The Destructive/Non-Destructive Identification of Enameled Pottery, Glass Artifacts and Associated Pigments—A Brief Overview
Abstract
1. Context
2. Pottery and Glass Technology: A Brief Survey
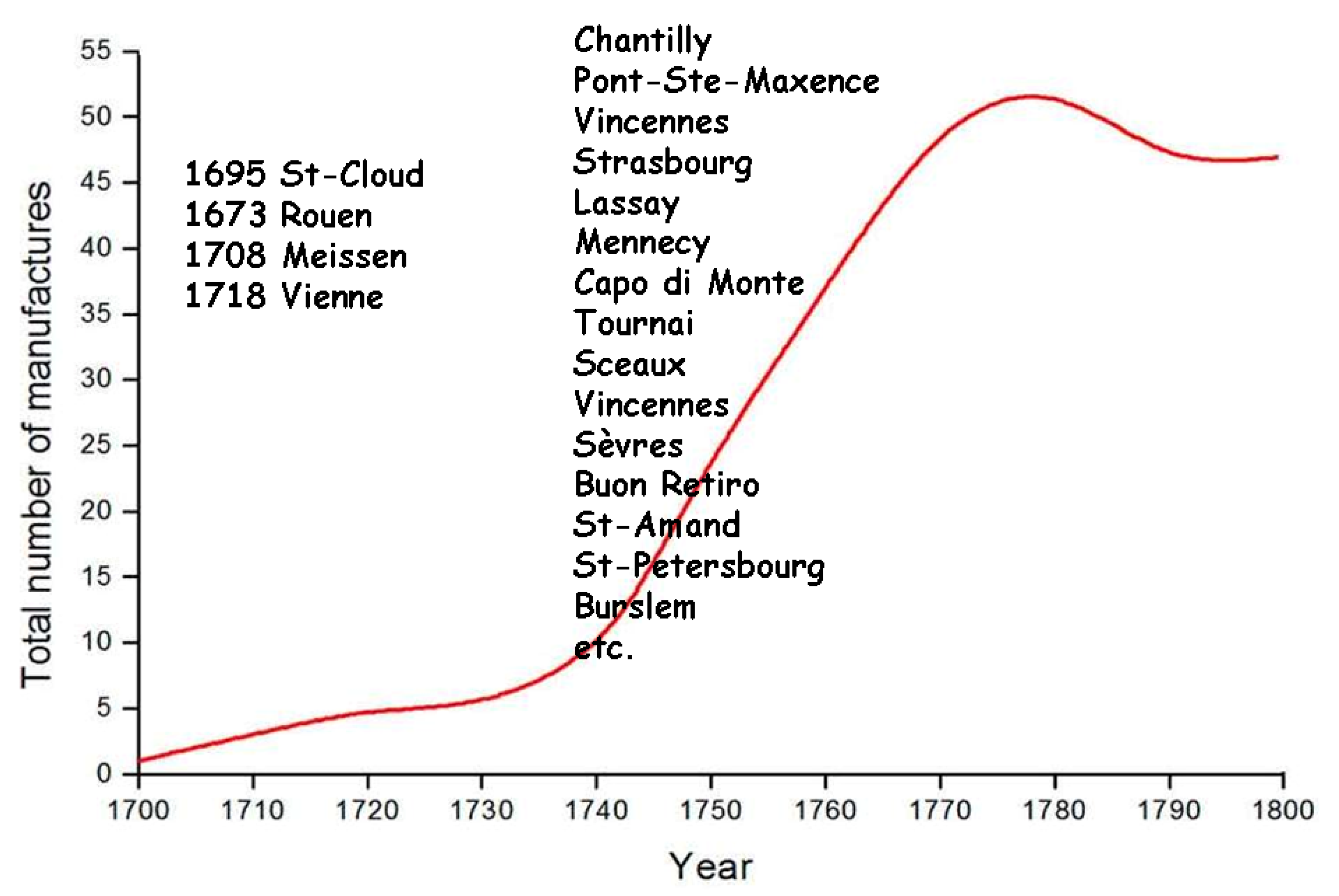
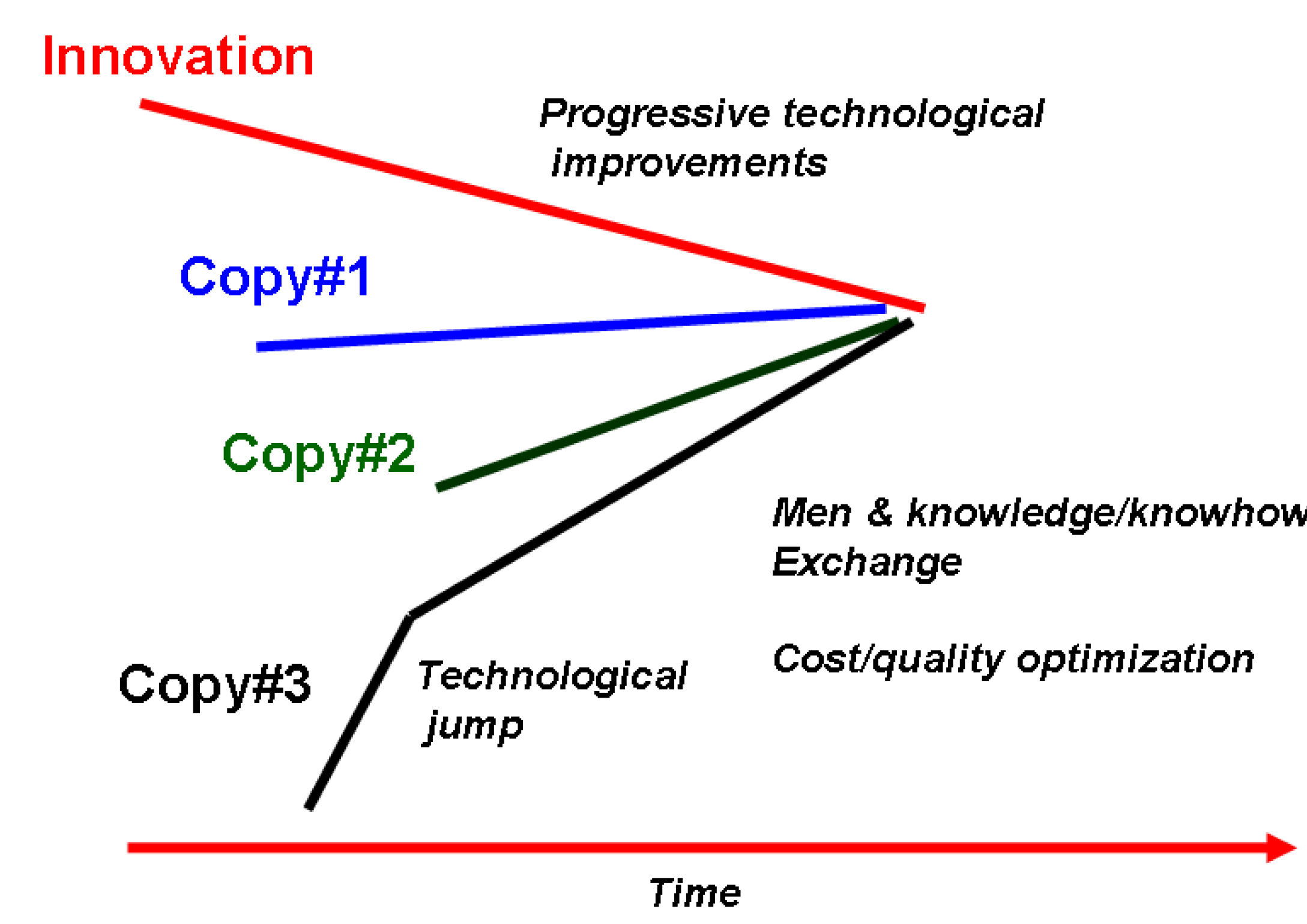
3. The Development of Solid State Chemistry and Related Technologies Offers Chronological/Technological Milestones
4. The Analytical Techniques of Artworks: Toward Non-Destructive Procedures
5. The Development of Mobile/Remote Raman Microspectroscopy

