ERG3-Encoding Sterol C5,6-DESATURASE in Candida albicans Is Required for Virulence in an Enterically Infected Invasive Candidiasis Mouse Model
Abstract
1. Introduction
2. Results
2.1. Loss of ERG3 in C. albicans Results in Attenuated Virulence in an Enterically Infected Invasive Candidiasis Mouse Model
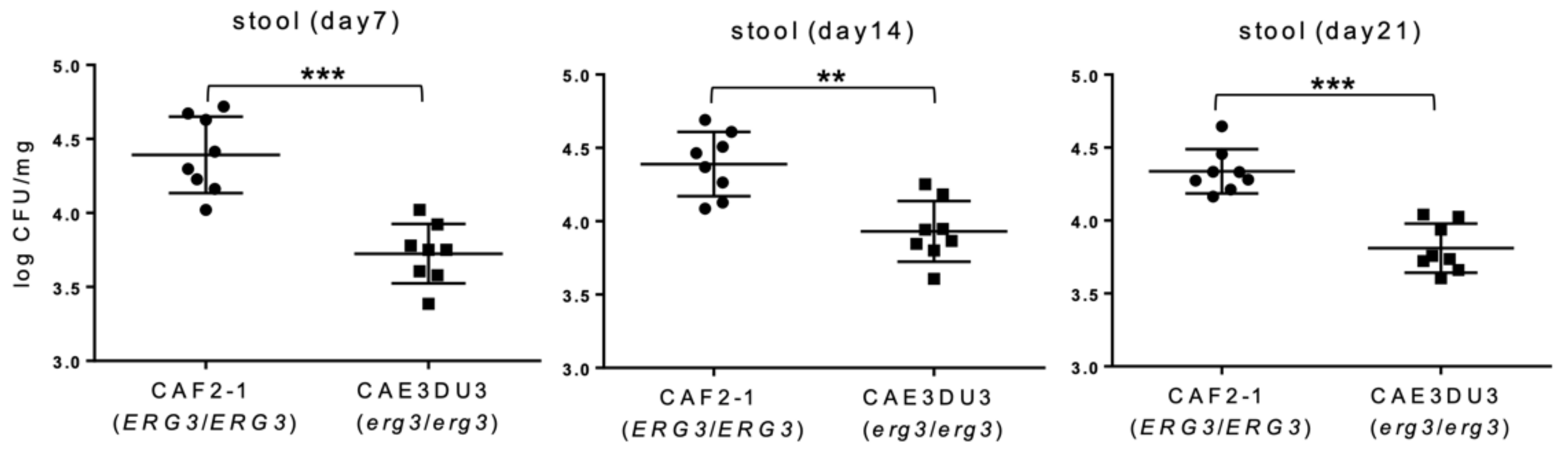
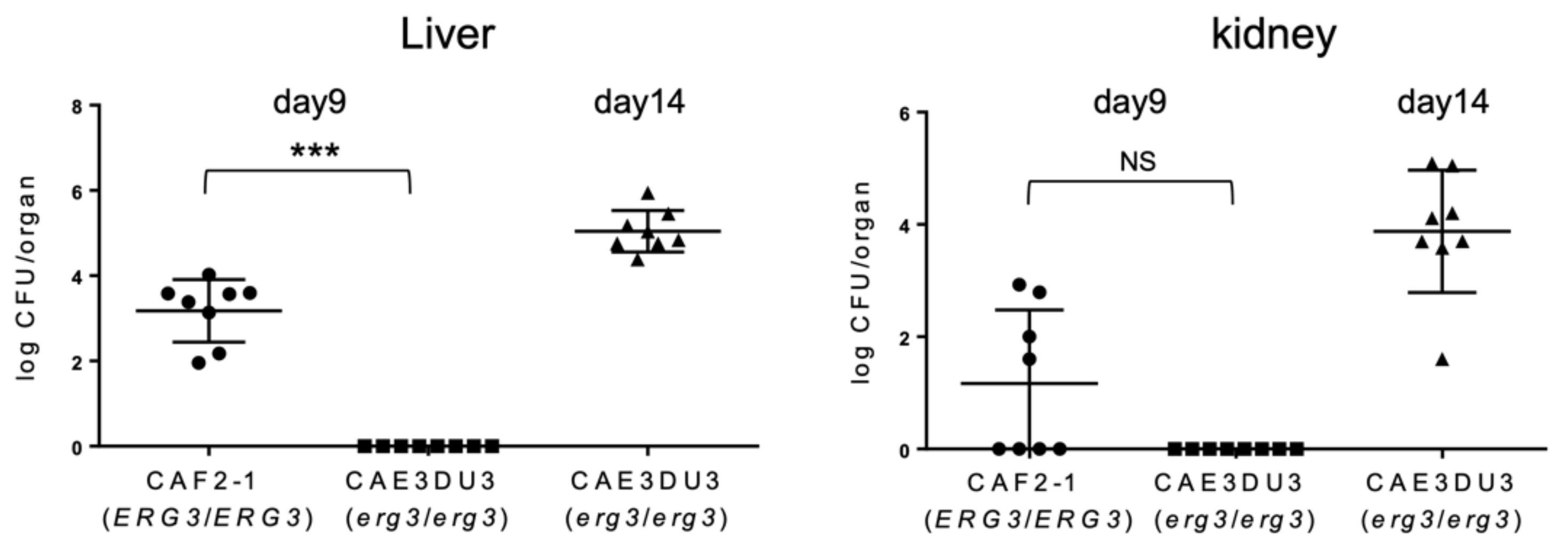
2.2. C. albicans erg3 Null Mutant Has a Lower Colonization and Dissemination Capacity in the Gut
2.3. Chemokines Levels in the Gut and Blood of Mice Infected with the erg3 Null Mutant Were Significantly Lower Than Those in Mice Infected with the Wild-Type Strain
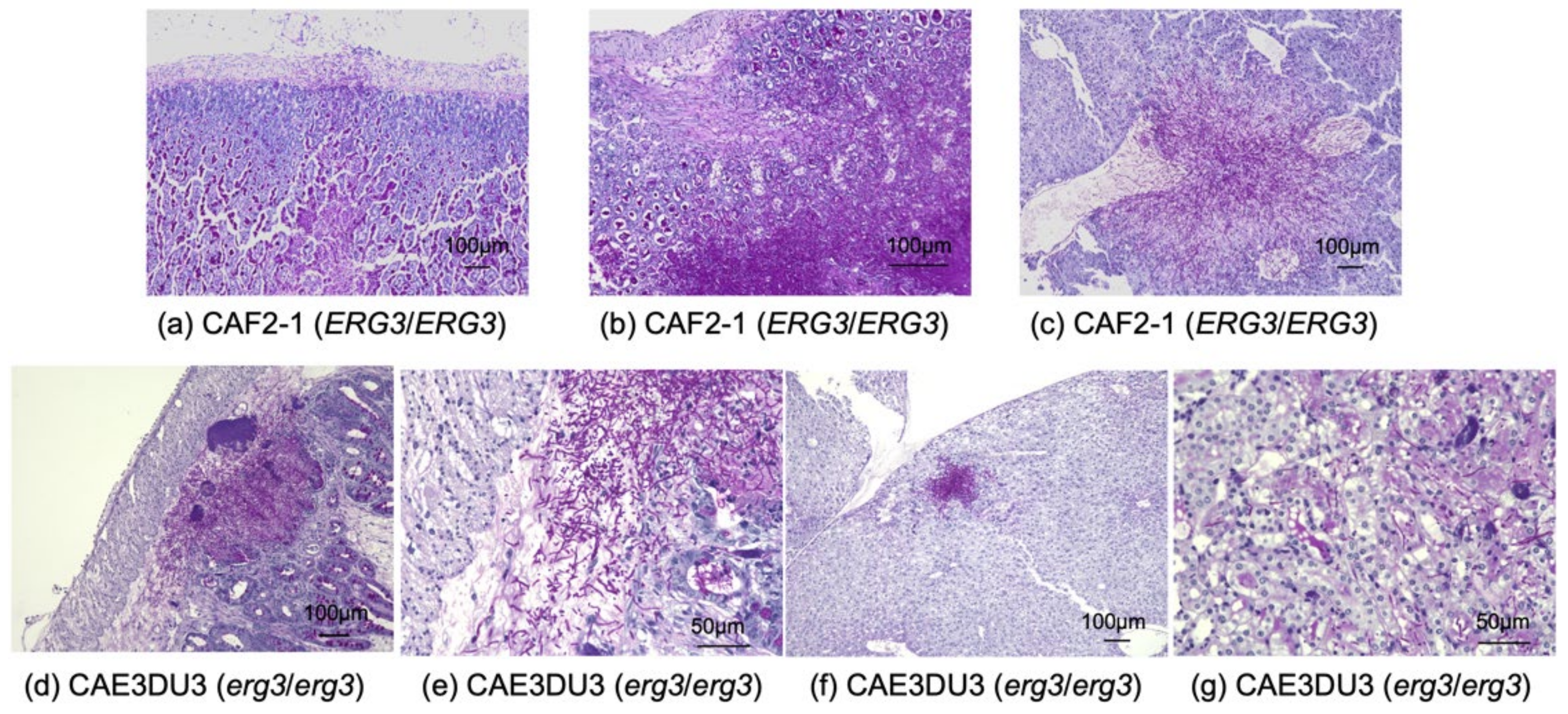
2.4. Histopathological Examination
3. Discussion
4. Materials and Methods
4.1. Candida Strains and Culture Conditions
4.2. Animals and Ethics Statement
4.3. Murine Model of Candidiasis from the Gut
4.4. Isolation of Candida Cells from the Blood, Liver, Kidney, and Stool
4.5. Chemokine Detection Assay
4.6. Histopathological Examination
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from a Prospective Nationwide Surveillance Study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.; Neofytos, D.; Diekema, D.; Azie, N.; Meier-Kriesche, H.-U.; Quan, S.-P.; Horn, D. Epidemiology and outcomes of candidemia in 3648 patients: Data from the Prospective Antifungal Therapy (PATH Alliance(R)) registry, 2004–2008. Diagn. Microbiol. Infect Dis. 2012, 74, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Hallen-Adams, H.E.; Suhr, M.J. Fungi in the healthy human gastrointestinal tract. Virulence 2017, 8, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Anaissie, E. Revisiting the Source of Candidemia: Skin or Gut? Clin. Infect. Dis. 2001, 33, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Kelly, S.L.; Lamb, D.C.; Corran, A.J.; Baldwin, B.C.; Kelly, D.E. Mode of action and resistance to azole antifungals associated with the formation of 14 al-pha-methylergosta-8,24(28)-dien-3 beta,6 alpha-diol. Biochem. Biophys. Res. Commun. 1995, 207, 910–915. [Google Scholar] [CrossRef]
