Abstract
We reviewed the evidence on community-based interventions for the prevention and control of cutaneous leishmaniasis (CL). Community initiatives tailored towards awareness and mobilisation are regarded as a priority area in the Neglected Tropical Disease Roadmap 2021–2030 by the World Health Organization. We searched nine electronic databases for intervention-based studies. Two independent reviewers screened and assessed the articles for methodological quality using predefined criteria. We conducted a meta-analysis using a random effects model, along with narrative synthesis. Thirteen articles were eligible for inclusion, of which 12 were quantitative studies (quasi-experimental with control group and pre-post interventions) and one qualitative study. All articles reported on health education interventions aimed at changing people’s knowledge, attitudes, and practices (KAP) in relation to CL. Participant groups included students, mothers, housewives, volunteer health workers, and residents in general. An increased score was recorded for all outcomes across all interventions: knowledge (SMD: 1.85, 95% CI: 1.23, 2.47), attitudes (SMD: 1.36, 95% CI: 0.56, 2.15), and practices (SMD: 1.73, 95% CI: 0.99, 2.47). Whilst our findings show that educational interventions improved people’s knowledge, attitudes, and practices about CL, we argue that this approach is not sufficient for the prevention and control of this disease. Knowledge does not always translate into action, particularly where other structural barriers exist. Therefore, we recommend the design of more innovative community-based interventions with a broader focus (e.g., stigma, financial barriers, and healthcare access).
1. Introduction
Cutaneous leishmaniasis (CL) is a parasitic disease caused by infection with a vector-borne protozoan parasite of the genus Leishmania spp. The parasite is transmitted by the bite of an infected phlebotomine sand fly. Infection results in skin lesions which take a long time to heal and may leave permanent, disfiguring scars (de Vries et al. 2015). CL is classified as a neglected tropical disease (NTD), and in common with several other NTDs, is associated with psychosocial effects including stigma, social exclusion, and declining mental health (Bailey et al. 2019; Bennis et al. 2018; Wenning et al. 2022). Emerging evidence suggests that people with CL are at a higher risk of experiencing anxiety, depression, decreased body satisfaction, loss of social status, and lower quality of life (Bennis et al. 2018; Yanik et al. 2004). The global mean age-standardised disability-adjusted life years (DALYs) lost by CL was 0.58 per 100,000 people (Karimkhani et al. 2016). Notably, this statistic only considers the physical effects of the lesions and does not account for the potentially considerable psychological and social effects of CL (Bailey et al. 2017; Bailey et al. 2019; Wenning et al. 2022).
Figures from the World Health Organization (WHO) show that CL is endemic in around 90 countries (WHO 2018), with 0.7–1.2 million new cases of CL recorded annually across the globe (Alvar et al. 2012). The actual number of new cases per year, however, is likely to be much higher due to underreporting in official surveillance data, e.g., in East Africa (Alvar et al. 2012). The burden of CL disproportionately affects the poorest communities in low- and middle- income countries (LMICs) (Du et al. 2016; Hayani et al. 2015), particularly areas with poor housing conditions and lack of sanitation (Alvar et al. 2006). As a result, people living in conflict zones and displacement areas are particularly vulnerable to CL risk (Al-Salem et al. 2016). Poverty is also closely linked to barriers in health-seeking, including low health literacy and lack of disease recognition, as well as geographic and financial inaccessibility of CL treatment (Alvar et al. 2006). In the absence of biomedical treatment, CL may be treated with home ‘cures’ ranging from herbal remedies to the application of battery acid, petroleum or cauterization with fire (Ramdas 2012).
In the recently published Roadmap for NTDs (2021–2030), the WHO established targets for 85% of all CL cases to be detected and reported, and for 95% of reported cases to be treated by 2030 (WHO 2020). To date, no vaccines or prophylactic drugs are available for human CL and treatment still relies on old drugs which are toxic, expensive, and difficult to administer (Santos et al. 2008). Preventive and control measures, on an individual and public health level, are therefore crucial to limit the number of infected people with CL.
Prevention and Control of CL
CL prevention currently centres around the reduction of contact between sand fly vectors and humans, as well as the reduction of vector abundance. Different strategies exist for this purpose, ranging from use of insecticides and environmental management to personal protection, including bed nets (González et al. 2015). Other measures involve decreasing the reservoir host populations for the parasite, including rodents and dogs (González et al. 2015).
The WHO Roadmap for NTDs calls for an integrated approach in which epidemiological surveillance and vector control measures are employed in conjunction with wider-ranging strategies. These may include community health education to build awareness around prevention and treatment-seeking behaviour, and training for health workers and volunteers on screening and treating NTDs (WHO 2020). This community-oriented approach, with an emphasis on training, education, and participation, is emphasised in the CL literature as a means of assuring compliance among people in the prevention and control of this disease (Alvar et al. 2006; González et al. 2015; Pérez et al. 2020).
Community-based interventions are pivotal in the field of public health and refer to a ‘set of interventions designed to create changes in community infrastructure and services, norms, attitudes, beliefs, and policies that would result in improved health status for community residents’ (Guttmacher et al. 2010). Interventions at the community level may take place in neighbourhoods, schools, churches, work sites, voluntary agencies, or other organizations (McLeroy et al. 2003). In the context of NTDs, Lassi and colleagues recommend interventions to be directed at community mobilisation, education and training, removal of financial barriers, and referral to health facilities (Lassi et al. 2014). They further argue that addressing these different facets will enhance people’s knowledge, attitudes, and practices (KAP) around the disease, improve access to and coverage of healthcare services, and ultimately reduce disease morbidity and mortality (Lassi et al. 2014).
The evidence available on vector control strategies for CL, as well as a range of therapeutic interventions, have already been synthesised in previous systematic reviews (González et al. 2015; Reveiz et al. 2013). However, no reviews to date have focused on community-based interventions in relation to CL, such as information and education campaigns, health education programmes, support groups, financial incentives, and other public health initiatives. This is especially pertinent in light of the literature on CL which highlights low levels of awareness and education, difficulties accessing treatment, and other barriers at the community level (Hejazi et al. 2010; Saberi et al. 2012).