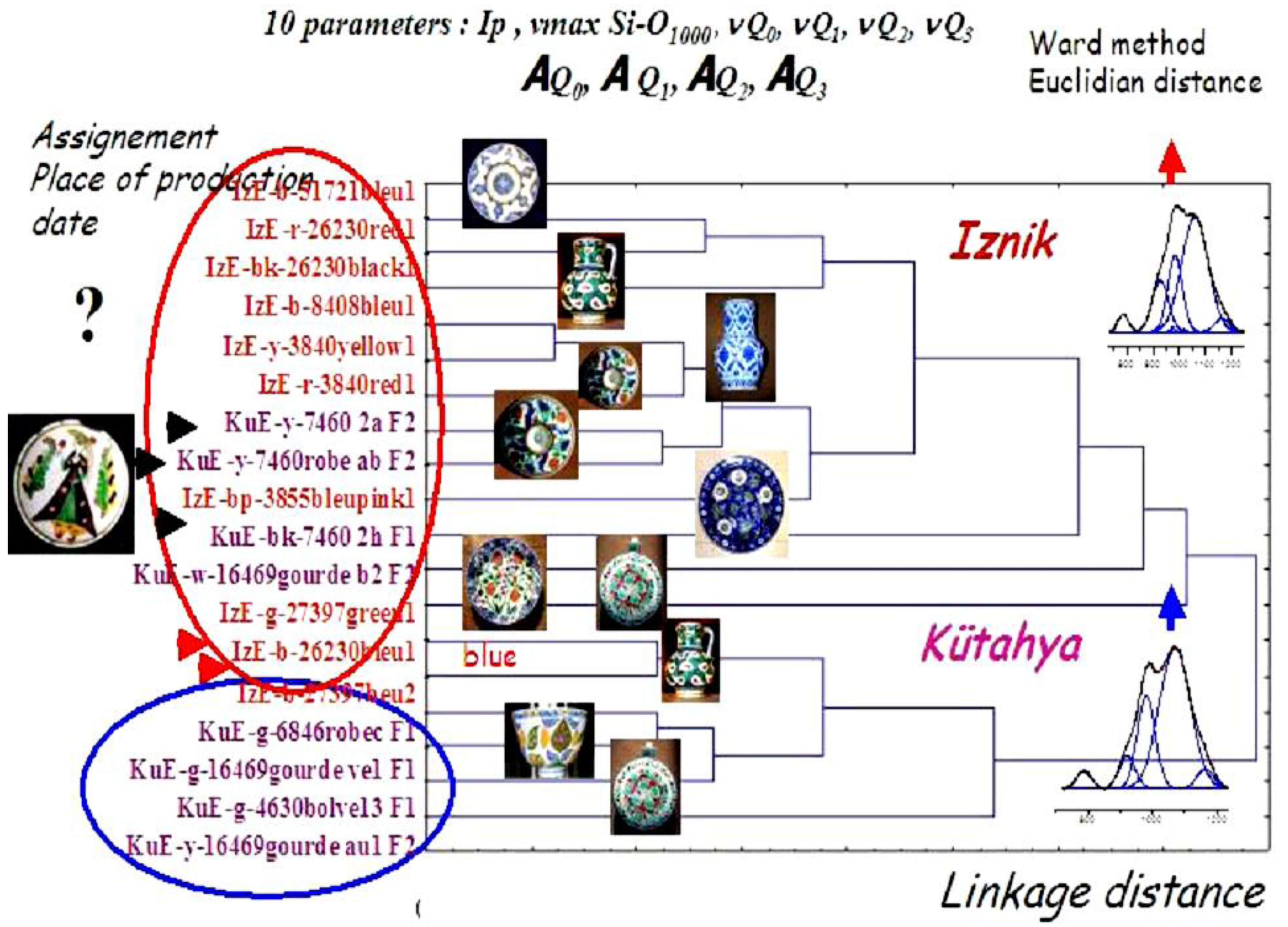
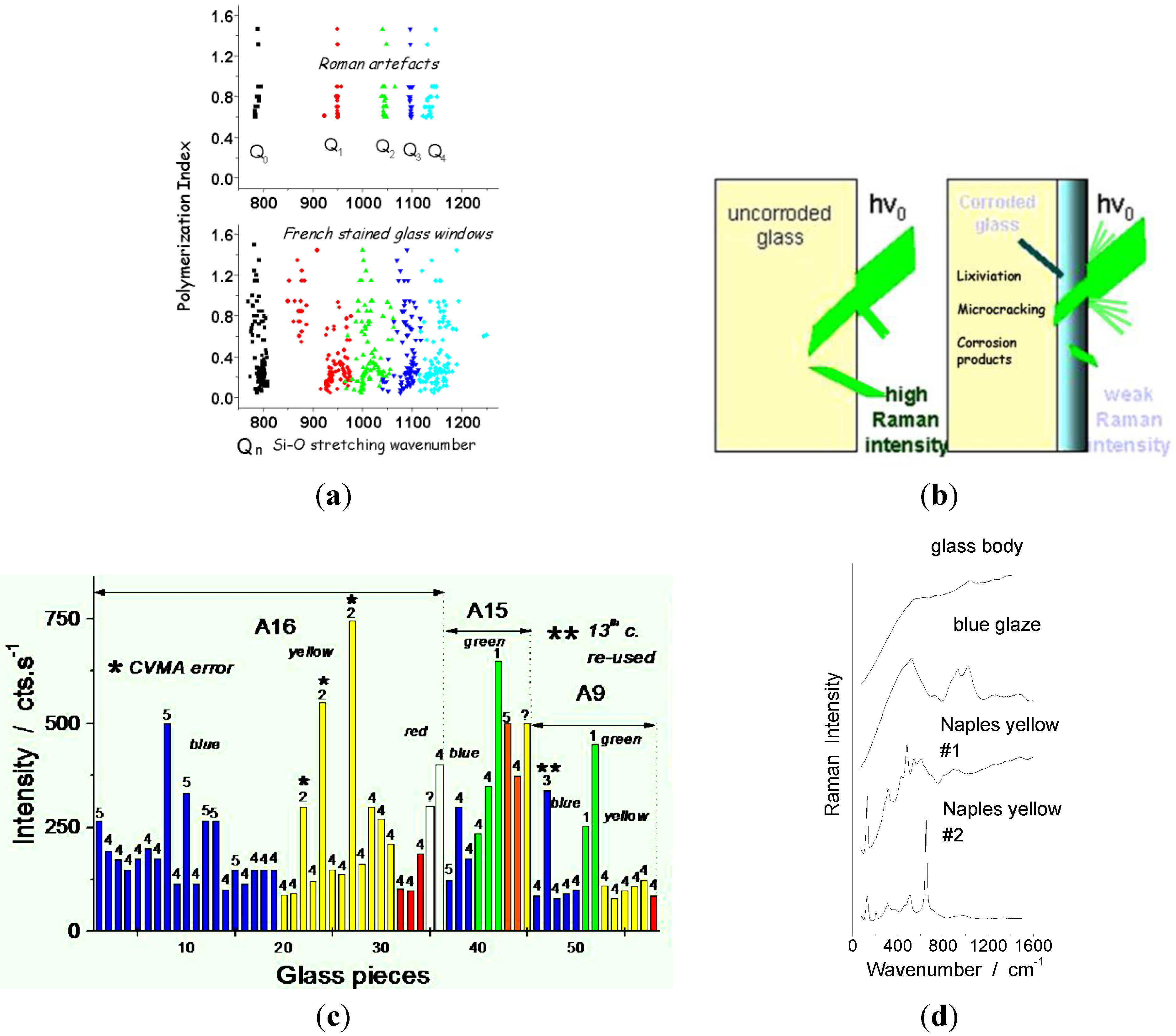
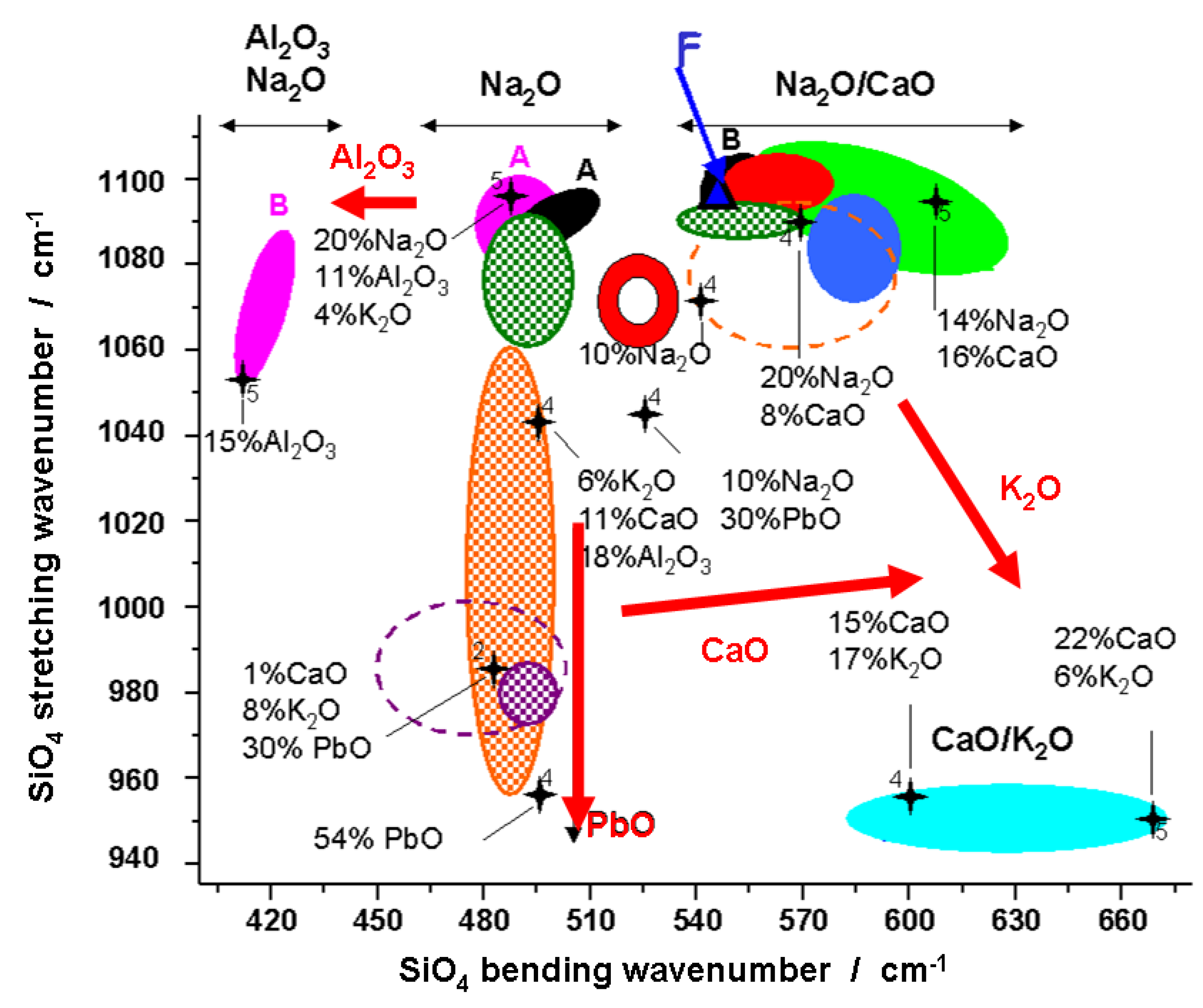
6. Case Studies: White, Blue, Yellow, Green, Red, and Black Pigments and Coloring Agents
6.1. White
| Color | Composition | Date |
|---|---|---|
| Bone white | Ca2(PO4)2 | Antiquity |
| Chalk (calcite) | CaCO3 | Antiquity |
| Cerussite | PbCO3 | Antiquity |
| Gypsum | CaSO4 2H2O | Antiquity |
| Bassanite | CaSO4 1/2H2O | ? |
| Anhydrite | CaSO4 | Antiquity |
| Antimoniate | CaSb2O7, CaSb2O6 | Antiquity |