- Sanglard, D.; Ischer, F.; Parkinson, T.; Falconer, D.; Bille, J. Candida albicans Mutations in the Ergosterol Biosynthetic Pathway and Resistance to Several Antifungal Agents. Antimicrob. Agents Chemother. 2003, 47, 2404–2412. [Google Scholar] [CrossRef]
- Chau, A.S.; Gurnani, M.; Hawkinson, R.; Laverdiere, M.; Cacciapuoti, A.; McNicholas, P.M. Inactivation of Sterol Δ5,6-Desaturase Attenuates Virulence in Candida albicans. Antimicrob. Agents Chemother. 2005, 49, 3646–3651. [Google Scholar] [CrossRef]
- Miyazaki, T.; Miyazaki, Y.; Izumikawa, K.; Kakeya, H.; Miyakoshi, S.; Bennett, J.E.; Kohno, S. Fluconazole Treatment Is Effective against a Candida albicans erg3/erg3 Mutant In Vivo Despite In Vitro Resistance. Antimicrob. Agents Chemother. 2006, 50, 580–586. [Google Scholar] [CrossRef]
- Vale-Silva, L.A.; Coste, A.T.; Ischer, F.; Parker, J.E.; Kelly, S.L.; Pinto, E.; Sanglard, D. Azole resistance by loss of function of the sterol Delta(5),(6)-desaturase gene (ERG3) in Candida albicans does not necessarily decrease virulence. Antimicrob. Agents Chemother. 2012, 56, 1960–1968. [Google Scholar] [CrossRef]
- Luna-Tapia, A.; Peters, B.M.; Eberle, K.E.; Kerns, M.E.; Foster, T.P.; Marrero, L.; Noverr, M.C.; Fidel, P.L.; Palmer, G.E. ERG2andERG24Are Required for Normal Vacuolar Physiology as Well as Candida albicans Pathogenicity in a Murine Model of Disseminated but Not Vaginal Candidiasis. Eukaryot. Cell 2015, 14, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Luna-Tapia, A.; Willems, H.M.E.; Parker, J.E.; Tournu, H.; Barker, K.S.; Nishimoto, A.T.; Rogers, P.D.; Kelly, S.L.; Peters, B.M.; Palmer, G.E. Loss of Upc2p-InducibleERG3Transcription Is Sufficient To Confer Niche-Specific Azole Resistance without CompromisingCandida albicansPathogenicity. mBio 2018, 9, e00225-18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liao, M.; Zhu, C.; Hu, Y.; Tong, T.; Peng, X.; Li, M.; Feng, M.; Cheng, L.; Ren, B.; et al. ERG3 and ERG11 genes are critical for the pathogenesis of Candida albicans during the oral mucosal infection. Int. J. Oral Sci. 