]
- Bosseno, M.F.; Barnabe, C.; Sierra, M.J.; Kengne, P.; Guerrero, S.; Lozano, F.; Ezequiel, K.; Gastelum, M.; Breniere, S.F. Wild ecotopes and food habits of Triatoma longipennis infected by Trypanosoma cruzi lineages I and II in Mexico. Am. J. Trop. Med. Hyg. 2009, 80, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Davalos, V.; Dangles, O.; Villacis, A.G.; Grijalva, M.J. Microdistribution of sylvatic triatomine populations in central-coastal Ecuador. J. Med. Entomol. 2010, 47, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Ocana-Mayorga, S.; Lobos, S.E.; Crespo-Perez, V.; Villacis, A.G.; Pinto, C.M.; Grijalva, M.J. Influence of ecological factors on the presence of a triatomine species associated with the arboreal habitat of a host of Trypanosoma cruzi. Parasit. Vectors 2018, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, D.M.; De Urioste-Stone, S.M.; Juarez, J.G.; Pennington, P.M. Ecological, social and biological risk factors for continued Trypanosoma cruzi transmission by Triatoma dimidiata in Guatemala. PLoS ONE 2014, 9, e104599. [Google Scholar] [CrossRef] [PubMed]
- Breniere, S.F.; Pietrokovsky, S.; Gastelum, E.M.; Bosseno, M.F.; Soto, M.M.; Ouaissi, A.; Kasten, F.L.; Wisnivesky-Colli, C. Feeding patterns of Triatoma longipennis Usinger (Hemiptera, Reduviidae) in peridomestic habitats of a rural community in Jalisco State, Mexico. J. Med. Entomol. 2004, 41, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Bosseno, M.F.; Garcia, L.S.; Baunaure, F.; Gastelum, E.M.; Gutierrez, M.S.; Kasten, F.L.; Dumonteil, E.; Breniere, S.F. Identification in triatomine vectors of feeding sources and Trypanosoma cruzi variants by heteroduplex assay and a multiplex miniexon polymerase chain reaction. Am. J. Trop. Med. Hyg. 2006, 74, 303–305. [Google Scholar] [CrossRef]
- Travi, B.L.; Jaramillo, C.; Montoya, J.; Segura, I.; Zea, A.; Goncalves, A.; Velez, I.D. Didelphis marsupialis, an important reservoir of Trypanosoma (Schizotrypanum) cruzi and Leishmania (Leishmania) chagasi in Colombia. Am. J. Trop. Med. Hyg. 1994, 50, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Acosta, N.; Llewellyn, M.; Sanchez, H.; Adamson, S.; Miles, G.A.; Lopez, E.; Gonzalez, N.; Patterson, J.S.; Gaunt, M.W.; et al. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int. J. Parasitol. 2005, 35, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.L.; Roque, A.L.; Arrais, R.C.; Santos, J.P.; Lima Vdos, S.; Xavier, S.C.; Cordeir-Estrela, P.; D’Andrea, P.S.; Jansen, A.M. Trypanosoma cruzi TcI and TcII transmission among wild carnivores, small mammals and dogs in a conservation unit and surrounding areas, Brazil. Parasitology 2013, 140, 160–170. [Google Scholar] [CrossRef]