| Kaolin | Al2O3, 2SiO2, 2H2O | Antiquity |
| Lead white ceruse | Pb(OH)2 | 500 BC |
| Cassiterite | SnO2 | 9th |
| Fluorite | CaF2 | 14th? |
| Quartz | SiO2 | 16th. |
| Arsenates | (Ca,Pb)1.5AsO4 | 17th |
| Zinc white | ZnO | 1781 (1834) |
| Lithopone | ZnS - BaSO4 | 1874 (Western) |
| Titanium white | TiO2 anatase TiO2 rutile | 1916 (1923) |
| 19th? | ||
| Freeman’ white | PbSO4 | 19th |
| Hamburg white | PbCO3, BaSO4 | 19th |
| Barium white | BaSO4 | 19th (Western) |
| Zirconia | ZrO2 | 20th |
| Zircon | ZrSiO4 | 20th |
6.2. Blue
| Color | Hue | Composition | Date |
|---|---|---|---|
| Egyptian blue | Light blue | CaCuSi4O10 | 3000 BC |
| Turquoise | Cu2+ in alkaline glass | 2000 BC | |
| Han blue | BaCuSi4O10 | 200–500 BC | |
| Han violet | BaCuSi2O6 | 200 BC | |
| Smalt | Light blue | CoO nSiO2; Co-containing glass | Antiquity |
| Lapis Lazuli (Lazurite) | ultramarine | Na8[Al6Si6O24]Sn | Roman |
| Cobalt,-manganese-iron ores | dissolved | 9th | |
| Indigo | C16H10N2O2 indigotin | <10th? | |
