Effect of Ivermectin Treatment on the Frequency of Seizures in Persons with Epilepsy Infected with Onchocerca volvulus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Participants
2.1.1. Aketi Health Zone, DRC
2.1.2. Maridi, South Sudan
2.1.3. Mahenge, Ulanga District, Tanzania
2.2. Skin Snip Testing
2.3. Ivermectin Treatment
2.4. Medical History and Clinical Examination
2.5. Statistical Analysis
2.6. Ethical Approval and Consent to Participate
3. Results
3.1. Baseline Characteristics of Participants
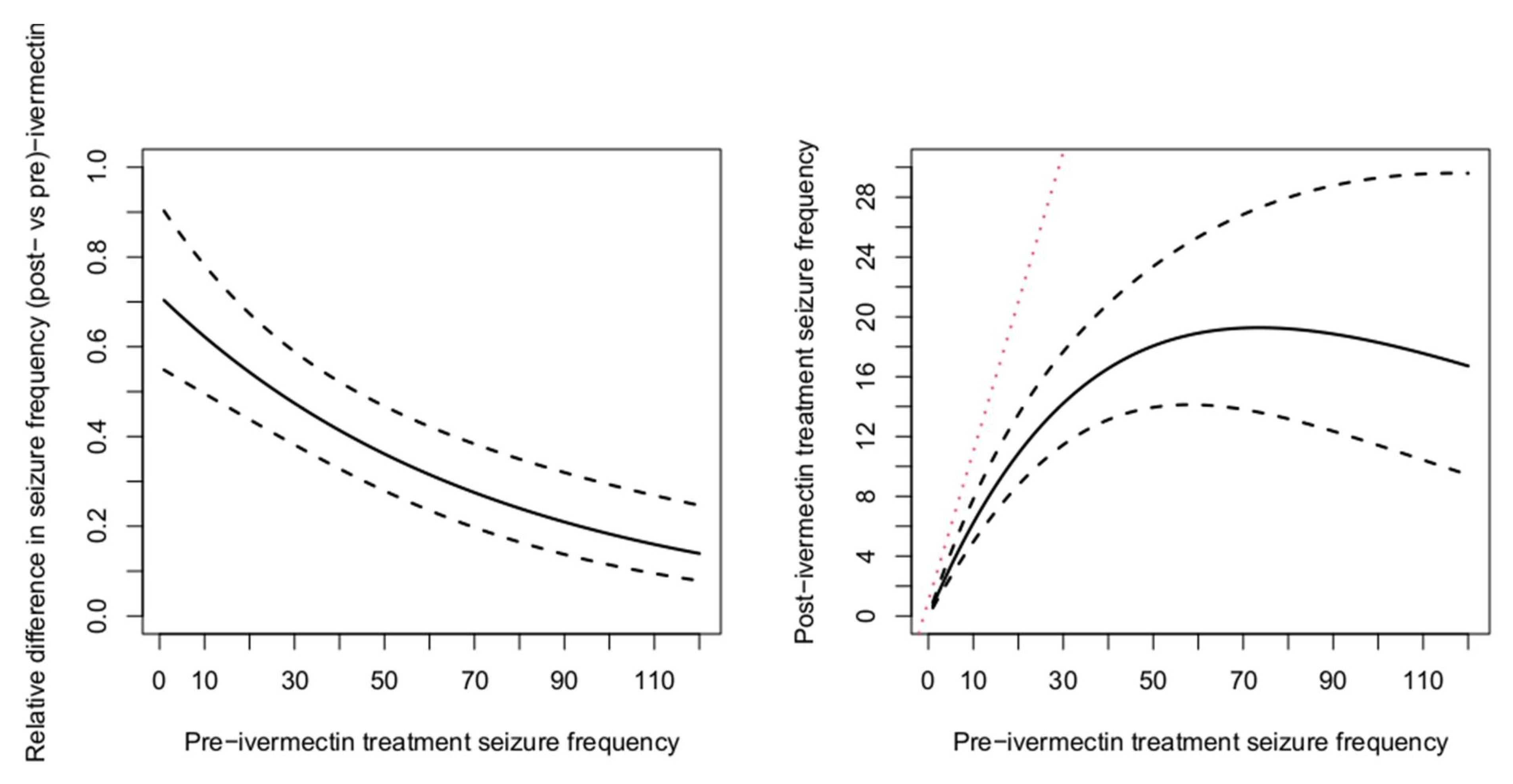
3.2. Pre- and Post-Ivermectin Microfilarial Density and Seizure Frequency
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colebunders, R.; Nelson Siewe, F.J.; Hotterbeekx, A. Onchocerciasis-associated epilepsy, an additional reason for strengthening onchocerciasis elimination programs. Trends Parasitol. 2018, 34, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Van Laethem, Y.; Lopes, C. Treatment of onchocerciasis. Drugs 1996, 52, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, C.D.; Geary, T.G.; Gerlach, J.A. Possible pathogenic pathways in the adverse clinical events seen following ivermectin administration to onchocerciasis patients. Filaria J. 2003, 2 (Suppl. 1), S5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Awadzi, K.; Dadzie, K.Y.; De Sole, G.; Remme, J. Reactions to ivermectin treatment in onchocerciasis patients. Acta Leiden 1990, 59, 193–199. [Google Scholar]
- De Sole, G.; Awadzi, K.; Remme, J.; Dadzie, K.Y.; Ba, O.; Giese, J.; Karam, M.; Keita, F.M.; Opoku, N.O. A community trial of ivermectin in the onchocerciasis focus of Asubende, Ghana. II. Adverse reactions. Trop. Med. Parasitol. 1989, 40, 375–382. [Google Scholar]
- Katabarwa, M.N.; Habomugisha, P.; Eyamba, A.; Byamukama, E.; Nwane, P.; Arinaitwe, A.; Musigire, J.; Tushemereirwe, R.; Khainza, A. Community-directed interventions are practical and effective in low-resource communities: Experience of ivermectin treatment for onchocerciasis control in Cameroon and Uganda, 2004–2010. Int. Health 2016, 8, 116–123. [Google Scholar] [CrossRef]
- Basanez, M.G.; Pion, S.D.; Boakes, E.; Filipe, J.A.; Churcher, T.S.; Boussinesq, M. Effect of single-dose ivermectin on Onchocerca volvulus: A systematic review and meta-analysis. Lancet Infect. Dis. 2008, 8, 310–322. [Google Scholar] [CrossRef]
- Kipp, W.; Burnham, G.; Kamugisha, J. Improvement in seizures after ivermectin. Lancet 1992, 340, 789–790. [Google Scholar] [CrossRef]
- Siewe Fodjo, J.N.; Mandro, M.; Mukendi, D.; Tepage, F.; Menon, S.; Nakato, S.; Nyisi, F.; Abhafule, G.; Wonya’rossi, D.; Anyolito, A.; et al. Onchocerciasis-associated epilepsy in the Democratic Republic of Congo: Clinical description and relationship with microfilarial density. PLoS Negl. Trop. Dis. 2019, 13, e0007300. [Google Scholar] [CrossRef]