- Curtis-Robles, R.; Snowden, K.F.; Dominguez, B.; Dinges, L.; Rodgers, S.; Mays, G.; Hamer, S.A. Epidemiology and Molecular Typing of Trypanosoma cruzi in Naturally-Infected Hound Dogs and Associated Triatomine Vectors in Texas, USA. PLoS Negl. Trop. Dis. 2017, 11, e0005298. [Google Scholar] [CrossRef]
- Freitas, Y.B.N.; Souza, C.; Magalhaes, J.M.E.; Sousa, M.L.R.; d’Escoffier, L.N.; Valle, T.Z.D.; Goncalves, T.C.M.; Gil-Santana, H.R.; Kazimoto, T.A.; Amora, S.S.A. Natural infection by Trypanosoma cruzi in triatomines and seropositivity for Chagas disease of dogs in rural areas of Rio Grande do Norte, Brazil. Rev. Soc. Bras. Med. Trop. 2018, 51, 190–197. [Google Scholar] [CrossRef]
- Elmayan, A.; Tu, W.; Duhon, B.; Marx, P.; Wolfson, W.; Balsamo, G.; Herrera, C.; Dumonteil, E. High prevalence of Trypanosoma cruzi infection in shelter dogs from southern Louisiana, USA. Parasit. Vectors 2019, 12, 322. [Google Scholar] [CrossRef]
- Porfirio, G.E.O.; Santos, F.M.; de Macedo, G.C.; Barreto, W.T.G.; Campos, J.B.V.; Meyers, A.C.; Andre, M.R.; Perles, L.; de Oliveira, C.E.; Xavier, S.; et al. Maintenance of Trypanosoma cruzi, T. evansi and Leishmania spp. by domestic dogs and wild mammals in a rural settlement in Brazil-Bolivian border. Int. J. Parasitol. Parasites Wildl. 2018, 7, 398–404. [Google Scholar] [CrossRef]
- Gurtler, R.E.; Cecere, M.C.; Rubel, D.N.; Petersen, R.M.; Schweigmann, N.J.; Lauricella, M.A.; Bujas, M.A.; Segura, E.L.; Wisnivesky-Colli, C. Chagas disease in north-west Argentina: Infected dogs as a risk factor for the domestic transmission of Trypanosoma cruzi. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 741–745. [Google Scholar] [CrossRef]
- Saldana, A.; Calzada, J.E.; Pineda, V.; Perea, M.; Rigg, C.; Gonzalez, K.; Santamaria, A.M.; Gottdenker, N.L.; Chaves, L.F. Risk factors associated with Trypanosoma cruzi exposure in domestic dogs from a rural community in Panama. Mem. Inst. Oswaldo Cruz 2015, 110, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Arce-Fonseca, M.; Carrillo-Sanchez, S.C.; Molina-Barrios, R.M.; Martinez-Cruz, M.; Cedillo-Cobian, J.R.; Henao-Diaz, Y.A.; Rodriguez-Morales, O. Seropositivity for Trypanosoma cruzi in domestic dogs from Sonora, Mexico. Infect. Dis. Poverty 2017, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Chacon, J.C.; Gutierrez-Cabrera, A.E.; Sanchez-Cordero, V.; Wirtz, R.A.; Ordonez, R.; Panzera, F.; Ramsey, J.M. Identification