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T.; Miyazaki, T.; Ito, Y.; Wakayama, M.; Shibuya, K.; Yamashita, K.; Takazono, T.; Saijo, T.; Shimamura, S.; Yamamoto, K.; et al. Virulence assessment of six major pathogenic Candida species in the mouse model of invasive candidiasis caused by fungal translocation. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Monteoliva, L.; Martinez-Lopez, R.; Pitarch, A.; Hernaez, M.L.; Serna, A.; Nombela, C.; Albar, J.P.; Gil, C. Quantitative Proteome and Acidic Subproteome Profiling ofCandida albicansYeast-to-Hypha Transition. J. Proteome Res. 2011, 10, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, I.D.; Wilson, D.; Wachtler, B.; Brunke, S.L.; Naglik, J.R.; Hube, B. Candida albicans dimorphism as a therapeutic target. Expert Rev. Anti Infect. Ther. 2012, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Morio, F.; Pagniez, F.; Lacroix, C.; Miegeville, M.; Le Pape, P. Amino acid substitutions in the Candida albicans sterol Delta5,6-desaturase (Erg3p) confer azole resistance: Charac-terization of two novel mutants with impaired virulence. J. Antimicrob. Chemother. 2012, 67, 2131–2138. [Google Scholar] [CrossRef]
- De Filippo, K.; Henderson, R.B.; Laschinger, M.; Hogg, N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macro-phages using distinct TLR signaling pathways. J. Immunol. 2008, 180, 4308–4315. [Google Scholar] [CrossRef]
- Chin, V.K.; Foong, K.J.; Maha, A.; Rusliza, B.; Norhafizah, M.; Chong, P.P. Early expression of local cytokines during systemic Candida albicans infection in a murine intravenous challenge model. Biomed. Rep. 2014, 2, 869–874. [Google Scholar] [CrossRef]
- Abe, Y.; Yamamoto, N.; Nakamura, K.; Arai, K.; Sakurai, C.; Hatsuzawa, K.; Ogura, Y.; Iseki, K.; Tase, C.; Kanemitsu, K. IL-13 attenuates early local CXCL2-dependent neutrophil recruitment for Candida albicans clearance during a severe murine systemic infection. Immunobiology 2019, 224, 15–29. [Google Scholar] [CrossRef]
- Witchley, J.N.; Penumetcha, P.; Abon, N.V.; Woolford, C.A.; Mitchell, A.P.; Noble, S.M. Candida albicans Morphogenesis Programs Control the Balance between Gut Commensalism and Invasive Infec-tion. Cell Host Microbe 2019, 25, 432–443.e6. [Google Scholar] [CrossRef] [PubMed]