- Colebunders, R.; Mandro, M.; Mukendi, D.; Dolo, H.; Suykerbuyk, P.; Van Oijen, M. Ivermectin treatment in patients with onchocerciasis-associated epilepsy: Protocol of a randomized clinical trial. JMIR Res. Protoc. 2017, 6, e137. [Google Scholar] [CrossRef]
- Mandro, M.; Siewe Fodjo, J.N.; Dusabimana, A.; Mukendi, D.; Haesendonckx, S.; Lokonda, R.; Nakato, S.; Nyisi, F.; Abhafule, G.; Wonya’rossi, D.; et al. Single versus multiple dose ivermectin regimen in onchocerciasis-infected persons with epilepsy treated with phenobarbital: A randomized clinical trial in the democratic Republic of Congo. Pathogens 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Dusabimana, A.; Bhwana, D.; Raimon, S.; Mmbando, B.P.; Hotterbeekx, A.; Tepage, F.; Mandro, M.; Siewe Fodjo, J.N.; Abrams, S.; Colebunders, R. Ivermectin treatment response in onchocerca volvulus infected persons with epilepsy: A three-country short cohort study. Pathogens 2020, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Levick, B.; Laudisoit, A.; Tepage, F.; Ensoy-Musoro, C.; Mandro, M.; Bonareri Osoro, C.; Suykerbuyk, P.; Kashama, J.M.; Komba, M.; Tagoto, A.; et al. High prevalence of epilepsy in onchocerciasis endemic regions in the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2017, 11, e0005732. [Google Scholar] [CrossRef] [PubMed]
- Mukendi, D.; Tepage, F.; Akonda, I.; Siewe, J.N.F.; Rotsaert, A.; Ndibmun, C.N.; Laudisoit, A.; Couvreur, S.; Kabutako, B.; Menon, S.; et al. High prevalence of epilepsy in an onchocerciasis endemic health zone in the Democratic Republic of the Congo, despite 14 years of community-directed treatment with ivermectin: A mixed-method assessment. Int. J. Infect. Dis. 2019, 79, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Colebunders, R.; Carter, J.Y.; Olore, P.C.; Puok, K.; Bhattacharyya, S.; Menon, S.; Abd-Elfarag, G.; Ojok, M.; Ensoy-Musoro, C.; Lako, R.; et al. High prevalence of onchocerciasis-associated epilepsy in villages in Maridi County, Republic of South Sudan: A community-based survey. Seizure 2018, 63, 93–101. [Google Scholar] [CrossRef]
- Mmbando, B.P.; Suykerbuyk, P.; Mnacho, M.; Kakorozya, A.; Matuja, W.; Hendy, A.; Greter, H.; Makunde, W.H.; Colebunders, R. High prevalence of epilepsy in two rural onchocerciasis endemic villages in the Mahenge area, Tanzania, after 20 years of community directed treatment with ivermectin. Infect. Dis. Poverty 2018, 7, 64. [Google Scholar] [CrossRef]
- WHO. African Programme for Onchocerciasis Control (APOC): Ivermectin. Available online: https://www.who.int/apoc/cdti/ivermectin/en/ (accessed on 6 March 2018).