of blood meal source and infection with Trypanosoma cruzi of Chagas disease vectors using a multiplex cytochrome b polymerase chain reaction assay. Vector Borne Zoonotic Dis. 2007, 7, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Gurtler, R.E.; Ceballos, L.A.; Ordonez-Krasnowski, P.; Lanati, L.A.; Stariolo, R.; Kitron, U. Strong host-feeding preferences of the vector Triatoma infestans modified by vector density: Implications for the epidemiology of Chagas disease. PLoS Negl. Trop. Dis. 2009, 3, e447. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, E.; Herrera, C.; Martini, L.; Grijalva, M.J.; Guevara, A.G.; Costales, J.A.; Aguilar, H.M.; Breniere, S.F.; Waleckx, E. Chagas Disease Has Not Been Controlled in Ecuador. PLoS ONE 2016, 11, e0158145. [Google Scholar] [CrossRef]
- Patterson, N.M.; Bates, B.R.; Chadwick, A.E.; Nieto-Sanchez, C.; Grijalva, M.J. Using the health belief model to identify communication opportunities to prevent Chagas disease in Southern Ecuador. PLoS Negl. Trop. Dis. 2018, 12, e0006841. [Google Scholar] [CrossRef]
- Gorchakov, R.; Trosclair, L.P.; Wozniak, E.J.; Feria, P.T.; Garcia, M.N.; Gunter, S.M.; Murray, K.O. Trypanosoma cruzi Infection Prevalence and Bloodmeal Analysis in Triatomine Vectors of Chagas Disease From Rural Peridomestic Locations in Texas, 2013–2014. J. Med. Entomol. 2016, 53, 911–918. [Google Scholar] [CrossRef]
- Lehane, M.J.; Schofield, C.J. Field experiments of dispersive flight by Triatoma infestans. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 399–400. [Google Scholar] [CrossRef]
- Schweigmann, N.; Vallve, S.; Muscio, O.; Ghillini, M.; Alberti, A.; Wisnivesky-Colli, C. Dispersal flight by Triatoma infestans in an arid area of Argentina. Med. Vet. Entomol. 1988, 2, 401–404. [Google Scholar] [CrossRef]
- Dumonteil, E.; Tripet, F.; Ramirez-Sierra, M.J.; Payet, V.; Lanzaro, G.; Menu, F. Assessment of Triatoma dimidiata dispersal in the Yucatan Peninsula of Mexico by morphometry and microsatellite markers. Am. J. Trop. Med. Hyg. 2007, 76, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Bremond, P.; Salas, R.; Waleckx, E.; Buitrago, R.; Aliaga, C.; Barnabe, C.; Depickere, S.; Dangles, O.; Breniere, S.F. Variations in time and space of an Andean wild population of T. infestans at a microgeographic scale. Parasit. Vectors 2014, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Schaub, G.A.; Boker, C.A.; Jensen, C.; Reduth, D. Cannibalism and coprophagy are modes of transmission of Blastocrithidia triatomae (Trypanosomatidae) between triatomines. J. Protozool. 1989, 36, 171–175. [Google Scholar] [CrossRef]