We, therefore, aimed to review the literature on community-based interventions for the prevention and control of CL. Specific objectives were to:
- (a)
- identify the characteristics of existing interventions
- (b)
- determine their effectiveness in terms of changing CL-related knowledge, attitudes, and practices, or improving the infection and/or treatment rates of CL
- (c)
- explore factors affecting the implementation of these interventions in the community
2. Materials and Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (Page et al. 2021). A protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42020189030).
2.1. Search Strategy
Nine electronic databases were searched (Cinahl Plus, Academic Search Complete, Global Index Medicus, MEDLINE, Embase, Web of Science, Scopus, Cochrane Central, and Global Health) from inception until 01 June 2020 and updated on 30 June 2021, using structured search strategies. The full MEDLINE search strategy can be found in Supplementary Table S1. Additional sources were identified by hand searching the reference lists of included studies and relevant systematic reviews. We also searched for grey literature using dissertation databases, trial registers, Eldis (research and policy document database relating to international development) and OpenGrey (grey literature database). No language restrictions were applied.
2.2. Eligibility Criteria
We included studies if they reported on a community-based intervention, defined as any intervention delivered at home, village, or any other community setting, but not in a health facility. This might include, for instance, training for lay health workers, health education to community members, or financial interventions to improve the uptake of health services. The study eligibility criteria are outlined in Table 1.

Table 1.
Inclusion and exclusion criteria adopted in this review.
2.3. Screening and Study Selection
The study selection process was tested and piloted a priori by members of the review team. Two authors independently reviewed all titles, abstracts, and full texts against predefined criteria. Discrepancies were resolved through discussion with a third reviewer. Where appropriate, translations of full-text studies were sought in order to determine eligibility.
2.4. Data Extraction and Quality Appraisal
After completing the study selection process, data extraction was completed by two reviewers independently using a customised data extraction form. Extracted data included population characteristics, study setting, aims and design, interventions, data collection methods, outcome measures, key findings, and study limitations. The quality of the studies was assessed independently by two reviewers, and any disagreements were discussed with a third reviewer until a consensus was reached. We used the National Institute of Health quality assessment toolkits for intervention-based studies (NIH 2021) and the Critical Appraisal Skills Programme (CASP) checklist for qualitative studies (CASP 2018). Ratings of high, moderate, or poor were applied according to the criteria stated in the toolkits.
2.5. Data Analysis
We used a meta-analysis to integrate available outcomes extracted from the quantitative studies. A random effects model was used to estimate pooled standardised mean difference (SMD) of outcomes between the experimental/post-intervention and control groups/pre-intervention with a 95% confidence interval (CI). Statistical heterogeneity was assessed using the Chi2 and I2 statistics, which can be interpreted as the percentage of the total variation between studies that is attributable to heterogeneity rather than to chance. Estimates with a p-value of <0.05 in Chi2 and I2 ≥ 50% were considered to have significant heterogeneity. In the case of one study, we suspected a typographical error in a value for post-intervention knowledge score (Jeihooni et al. 2019). We contacted the corresponding author twice to seek clarification, and due to receiving no response, we decided not to include the study in the analysis. When quantitative analyses were not appropriate (e.g., because of heterogeneity, insufficiency, or variation in outcome reporting), we synthesised data narratively.
3. Results
The electronic database search identified a total of 7348 unique citations, with 37 additional studies identified through reference checking. After removing duplicates, 3515 studies were screened by title and abstract, followed by an assessment of 40 full-text articles for inclusion. Thirteen studies met the full eligibility criteria. The flow of the studies through the review process, with reasons for exclusion, is presented in Figure 1.
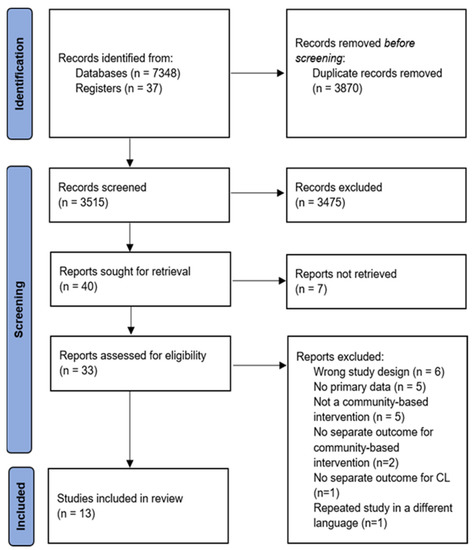
Figure 1.
PRISMA flow chart of screening process and selected studies.
3.1. Characteristics of Included Studies
Of the 13 included articles, eight consisted of quasi-experimental studies with a control group, four were pre-post intervention studies, and one was a qualitative study. The majority of articles stated that the studies were carried out between 2006 and 2017. All studies were conducted in Iran, except for one in Brazil; all in regions which are highly endemic for CL. The number of participants in each study ranged from 20 to 450 people, with a total of 2050 participants across all 13 studies. These participants included residents of the region (Reis et al. 2006), students (Ghodsi et al. 2019; Motamedi et al. 2010; Saghafipour et al. 2017), health-related personnel (Kashfi et al. 2012; Tafti et al. 2011), volunteer health workers (Hazavehei et al. 2014; Zeinali et al. 2019), mothers (Baghianimoghadam et al. 2016; Kavoosi and Shojaeizadeh 2016), housewives (Jeihooni et al. 2019; Nazari et al. 2016), and parents (Dehghani-Tafti et al. 2017). Although the interventions were delivered primarily to these target populations, in five studies, intervention delivery was also aimed at a further secondary group made up of people considered to be influential in the community, including family members, health staff, teachers, and local dignitaries (Ghodsi et al. 2019; Hazavehei et al. 2014; Jeihooni et al. 2019; Kashfi et al. 2012; Motamedi et al. 2010). In three studies, having a history of CL excluded individuals from participation (Ghodsi et al. 2019; Hazavehei et al. 2014; Jeihooni et al. 2019). In contrast, Baghianimoghadam et al.’s (2016) study only included participants with a CL history. Saghafipour et al. (2017) reported that 95.8% of participants did not have a CL history, whilst 37% of Motamedi et al.’s (2010) participants reported having a CL history. This information was not present in seven of the included studies. Table 2 describes the characteristics of the final included studies, including author, year, country, setting, population, method, design, and sample characteristics.