- Fonzi, W.A.; Irwin, M.Y. Isogenic Strain Construction and Gene Mapping in Candida Albicans. Genetics 1993, 134, 717–728. [Google Scholar] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals of the Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council. Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 2011. Available online: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf (accessed on 28 December 2020).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirayama, T.; Miyazaki, T.; Sumiyoshi, M.; Ashizawa, N.; Takazono, T.; Yamamoto, K.; Imamura, Y.; Izumikawa, K.; Yanagihara, K.; Kohno, S.; et al. ERG3-Encoding Sterol C5,6-DESATURASE in Candida albicans Is Required for Virulence in an Enterically Infected Invasive Candidiasis Mouse Model. Pathogens 2021, 10, 23. https://doi.org/10.3390/pathogens10010023
Hirayama T, Miyazaki T, Sumiyoshi M, Ashizawa N, Takazono T, Yamamoto K, Imamura Y, Izumikawa K, Yanagihara K, Kohno S, et al. ERG3-Encoding Sterol C5,6-DESATURASE in Candida albicans Is Required for Virulence in an Enterically Infected Invasive Candidiasis Mouse Model. Pathogens. 2021; 10(1):23. https://doi.org/10.3390/pathogens10010023
Chicago/Turabian StyleHirayama, Tatsuro, Taiga Miyazaki, Makoto Sumiyoshi, Nobuyuki Ashizawa, Takahiro Takazono, Kazuko Yamamoto, Yoshifumi Imamura, Koichi Izumikawa, Katsunori Yanagihara, Shigeru Kohno, and et al. 2021. "ERG3-Encoding Sterol C5,6-DESATURASE in Candida albicans Is Required for Virulence in an Enterically Infected Invasive Candidiasis Mouse Model" Pathogens 10, no. 1: 23. https://doi.org/10.3390/pathogens10010023
APA StyleHirayama, T., Miyazaki, T., Sumiyoshi, M., Ashizawa, N., Takazono, T., Yamamoto, K., Imamura, Y., Izumikawa, K., Yanagihara, K., Kohno, S., & Mukae, H. (2021). ERG3-Encoding Sterol C5,6-DESATURASE in Candida albicans Is Required for Virulence in an Enterically Infected Invasive Candidiasis Mouse Model. Pathogens, 10(1), 23. https://doi.org/10.3390/pathogens10010023