- Torres, M.; Cardenas, E.; Perez, S.; Morales, A. Haematophagy and cleptohaematophagy of Clerada apicicornis (Hemiptera: Lygaeidae), a potential biological control agent of Rhodnius prolixus (Hemiptera: Reduviidae). Mem. Inst. Oswaldo Cruz 2000, 95, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, C.M.; Joya, M.I.; Gutierez, R.; Angulo, V.M. Cleptohaematophagy of the Triatomine bug Belminus herreri. Med. Vet. Entomol. 2000, 14, 100–101. [Google Scholar] [CrossRef] [PubMed]
- Pojo De Rego, I.; Walter, A.; Ferreira, A.J.; Rangel, M.; Girard-Ferreira, E.; Noireau, F. Peridomestic structure, farming activity and triatomine infestation. Parasite 2006, 13, 237–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guhl, F.; Pinto, N.; Aguilera, G. Sylvatic triatominae: A new challenge in vector control transmission. Mem. Inst. Oswaldo Cruz 2009, 104 (Suppl. 1), 71–75. [Google Scholar] [CrossRef]
- Diotaiuti, L. Triatomine-Vector of Trypanosoma cruzi Infection. In Emerging Chagas Disease; Teixeira, A., Vinaud, M., Castro, A.M., Eds.; Bentham Science: Sharjah, UAE, 2009. [Google Scholar] [CrossRef]
- Rabinovich, J.E.; Kitron, U.D.; Obed, Y.; Yoshioka, M.; Gottdenker, N.; Chaves, L.F. Ecological patterns of blood-feeding by kissing-bugs (Hemiptera: Reduviidae: Triatominae). Mem. Inst. Oswaldo Cruz 2011, 106, 479–494. [Google Scholar] [CrossRef]
- Gurtler, R.E.; Cecere, M.C.; Vazquez-Prokopec, G.M.; Ceballos, L.A.; Gurevitz, J.M.; Fernandez Mdel, P.; Kitron, U.; Cohen, J.E. Domestic animal hosts strongly influence human-feeding rates of the Chagas disease vector Triatoma infestans in Argentina. PLoS Negl. Trop. Dis. 2014, 8, e2894. [Google Scholar] [CrossRef]


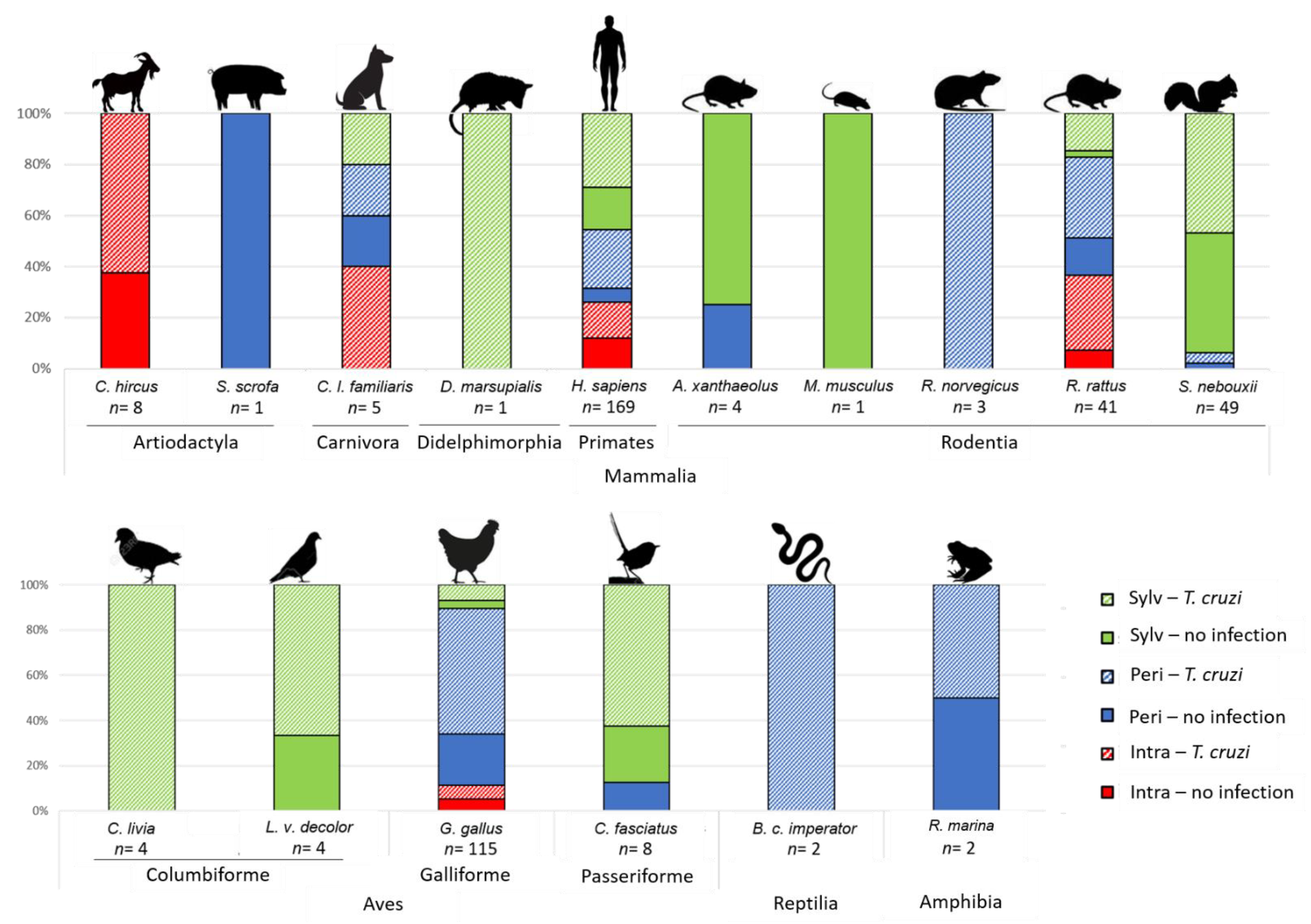
| Vertebrate Blood Source | I n (Tc %) | P n (Tc %) | S n (Tc %) | Total n (Tc %) | |
|---|---|---|---|---|---|
| Species | Common Name | ||||
| Mammalia | |||||
| Artiodactyla | |||||
| Capra hircus | Goat | 8 (62.5) | - | - | 8 (62.5) |
| Sus scrofa | Pig | - | 1 (0.0) | - | 1 (0.0) |
| Carnivora | |||||
| Canis lupus familiaris | Dog | 2 (100.0) | 2 (50) | 1 (100.0) | 5 (80.0) |
| Didelphimorphia | |||||
| Didelphis marsupialis | Opossum | - | - | 1 (100.0) | 1 (100.0) |
| Primate | |||||
| Homo sapiens | Human | 45 (53.3) | 47 (83.0) | 78 (62.8) | 170 (65.9) |
| Rodentia | |||||
| Aegialomys xanthaeolus | Yellowish rice rat | - | 1 (0.0) | 3 (0.0) | 4 (0.0) |
| Mus musculus | Mouse | - | - | 1 (0.0) | 1 (0.0) |