Table 2.
Characteristics of included studies.
3.2. Characteristics of Interventions
Community-based interventions across all studies were exclusively of an educational nature. While interventions varied in terms of design, content, and activities, they all shared similar aims of improving the understanding of CL among people living in endemic areas, and thereby enhancing CL-related preventive and health-seeking behaviours. Various health education activities were used in the interventions, including lectures, slideshows, videos, banners and/or posters, group discussions, demonstrations, Question and Answer sessions, brainstorming, role-playing, thematic workshops, and distributions of pamphlets, booklets, and manuals. The most commonly used were lectures, adopted in eight interventions (Baghianimoghadam et al. 2016; Ghodsi et al. 2019; Jeihooni et al. 2019; Kavoosi and Shojaeizadeh 2016; Motamedi et al. 2010; Nazari et al. 2016; Tafti et al. 2011; Zeinali et al. 2019) and group discussions (Baghianimoghadam et al. 2016; Ghodsi et al. 2019; Jeihooni et al. 2019; Kavoosi and Shojaeizadeh 2016; Motamedi et al. 2010; Nazari et al. 2016; Saghafipour et al. 2017) which were used in six interventions, across the thirteen studies. Overall, each intervention combined multiple educational activities, with the exception of Reis et al.’s (2006) intervention, which was only based on thematic workshops. Kavoosi and Shojaeizadeh (2016) assigned participants to two experimental groups—one group attending a lecture and the other taking part in a group discussion—to compare the effectiveness of these two activities.
The content of educational interventions, with the exception of three studies (Kavoosi and Shojaeizadeh 2016; Tafti et al. 2011; Zeinali et al. 2019), was guided by a theoretical model. These theories stemmed from the fields of education, public health, health promotion, and psychology, which in turn, influenced the outcomes reported. Five studies used the Beliefs, Attitudes, Subjective Norms, and Enabling Factors (BASNEF) model (Dehghani-Tafti et al. 2017; Ghodsi et al. 2019; Hazavehei et al. 2014; Kashfi et al. 2012; Saghafipour et al. 2017). This educational model is used to study behaviours, offer plans for change, and define the factors influencing individuals’ decision-making (Hubley 1988). Two studies used the Health Belief Model (HBM) (Ghodsi et al. 2019; Motamedi et al. 2010), which assumes that people’s willingness to change their health behaviours is primarily due to their health perceptions (Becker 1974). The outcomes of these studies include reporting knowledge, perceived susceptibility, perceived severity, perceived benefits, perceived barriers, and cues to action. Another two studies were informed by elements of the PRECEDE-PROCEED framework (Jeihooni et al. 2019; Nazari et al. 2016), which is used to assess health needs for the design, implementation, and evaluation of a health promotion intervention (Crosby and Noar 2011). The focus of these studies was on knowledge, attitude, subjective norms, enabling factors, and behaviour. Only one study used the Theory of Planned Behaviour (Baghianimoghadam et al. 2016) which posits that behaviours are immediately determined by behavioural intentions, and under certain circumstances, perceived behavioural control (Conner 2020). In this case, the outcomes reported were knowledge, attitude, subjective norms, behavioural intention, and perceived behaviour. Reis et al.’s (2006) qualitative study was informed by social representation theory as they sought to (re)construct participants’ social representations of CL.
Overall, topics covered in the education interventions included the status of CL in the local context (Ghodsi et al. 2019), general description of the disease, its vector, and modes of transmission (Dehghani-Tafti et al. 2017; Ghodsi et al. 2019; Hazavehei et al. 2014; Kavoosi and Shojaeizadeh 2016; Reis et al. 2006), measures of preventing and controlling CL (Dehghani-Tafti et al. 2017; Ghodsi et al. 2019; Hazavehei et al. 2014; Jeihooni et al. 2019; Nazari et al. 2016), and treatment-seeking for CL (Ghodsi et al. 2019; Nazari et al. 2016; Reis et al. 2006). No information about the educational content was present in four articles (Baghianimoghadam et al. 2016; Dehghani-Tafti et al. 2011; Saghafipour et al. 2017; Zeinali et al. 2019). Interventions delivered to community volunteer workers and health workers in Hazavehei et al.’s (2014) and Kashfi et al.’s (2012) studies also covered interpersonal and communication skills training to enhance participants’ capability in training other members of their community.
The majority of studies did not specify who delivered the interventions. Of those which did, the research team, public health experts, and other local officials were mentioned (Jeihooni et al. 2019; Kavoosi and Shojaeizadeh 2016; Saghafipour et al. 2017). Overall, the interventions varied in frequency and length of sessions, ranging from only one session to one session per week over two months, each with a duration between 10–120 min. Details around the activities pertaining to each intervention, together with the respective theoretical model are summarised in Table 3.

Table 3.
Description of educational interventions.
In order to assess the intervention outcomes in the quantitative studies, a variety of questionnaires were used in response to the different aims and models/theories. In seven studies (Ghodsi et al. 2019; Hazavehei et al. 2014; Jeihooni et al. 2019; Kavoosi and Shojaeizadeh 2016; Motamedi et al. 2010; Nazari et al. 2016; Saghafipour et al. 2017), the research team designed their own questionnaire, whilst also explaining how the instrument’s assessment of validity and reliability, as well as scoring, was performed. One of these studies did not provide such details (Jeihooni et al. 2019). In three studies, an existing questionnaire was used (Baghianimoghadam et al. 2016; Dehghani-Tafti et al. 2017; Kashfi et al. 2012); however, only Baghianimoghadam et al.’s (2016) revalidated an existing tool for their particular study population. The instruments used contained a mean of 61 questions, ranging from 25 to 79. These included demographic information and questions that pointed to the outcomes evaluated according to the model/theory used. The questionnaires were answered first before the intervention and second after the intervention, usually after two months, although this time varied in each study (between one and nine months). The qualitative study by Reis et al. (2006), on the other hand, tested participants’ knowledge two months after completing the intervention, via an interview to check if participants maintained their initial position.
3.3. Outcomes
Each study contained multiple outcome measures, largely corresponding to the models/theories informing the intervention. For the purpose of this review, we will be reporting results on the pre-specified outcomes highlighted earlier in Table 1.