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Bind, M.A.; Vanderweele, T.J.; Coull, B.A.; Schwartz, J.D. Causal mediation analysis for longitudinal data with exogenous exposure. Biostatistics 2016, 17, 122–134. [Google Scholar] [CrossRef]
- Abd-Elfarag, G.; Carter, J.Y.; Raimon, S.; Sebit, W.; Suliman, A.; Siewe Fodjo, J.N.; Claver Olore, P.; Biel, K.P.; Ojok, M.; Logora, M.Y.; et al. Persons with onchocerciasis-associated epilepsy with nodding seizures have a more severe form of epilepsy, with more cognitive impairment and higher levels of Onchocerca volvulus infection. Epileptic Disord. 2020, in press. [Google Scholar] [CrossRef]
- Chandler, R.E. Serious neurological adverse events after ivermectin–Do they occur beyond the indication of onchocerciasis? Am. J. Trop. Med. Hyg. 2018, 98, 382–388. [Google Scholar] [CrossRef]
- Okonkwo, P.O.; Ogbuokiri, J.E.; Ofoegbu, E.; Klotz, U. Protein binding and ivermectin estimations in patients with onchocerciasis. Clin. Pharmacol. Ther. 1993, 53, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Diazgranados-Sanchez, J.A.; Mejia-Fernandez, J.L.; Chan-Guevara, L.S.; Valencia-Artunduaga, M.H.; Costa, J.L. Ivermectin as an adjunct in the treatment of refractory epilepsy. Rev. Neurol. 2017, 65, 303–310. [Google Scholar] [PubMed]
| Characteristic | Maridi (n = 105) | Aketi (n = 87) | Mahenge (n = 23) | All Participants (n = 215) |
|---|---|---|---|---|
| Age (years): median (IQR) | 17.0 (14.0–18.0) | 17.0 (15.0–20.0) | 33.0 (27.3–42.0) | 17.0 (15.0–21.0) |
| Gender: Male, n (%) | 53 (50.5) | 40 (49.4) | 9 (39.1) | 102 (48.8) |
| Weight (kg): median (IQR) | 37.0 (30.0–48.0) | 39.0 (30.0–43.0) | 45.0 (40.5–49. 5) | 40.0 (31.0–48.0) |
| History of anti-seizure drug use at enrolment, n (%) | 97 (93.3) | 55 (63.2) | 21 (95.5) | 173 (81.2) |
| Most frequent types of seizure | ||||
| Generalized tonic-clonic seizures: n (%) | 58 (55.2) | 74 (85.1) | 17 (73.9) | 149 (69.3) |
| Atonic seizures (drop attacks): n (%) | 1 (0.9) | 0 (0.0) | 1 (4.3) | 2 (0.9) |
| Absences: n (%) | 3 (2.9) | 4 (4.6) | 3 (13.0) | 10 (4.6) |
| Nodding seizures: n (%) | 25 (23.8) | 7 (8.0) | 2 (8.7) | 34 (15.8) |
| Generalized tonic-clonic with nodding seizures: n (%) | 18 (17.1) | 2 (2.3) | 0 (0.0) | 20 (9.3) |
| Ivermectin use in 2018, n (%) | 41 (39.0) | 81 (93.1) | 18 (78.3) | 140 (65.1) |
| Variables | Before Ivermectin Intake | 3 Months after Ivermectin Intake | p-Value |
|---|---|---|---|
| Positive skin snip for mf: n (%) | 87 (100) | 18 (22.2) | <0.001 a |
| mf density: median (IQR) | 12.0 (4.0–63.0) | 0.0 (0.0–0.0) | <0.001 b |
| Seizures per month: median (IQR) | 1.0 (0.5–2.0) | 1.0 (0.0–2.0) | <0.001 b |
| Number of PWE with <1 seizure per month: n (%) | 10 (11.5) | 55 (63.3) | <0.001 a |
| % reduction in frequency of seizures: median (IQR) | NA | 100 (50.0–100) | NA |
| Variables | Before Ivermectin Intake | 5 Months after Ivermectin Intake | p-Value |
|---|---|---|---|
| Positive skin snip for mf: n (%) | 105 (100) | 83/105 (79.0%) | <0.001 a |
| mf density: median (IQR) | 52.0 (29.0–84.0) | 3.0 (1.0–10.0) | <0.001 b |
| Seizures per month: median (IQR) | 12.0 (2.0–90.0) | 8.0 (2.0–20.0) | <0.001 b |