| Rattus norvegicus | Brown rat | - | 3 (100.0) | - | 3 (100.0) |
| Rattus rattus | Black rat | 15 (80.0) | 19 (68.4) | 7 (85.7) | 41 (75.6) |
| Simosciurus nebouxii | Guayaquil squirrel | - | 3 (66.7) | 45 (51.1) | 48 (52.1) |
| Total mammalia | 70 (61.4) | 76 (76.3) | 136 (58.8) | 282 (64.2) | |
| Aves | |||||
| Columbiforme | |||||
| Columba livia | Pigeon | - | - | 4 (100.0) | 4 (100.0) |
| Leptotila verreauxi decolor | White-tipped dove | - | - | 3 (66.7) | 3 (66.77) |
| Galliforme | |||||
| Gallus gallus | Chicken | 13 (53.8) | 90 (71.1) | 12 (66.7) | 115 (68.7) |
| Passeriforme | |||||
| Campylorhyncus fasciatus | Fasciated wren | - | 1 (0.0) | 7 (71.4) | 8 (62.5) |
| Total aves | 13 (53.8) | 91 (70.3) | 26 (73.1) | 130 (69.2) | |
| Reptilia | |||||
| Squamata | |||||
| Boa constrictor imperator | Boa | - | 2 (100.0) | - | 2 (100.0) |
| Total reptilian | - | 2 (100.0) | - | 2 (100.0) | |
| Amphibia | |||||
| Anura | |||||
| Rhinella marina | Cane toad | - | 2 (50.0) | - | 2 (50.0) |
| Total amphibian | - | 2 (50.0) | - | 2 (50.0) | |
| Triatomines | |||||
| TOTAL | 83 (60.2) | 171 (73.1) | 162 (61.1) | 416 (65.9) | |
| Adults | 32 (53.1) | 88 (71.6) | 68 (69.1) | 188 (67.6) | |
| Nymphs | 51 (64.7) | 83 (74.7) | 94 (55.3) | 228 (64.5) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocaña-Mayorga, S.; Bustillos, J.J.; Villacís, A.G.; Pinto, C.M.; Brenière, S.F.; Grijalva, M.J. Triatomine Feeding Profiles and Trypanosoma cruzi Infection, Implications in Domestic and Sylvatic Transmission Cycles in Ecuador. Pathogens 2021, 10, 42. https://doi.org/10.3390/pathogens10010042
Ocaña-Mayorga S, Bustillos JJ, Villacís AG, Pinto CM, Brenière SF, Grijalva MJ. Triatomine Feeding Profiles and Trypanosoma cruzi Infection, Implications in Domestic and Sylvatic Transmission Cycles in Ecuador. Pathogens. 2021; 10(1):42. https://doi.org/10.3390/pathogens10010042
Chicago/Turabian StyleOcaña-Mayorga, Sofía, Juan José Bustillos, Anita G. Villacís, C. Miguel Pinto, Simone Frédérique Brenière, and Mario J. Grijalva. 2021. "Triatomine Feeding Profiles and Trypanosoma cruzi Infection, Implications in Domestic and Sylvatic Transmission Cycles in Ecuador" Pathogens 10, no. 1: 42. https://doi.org/10.3390/pathogens10010042
APA StyleOcaña-Mayorga, S., Bustillos, J. J., Villacís, A. G., Pinto, C. M., Brenière, S. F., & Grijalva, M. J. (2021). Triatomine Feeding Profiles and Trypanosoma cruzi Infection, Implications in Domestic and Sylvatic Transmission Cycles in Ecuador. Pathogens, 10(1), 42. https://doi.org/10.3390/pathogens10010042