3.3.1. Change in Knowledge, Attitudes, and Practices
Knowledge
Knowledge about CL (e.g., description of the disease, transmission routes, methods of prevention, and treatment) was an outcome evaluated in all studies. An improvement in knowledge was observed after the intervention or in comparison with the control group across all studies. A meta-analysis of 11 quantitative studies showed a significant improvement in the overall mean scores of knowledge after the intervention (SMD: 1.85, 95% CI: 1.23, 2.47). The mean score was notably larger for Hazavehei et al.’s (2014) and Dehghani-Tafti et al.’s (2017) studies, and the lowest for Nazari et al.’s (2016) work (SMD: 4.6 and 3.44 versus 0.31) (see Table 4 and Figure 2a). Kavoosi and Shojaeizadeh’s (2016) study, which incorporated two intervention groups (i.e., one group of mothers attended a lecture, and another participated in a group discussion), did not show a significant difference in scores post-intervention. Nonetheless, mean knowledge scores were slightly higher among those who attended ‘group discussion’. Finally, Reis et al.’s (2006) qualitative study also showed that new knowledge about CL was retained following the health education intervention.

Table 4.
Summary estimates of standardised mean difference (SMD) for the overall and subgroups studies.
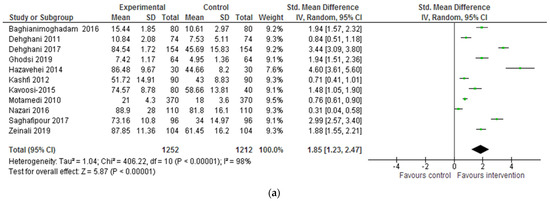
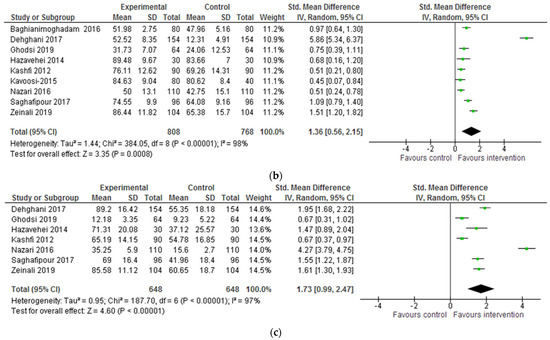
Figure 2.
(a) Forest plot for the impact of the intervention on CL-related knowledge. (b) Forest plot for the impact of the intervention on CL-related attitudes. (c) Forest plot for the impact of the intervention on CL-related behaviours/ practices.
Attitudes
Attitudes towards CL (e.g., beliefs about transmission and severity of disease) were evaluated in ten of 12 studies (Baghianimoghadam et al. 2016; Dehghani-Tafti et al. 2017; Ghodsi et al. 2019; Hazavehei et al. 2014; Jeihooni et al. 2019; Kashfi et al. 2012; Kavoosi and Shojaeizadeh 2016; Nazari et al. 2016; Saghafipour et al. 2017; Zeinali et al. 2019). An increase in scores was observed in all ten studies. Nine studies were pooled for the SMD scores of attitudes, which showed significantly improved mean scores of attitudes after the intervention (SMD: 1.36, 95% CI: 0.56, 2.15). This was highest for Dehghani-Tafti et al.’s (2017) study, suggesting that their intervention consisting of two training sessions featuring an education film and the distribution of pamphlets, produced better results in terms of changing attitudes towards CL (See Table 4 and Figure 2b). In this instance, a significant difference was noted between the ‘lecture’ and ‘group discussion’ intervention groups in Kavoosi and Shojaeizadeh’s (2016) study, with the participants in the group discussion scoring significantly higher in terms of attitudes.
Behaviours/Practices
Behavioural changes to prevent and control CL (e.g., use of insecticide-treated bed nets and insect repellent products and treatment-seeking) were evaluated in nine of 12 quantitative studies (Dehghani-Tafti et al. 2017; Ghodsi et al. 2019; Hazavehei et al. 2014; Jeihooni et al. 2019; Kashfi et al. 2012; Motamedi et al. 2010; Nazari et al. 2016; Saghafipour et al. 2017; Dehghani-Tafti et al. 2011), with a change observed in all of them. The results of seven studies were pooled for this outcome measure. All seven showed significant improvement in the overall mean scores of practices after the intervention (SMD: 1.73, 95% CI: 0.99, 2.47). The mean scores of practices were notably larger in Nazari et al.’s (2016) study, and lowest in Kashfi et al. (2012) and Ghodsi et al.’s (2019) (4.47 versus 0.67) studies (see Table 4 and Figure 2c). Kavoosi and Shojaeizadeh’s (2016) analysis importantly identified no correlation between the increase in knowledge and practice scores post-intervention. This information was not reported in the remainder of the included studies.
3.3.2. Change in Number of Cases, Incidence, or Cure Rate of CL
All studies included in the review measured the outcomes through self-reported questionnaires. No data about the incidence or cure rate of CL were documented.
3.3.3. Barriers or Facilitators to Implementation
None of the included studies offered an in-depth discussion about the implementation phase of the educational interventions. However, some barriers and facilitators were documented across the articles. The considerable demand to produce educational materials, together with a lack of time availability, was identified by Reis et al. (2006) as a barrier to the delivery of an intervention based on social representations. Moreover, Saghafipour et al. (2017) highlighted the bureaucratic processes to gain permission as a key barrier to delivering educational interventions, especially to students given the importance for this population to be easily accessible for teaching and learning. The involvement of people considered influential by the target population, in the implementation of educational interventions, was saliently recognised as a facilitator (Ghodsi et al. 2019; Hazavehei et al. 2014). The lack of involvement of these influential groups was conversely viewed as a barrier (Dehghani-Tafti et al. 2017; Nazari et al. 2016).