| Number of PWE with <1 seizure per month: n (%) | 8 (7.6) | 45 (42.9) | NA |
| % reduction in frequency of the seizures: median (IQR) | NA | 73.4 (26.0–90.0) | NA |
| Parameters | Before Ivermectin Intake | 3 Months after Ivermectin Intake | p-Value |
|---|---|---|---|
| Positive skin snip for mf: n (%) | 23 (100) | 11/23 (47.8) | 0.002 a |
| mf density: median (IQR) | 3.0 (1.0–5.0) | 0.0 (0.0–1.0) | <0.001 b |
| Seizures per month: median (IQR) | 2.0 (1.0–2.0) | 0.0 (0.0–1.0) | 0.093 b |
| Number of PWE with <1 seizure per month: n (%) | 2 (8.7) | 12 (52.2) | <0.001 a |
| % reduction in frequency of seizures: median (IQR) | NA | 100 (85.8–100.0) | NA |
| Effect | Estimate | 95% CI | p-Value | |
|---|---|---|---|---|
| Intercept | 2.322 | 0.736 | 3.908 | 0.004 |
| Age (years) | −0.048 | −0.107 | 0.012 | 0.114 |
| Gender (male vs female) | −0.309 | −0.833 | 0.215 | 0.245 |
| Site (Mahenge vs Aketi) | −0.610 | −2.207 | 0.987 | 0.452 |
| Site (Maridi vs Aketi) | 1.646 | 0.908 | 2.385 | <0.001 |
| Baseline mf/skin snip | 0.002 | −0.003 | 0.006 | 0.471 |
| After ivermectin treatment (vs before) | −0.338 | −0.592 | −0.084 | 0.009 |
| Seizure before ivermectin * After ivermectin treatment (vs before) | −0.014 | −0.019 | −0.008 | <0.001 |
| Previous ivermectin (used vs not used) | −0.371 | −1.009 | 0.268 | 0.253 |
| Anti-seizure drug use with ivermectin | 0.144 | −0.436 | 0.724 | 0.624 |
| Var[b0] (se) | 2.129 (0.325) | |||
| Estimates | Estimate | 95% CI | |
|---|---|---|---|
| Indirect effect | −0.014 | −0.020 | −0.006 |
| Direct effect | −0.715 | −0.955 | −0.465 |
| Total effect | −0.730 | −0.970 | −0.470 |
| Percentage mediated | 1.867 | 1.363 | 2.087 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dusabimana, A.; Tsebeni Wafula, S.; Raimon, S.J.; Fodjo, J.N.S.; Bhwana, D.; Tepage, F.; Abd-Elfarag, G.; Hotterbeekx, A.; Abrams, S.; Colebunders, R. Effect of Ivermectin Treatment on the Frequency of Seizures in Persons with Epilepsy Infected with Onchocerca volvulus. Pathogens 2021, 10, 21. https://doi.org/10.3390/pathogens10010021
Dusabimana A, Tsebeni Wafula S, Raimon SJ, Fodjo JNS, Bhwana D, Tepage F, Abd-Elfarag G, Hotterbeekx A, Abrams S, Colebunders R. Effect of Ivermectin Treatment on the Frequency of Seizures in Persons with Epilepsy Infected with Onchocerca volvulus. Pathogens. 2021; 10(1):21. https://doi.org/10.3390/pathogens10010021
Chicago/Turabian StyleDusabimana, Alfred, Solomon Tsebeni Wafula, Stephen Jada Raimon, Joseph Nelson Siewe Fodjo, Dan Bhwana, Floribert Tepage, Gasim Abd-Elfarag, An Hotterbeekx, Steven Abrams, and Robert Colebunders. 2021. "Effect of Ivermectin Treatment on the Frequency of Seizures in Persons with Epilepsy Infected with Onchocerca volvulus" Pathogens 10, no. 1: 21. https://doi.org/10.3390/pathogens10010021
APA StyleDusabimana, A., Tsebeni Wafula, S., Raimon, S. J., Fodjo, J. N. S., Bhwana, D., Tepage, F., Abd-Elfarag, G., Hotterbeekx, A., Abrams, S., & Colebunders, R. (2021). Effect of Ivermectin Treatment on the Frequency of Seizures in Persons with Epilepsy Infected with Onchocerca volvulus. Pathogens, 10(1), 21. https://doi.org/10.3390/pathogens10010021