Quality Assessment of Included Studies
In general, included studies were of low (n = 8) or moderate (n = 5) methodological quality. Table 5 presents a combined result of the quality assessment of the pre-post intervention and quasi-experimental studies as well as the qualitative study. Overall, there were elements which were commonly absent from the studies, accounting for high or unclear risk of bias, including the loss of follow-up with the participants, the blinding of the people who analysed the results of the outcomes, and the calculation of the sample size. With respect to pre-post intervention studies, high-risk elements included a lack of relevant information regarding selection criteria and participant enrolment, representability of the population, and the validity and reliability of questionnaires. In the quasi-experimental studies, two important elements were generally missing, including adherence to the intervention, which is crucial since multiple sessions were involved, and the absence of other interventions in both groups that could lead to potential confounders in the results. Finally, the qualitative study was strong in terms of methodology and methods but lacked minor details around recruitment, ethical considerations, and reflexivity.

Table 5.
Quality appraisal of pre and post-intervention and quasi-experimental studies.
4. Discussion
This is the first systematic review of published studies on community-based interventions to prevent and control CL, transcending vector and reservoir host control measures. However, the significance of these initiatives is apparent in the recently published NTD road map (2021–2030) where ‘social mobilisation and community education to build awareness about skin NTDs and encourage early reporting and treatment-seeking’ is recognised as a priority area (WHO 2020). In the context of CL, this is especially crucial given the myriad of studies conducted across a wide range of endemic areas showing low to moderate knowledge, attitudes, and practices scores. According to Lassi et al.’s conceptual framework, community-based interventions for the prevention and control of infectious diseases of poverty should not only aim at education and health promotion but also ensure the removal of financial barriers and access to health facilities (Lassi et al. 2014). The intended outcomes of such interventions should be improved KAP, improved coverage, improved access, cost-effective care, and reduced inequity. This broad framework informed our review question and search strategy.
The thirteen studies included in this review consisted of eight quasi-experimental with control group studies, four pre- and post-intervention studies, and one qualitative study. Despite the broad framing of our review question, all studies meeting the eligibility criteria comprised of educational interventions. These interventions included a combination of various individual and team-based activities which involved diverse groups of study participants. All studies reported improvement in KAP scores, with our meta-analysis indicating that the most significant improvement in knowledge scores was noted in Hazavahei et al.’s intervention with volunteer health workers (Hazavehei et al. 2014); attitudes scores in Dehghani-Tafti et al.’s (2017) study with parents; and practices in Nazari et al.’s (2016) study with housewives. Kavoosi and Shojaeizadeh’s (2016) study which compared the effectiveness of two activities—lecturing and group discussions—found the latter to be more effective for bringing about changes in attitudes. This is likely due to the active and interactive way in which learning took place. Due to the heterogeneity, lack of information reported, and general low to moderate quality across the studies, it is difficult to determine which components of these interventions contributed to their effectiveness.
Our findings build on Souza et al.’s (2011) systematic review, which similarly looked at health education relating to leishmaniasis in the South American context. A total of four studies about CL specifically were included in their review, carried out in Peru, Colombia, and Brazil. While one study by Reis et al. (2006) has also been included in our review, the others were not eligible for inclusion due to their study design. With the exception of one survey study (Isaza et al. 1999), the other two studies delivered educational interventions among tourist and school populations, respectively, (Bauer 2002; Uchôa et al. 2004). Bauer’s (2002) intervention involved the delivery of leaflets about preventive measures to tourists visiting a National Park; however, no significant differences were reported between the intervention and control groups. This contrasts with the results of our included studies. Uchôa et al.’s (2004) intervention, on the other hand, involved lectures, booklets, and other educational activities for students. However, no baseline measure was recorded in order to determine the effect of this intervention.
The exclusive focus on health education, more specifically KAP scores, evident across all the included studies in this review, is not surprising, as the KAP design is predominant in the CL literature, demonstrated by the innumerable published studies adopting such design. The attractiveness of KAP surveys may be attributed to their easy design, quantifiable data, ease of interpretation and presentation of results, generalisability of small sample results to a wider population, cross-cultural comparability, and rapid implementation (Launiala 2009). However, this highlights the narrow nature of community based KAP interventions in this research area. Whilst raising awareness and educating the public about CL is indeed indispensable, this only represents one part of the solution. As is widely recognised in public health, a myriad of social, cultural, financial and political factors influence the prevention and control of NTDs, which are overlooked in these community-based KAP interventions (Lassi et al. 2014; Launiala 2009). The assumption that a direct relationship exists between knowledge and action is rather simplistic (Hausmann-Muela et al. 2003). The adoption of preventive measures and health-seeking practices do not depend on knowledge alone (Launiala 2009). This is confirmed in Kavoosi and Shojaeizadeh’ (2016) study, whereby no correlation was found between mothers’ increased knowledge of CL and an increase in health-seeking behaviour. Moreover, Ghodsi et al. (2019) also draw attention to the lack of resources such as money for the study population to buy protective equipment against sand flies (e.g., bed nets and insect repellents), which ultimately would not allow individuals to fully apply what they have learned as part of the educational intervention in real life. Furthermore, since the effectiveness of these interventions was measured only via self-report questionnaires, it is not possible to know the extent to which improved knowledge, attitudes, and practice translated into applied and long-term impact. This is especially so since none of the studies included incidence and/or cure rates of CL as outcome measures.
Strengths and Limitations
We used a robust methodology following PRISMA guidelines and a comprehensive search strategy, with no geographical and language restrictions. Findings, therefore, reflect what has been published on this topic worldwide. Nonetheless, the major limitation of this review is that all included studies were conducted in Iran, with the exception of one undertaken in Brazil. This does not allow broad generalisation to be made to different epidemiological and cultural settings. In the preliminary stages of this review, we sought to address this limitation by broadening our eligibility criteria. However, this adjustment did not have the desired effect. Ultimately, we consider this geographical imbalance to be a valuable finding in and of itself, by highlighting an important evidence gap in CL-related research and public health interventions. An additional limitation of our systematic review concerns the low methodological quality of the majority of included studies. Publication bias whereby studies with negative results are less likely to be published may also have affected the overall results. We also identified a need to include further contextual details in future studies, particularly highlighting the facilitators and barriers in the implementation of such interventions. Addressing these limitations in future CL-related research is especially crucial in light of the significance that community-based interventions are given in the WHO Roadmap for NTDs (2021–2030) (WHO 2020).
5. Conclusions
Our review clearly shows a lack of research on the prevention and control of CL at a community level, confirming the neglected status of this disease. Designing more innovative community-based interventions, with a more comprehensive focus—such as addressing stigma, financial barriers, and healthcare access—is crucial. Whilst interventions reported in this review were demonstrated to have a positive impact on people’s levels of knowledge, attitudes, and practices regarding CL, adopting preventive measures and accessing treatment are also influenced by various structural factors, which may be especially pertinent in LMICs where this disease prevails. Individual behaviour is located within a wider socio-political context, including interpersonal relationships, neighbourhoods, organisations, and public policy (McLeroy et al. 2003). For the ‘community’ to be truly at the heart of these interventions, future research needs to look beyond the individual level in order to tackle the wider determinants of CL vulnerability. This systematic review highlights the urgent need for methodologically rigorous, high-quality studies on designing and implementing more effective community-based interventions using a holistic approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/socsci11100490/s1, Ovid MEDLINE(R) and In-Process & Other Non-Indexed Citations 1946 to 3 June 2020.
Author Contributions
In this article, K.P. and B.W. share first authorship and H.P. and L.D. share senior authorship. Conceptualization, K.P., H.P. and L.D.; methodology, K.P., A.R.-C., B.D. and J.P.; formal analysis, H.A.; writing—original draft preparation, K.P., B.W., S.G. and M.M.; writing—review and editing, K.P., B.W., H.P. and L.D.; supervision, H.P. and L.D. All authors have read and agreed to the published version of the manuscript.
Funding
Authors K.P., B.W., S.G., H.A., M.M., H.P. and L.D. are funded through the ECLIPSE programme. The ECLIPSE programme is funded by the National Institute for Health and Care Research (NIHR) (NIHR200135) using UK aid from the UK Government to support global health research. The views expressed in this article are those of the authors and not necessarily those of the NIHR or the UK Department of Health and Social Care.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analysed in this review are included in this article and its supplementary information.
Acknowledgments
The authors would like to thank the NIHR for supporting the work of the ECLIPSE program, including the creation of this systematic literature review.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Al-Salem, Waleed S., David M. Pigott, Krishanthi Subramaniam, Lee Rafuse Haines, Louise Kelly-Hope, David H. Molyneux, Simon I. Hay, and Alvaro Acosta-Serrano. 2016. Cutaneous leishmaniasis and conflict in Syria. Emerging Infectious Diseases 22: 931. [Google Scholar] [CrossRef] [PubMed]
- Alvar, Jorge, Iván D. Vélez, Caryn Bern, Mercé Herrero, Philippe Desjeux, Jorge Cano, Jean Jannin, Margriet den Boer, and WHO Leishmaniasis Control Team. 2012. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7: e35671. [Google Scholar] [CrossRef] [PubMed]
- Alvar, Jorge, Sergio Yactayo, and Caryn Bern. 2006. Leishmaniasis and poverty. Trends in Parasitology 22: 552–57. [Google Scholar] [CrossRef] [PubMed]
- Baghianimoghadam, Mohammed, Banafsheh Tavakoli, Jamshid Ayatollahi, and Masoud Mirzaei. 2016. The effect of education based on the theory of planned behavior on preventive behaviors of cutaneous Leishmaniasis in mothers living in endemic city of Natanz. Tolooebehdasht 15: 54–56. Available online: http://tbj.ssu.ac.ir/article-1-2271-en.html (accessed on 12 July 2019).
- Bailey, Freddie, Karina Mondragon-Shem, Lee Rafuse Haines, Amina Olabi, Ahmed Alorfi, Jose Antonio Ruiz-Postigo, Jorge Alvar, Peter Hotez, Emily R Adams, Iván D Vélez, and et al. 2019. Cutaneous leishmaniasis and co-morbid major depressive disorder: A systematic review with burden estimates. PLoS Neglected Tropical Diseases 13: e0007092. [Google Scholar] [CrossRef]
- Bailey, Freddie, Karina Mondragon-Shem, Peter Hotez, Jose Antonio Ruiz-Postigo, Waleed Al-Salem, Alvaro Acosta-Serrano, and David H. Molyneux. 2017. A new perspective on cutaneous leishmaniasis—Implications for global prevalence and burden of disease estimates. PLoS Neglected Tropical Diseases 11: 30005739. [Google Scholar] [CrossRef]
- Bauer, Irmgard L. 2002. Knowledge and behavior of tourists to Manu National Park, Peru, in relation to Leishmaniasis. Journal of Travel Medicine 9: 173–79. [Google Scholar] [CrossRef]
- Becker, Marshall H. 1974. The health belief model and sick role behavior. Health Education Monographs 2: 409–19. [Google Scholar] [CrossRef]
- Bennis, Issam, Vincent De Brouwere, Zakaria Belrhiti, Hamid Sahibi, and Marleen Boelaert. 2018. Psychosocial burden of localised cutaneous Leishmaniasis: A scoping review. BMC Public Health 18: 358. [Google Scholar] [CrossRef]
- CASP (Critical Appraisal Skills Programme). 2018. CASP Qualitative Checklist. Available online: https://casp-uk.net/wp-content/uploads/2018/01/CASP-Qualitative-Checklist-2018.pdf (accessed on 30 November 2019).
- Conner, Mark. 2020. Theory of planned behavior. In Handbook of Sport Psychology. San Francisco: John Wiley & Sons Inc., pp. 1–8. [Google Scholar] [CrossRef]
- Crosby, Richard, and Seth M. Noar. 2011. What is a planning model? An introduction to PRECEDE-PROCEED. Journal of Public Health Dentistry 71: S7–S15. [Google Scholar] [CrossRef]
- de Vries, Henry J. C., Sophia H. Reedijk, and Henk DFH Schallig. 2015. Cutaneous leishmaniasis: Recent developments in diagnosis and management. American Journal of Clinical Dermatology 16: 99–109. [Google Scholar] [CrossRef] [PubMed]
- Dehghani-Tafti, Abasali, Arefeh Dehghani-Tafti, Mohammad Zobeydi, and Syed Abed Tofighyan. 2017. The Effect of Education Based on the Pattern of Behaviors in Promoting Preventive Behaviors of Cutaneous Leishmaniasis in Between Parent Families Living in the City Rāmhormoz in 2015. Tolooebehdasht 16: 62–74. Available online: http://tbj.ssu.ac.ir/article-1-2075-en.html (accessed on 13 July 2019).
- Dehghani-Tafti, Mohammad Hussain, Hussain Forghani, Mohammad Hussain Baghiani Moghadam, Parisa Khani, Mohammad Taghi Noorbala, and Saman Mohammadi. 2011. A survey on effect of health education on health volunteer performance and knowledge in prevention of cutaneous leishmaniasis in Yazd. Journal of Pakistan Association of Dermatologists 21: 27–32. [Google Scholar]
- Du, Rebecca, Peter J. Hotez, Waleed S. Al-Salem, and Alvaro Acosta-Serrano. 2016. Old world cutaneous leishmaniasis and refugee crises in the Middle East and North Africa. PLoS Neglected Tropical Diseases 10: e0004545. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, Musalreza, Mina Maheri, Hamid Joveini, Mohammad Hassan Rakhshani, and Ali Mehri. 2019. Designing and evaluating educational intervention to improve preventive behavior against Cutaneous Leishmaniasis in endemic areas in Iran. Osong Public Health and Research Perspectives 10: 253. [Google Scholar] [CrossRef]
- González, Urbà, Mariona Pinart, David Sinclair, Alireza Firooz, Claes Enk, Ivan D. Vélez, Tonya M. Esterhuizen, Mario Tristan, and Jorge Alvar. 2015. Vector and reservoir control for preventing leishmaniasis. Cochrane Database of Systematic Reviews 8. [Google Scholar] [CrossRef]
- Guttmacher, Sally, Patricia Kelly Vana, and Yumary Ruiz-Janecko. 2010. Community-Based Health Interventions. San Francisco: John Wiley & Sons. [Google Scholar]
- Hausmann-Muela, Susanna, Joan Muela Ribera, and Isaac Nyamongo. 2003. Health-Seeking Behaviour and the Health System’s Response. DCPP Working Paper no. 14. Available online: http://www.dcp2.org/file/29/wp14.pdf (accessed on 15 July 2019).
- Hayani, Kinan, Anwar Dandashli, and Elke Weisshaar. 2015. Cutaneous leishmaniasis in Syria: Clinical features, current status and the effects of war. Acta Dermato-Venereologica 95: 62–66. [Google Scholar] [CrossRef]
- Hazavehei, Mohammad Mahdi, Heshmati Hashem, Hasanzadeh Akbar, Pourmazar S Alireza, and Maghsoodlou Dorsa. 2014. The effect of volunteer health workers educational program on the basis of BASNEF model on promotion of their practices about cutaneous leishmaniasis. Zahedan Journal of Research in Medical Sciences 16: 16–21. Available online: https://sites.kowsarpub.com/zjrms/articles/1563.html (accessed on 13 July 2019).
- Hejazi, Seyed Hossein, Seyyed Morteza Hazavei, Leila Shirani Bidabadi, Ali Shademani, Amir Hossein Siadat, Azadeh Zolfaghari-Baghbaderani, Mohammed Ali Nilforoushzadeh, and Sayed Mohsen Hosseini. 2010. Evaluation of knowledge, attitude and performance of the mothers of children affected by cutaneous leishmaniasis. Infectious Diseases: Research and Treatment 3: IDRT-S3786. [Google Scholar] [CrossRef]
- Hubley, John. 1988. Understanding behaviour: The key to successful health education. Tropical Doctor 18: 134–38. [Google Scholar] [CrossRef]
- Isaza, Diana María, Berta Nelly Restrepo, Margarita Arboleda, Eudoro Casas, Herminio Hinestroza, and Tufik Yurgaqui. 1999. La leishmaniasis: Conocimientos y prácticas en poblaciones de la costa del Pacífico de Colombia. Revista Panamericana de Salud Pública 6: 177–84. Available online: http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S1020-49891999000800005&lng=pt&nrm=iso (accessed on 25 July 2019). [CrossRef]
- Jeihooni, Ali Khani, Pooyan Afzali Harsini, Seyyed Mansour Kashfi, and Tayebe Rakhshani. 2019. Effect of educational intervention based on the PRECEDE-PROCEED model on preventive behaviors of cutaneous leishmaniasis among housewives. Cadernos de Saúde Pública 35: e00158818. [Google Scholar] [CrossRef] [PubMed]
- Karimkhani, Chante, Valentine Wanga, Luc E. Coffeng, Paria Naghavi, Robert P. Dellavalle, and Mohsen Naghavi. 2016. Global burden of cutaneous leishmaniasis: A cross-sectional analysis from the Global Burden of Disease Study 2013. The Lancet Infectious Diseases 16: 584–91. [Google Scholar] [CrossRef]
- Kashfi, Seyyed Mansour, Ali Khani Jeihooni, and Abbas Rezaeianzade. 2012. Effect of health workers’ training programs on preventive behavior of leishmaniosis based on BASNEF model. Journal of Research in Health Sciences 12: 114–18. [Google Scholar] [PubMed]
- Kavoosi, Fatemeh, and Davoud Shojaeizadeh. 2016. Effect of the educational interventions on mother’s knowledge and attitude towards cutaneous leishmaniasis in Mashhad. Dermatology and Cosmetics 6: 209–20. Available online: https://www.sid.ir/en/journal/ViewPaper.aspx?ID=532582 (accessed on 12 July 2019).
- Lassi, Zohra S., Rehana A. Salam, Jai K. Das, and Zulfiqar A. Bhutta. 2014. The conceptual framework and assessment methodology for the systematic reviews of community-based interventions for the prevention and control of infectious diseases of poverty. Infectious Diseases of Poverty 3: 22. [Google Scholar] [CrossRef]
- Launiala, Annika. 2009. How much can a KAP survey tell us about people’s knowledge, attitudes and practices? Some observations from medical anthropology research on malaria in pregnancy in Malawi. Anthropology. Matters 11. [Google Scholar] [CrossRef]
- McLeroy, Kenneth R., Barbara L. Norton, Michelle C. Kegler, James N. Burdine, and Ciro V. Sumaya. 2003. Community-based interventions. American Journal of Public Health 93: 529–33. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, Neda, Seyed Hossein Hejazi, Seyed Mohammad Mahdi Hazavahei, Ahmad Reza Zamani, Sedigheh Saberi, and Ea Rahimi. 2010. Effect of Education Based on Health Belief Model on Promoting Preventive Behavior of Cutaneous Leishmaniasis. Available online: www.sid.ir/en/journal/ViewPaper.aspx?id=173596 (accessed on 13 July 2019).
- Nazari, Mahin, Goli Taravatmanesh, Mohammad Hossein Kaveh, Abouzar Soltani, and Haleh Ghaem. 2016. The Effect of Educational Intervention on Preventive Behaviors towards Cutaneous Leishmaniasis at Kharameh City in 2014. Shiraz E-Medical Journal 17: e39957. [Google Scholar] [CrossRef]
- NIH (National Institute of Health). 2021. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 29 November 2019).
- Page, Matthew J., Joanne E. McKenzie, Patrick M. Bossuyt, Isabelle Boutron, Tammy C. Hoffmann, Cynthia D. Mulrow, and Larissa Shamseer. 2021. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Systematic Reviews 10: 1–11. [Google Scholar] [CrossRef]
- Pérez, Isabel, Erick Durán, Freddy Pérez, Mei L. Trueba, and Renata Mendizábal-Cabrera. 2020. Strengthening Cutaneous Leishmaniasis control in Guatemala: Policy Recommendations. PRISMA Statement. Available online: http://www.prisma-statement.org/PRISMAStatement/PRISMAStatement (accessed on 5 September 2019).
- Ramdas, Sahienshadebie. 2012. Cruel disease, cruel medicine: Self-treatment of cutaneous leishmaniasis with harmful chemical substances in Suriname. Social Science & Medicine 75: 1097–105. [Google Scholar] [CrossRef][Green Version]
- Reis, Dener Carlos dos, Andréa Gazzinelli, Carolina Angélica de Brito Silva, and Maria Flávia Gazzinelli. 2006. Health education and social representation: An experience with the control of tegumentary leishmaniasis in an endemic area in Minas Gerais, Brazil. Cadernos de Saude Publica 22: 2301–10. [Google Scholar] [CrossRef]
- Reveiz, Ludovic, Ana Nilce Silveira Maia-Elkhoury, Rubén Santiago Nicholls, Gustavo Adolfo Sierra Romero, and Zaida E. Yadon. 2013. Interventions for American cutaneous and mucocutaneous leishmaniasis: A systematic review update. PLoS ONE 8: e61843. [Google Scholar] [CrossRef] [PubMed]
- Saberi, Sedigheh, Ahmadreza Zamani, Neda Motamedi, Mohammad Ail Nilforoushzadeh, Fariba Jaffary, Ezatollah Rahimi, and Seyed Hossein Hejazi. 2012. The knowledge, attitude, and prevention practices of students regarding cutaneous leishmaniasis in the hyperendemic region of the Shahid Babaie Airbase. Vector-Borne and Zoonotic Diseases 12: 306–9. [Google Scholar] [CrossRef] [PubMed]
- Saghafipour, Abedin, Jalil Nejati, Ehssan Mozaffari, Fatemeh Rezaei, Zabihollah Gharlipour, and Mahdi Mirheydari. 2017. The effectiveness of education based on BASNEF model on promoting preventive behavior of cutaneous leishmaniasis in students. International Journal of Pediatrics 5: 5125–36. [Google Scholar] [CrossRef]
- Santos, Dilvani O., Carlos E. R. Coutinho, Maria F. Madeira, Carolina G. Bottino, Rodrigo T. Vieira, Samara B. Nascimento, and Alice Bernardino. 2008. Leishmaniasis treatment—A challenge that remains: A review. Parasitology Research 103: 1–10. [Google Scholar] [CrossRef] [PubMed]
- Souza, Claudia Teresa Vieira de, Carlos Augusto Ferreira de Andrade, Dinair Leal da Hora, Eloisa Leal da Hora, Idanir Antônio Momesso Neto, Michelle Campos de Matos, Maria de Fátima Moreira Martins, and Sandro Javier Bedoya-Pacheco. 2011. Health education in South America regarding leishmaniasis: A systematic review. Revista da Patologia Tropical 44: 111–23. [Google Scholar] [CrossRef][Green Version]
- Uchôa, Claudia Maria Antunes, Cathia Maria Barrientos Serra, Ciléia de Melo Magalhães, Roger Magno Macedo da Silva, Letícia Pinto Figliuolo, Cristianni Antunes Leal, and Maria de Fátima Madeira. 2004. Educação em saúde: Ensinando sobre a leishmaniose tegumentar americana. Cadernos de Saúde pública 20: 935–41. [Google Scholar] [CrossRef][Green Version]
- Wenning, Brianne, Helen Price, Hasara Nuwangi, Kelemework Tafere Reda, Ben Walters, Reem Ehsanullah, Greice Viana, Alina Andras, and Lisa Dikomitis. 2022. Exploring the cultural effects of gender on perceptions of cutaneous leishmaniasis: A systematic literature review. Global Health Research and Policy 7: 34. [Google Scholar] [CrossRef]
- WHO (World Health Organization). 2018. Global Leishmaniasis Surveillance, 2017–2018. Available online: https://www.who.int/publications/i/item/who-wer9525 (accessed on 6 January 2020).
- WHO (World Health Organization). 2020. Ending the Neglect to Attain the Sustainable Development Goals. A Road Map for Neglected Tropical Diseases 2021–2030. Available online: https://www.who.int/neglected_diseases/Ending-the-neglect-to-attain-the-SDGs--NTD-Roadmap.pdf (accessed on 6 January 2020).
- Yanik, Medaim, Mehmet Salih Gurel, Zeki Simsek, and Mahmut Kati. 2004. The psychological impact of cutaneous leishmaniasis. Clinical and Experimental Dermatology: Clinical Dermatology 29: 464–67. [Google Scholar] [CrossRef]
- Zeinali, Mohammad, Mehdi Mohebali, Mahmoud Mahmoudi, Gholam Reza Hassanpour, and Mohammad Reza Shirzadi. 2019. Study on Knowledge, Attitude and Practice of Health Workers of East Azerbaijan, Ilam and Khorasan Razavi Provinces about Leishmaniasis During 2015–2016: A Comparative Study Before and After Intervention. Archives of Clinical Infectious Diseases 4: e64282. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).