| Prussian blue | Fe4[Fe(CN)6]3 14–16H2O | 1704 | |
| Cobalt blue | Dark blue | CoSiO2 olivine | 18th |
| Cobalt blue | Dark blue | CoAl2O4 spinel | 1775 (1802) |
| Cerulean | Dark blue | Co2SnO4 spinel | 1810 (1821) |
| Cobalt chromite | Co(Al,Cr)2O4 spinel | ||
| Synthetic ultramarine | 1826 | ||
| Cobalt spinel | (Co,Zn)Al2O4 spinel CoAl2O4/Co2SnO4 spinel | 19th | |
| Spinel aluminate blue | Blue | Al2O3; 0,5 ZnO; 0,5 CoO | ~1900 |
| Turquoise blue | Al2O3; 0,2–0,4 CoO; 0,6–0,8 ZnO | ||
| Dark blue | Al2O3, 2 (PO4)2Co3 ou 2 (AsO4)3Co3 | ||
| Violet | Al2O3; 0,5 CoO; 0,5–1 MgO | ||
| Blue grey | Al2O3; 0,6–0,8 CoO; 0,4–1,2 NiO | ||
| Dark blue green | Al2O3; CoO; Cr2O3 | ||
| Light blue green | Al2O3; CrO4; CoO; ZnO | ||
| Zircon blue | Light blue | ZrO2, SiO2 + V2O5 (<1%) | ~1950 |
| Turquoise | + NaCl or NaF | ||
| Light blue | + Na2CO3 | ||
| Cobalt oxide | Dark blue | Co3O4 | |
| Zincochromite | Dark blue | ZnCr2O4 | |
| Cobalt zinc oxide | Light blue | ZnCo2O4 | |
| Posnjakite | CuSO4 3Cu(OH)2 H2O | ||
| Cyprium | Na3Ca(Al3Si3O12) | ||
| Vivianite | Fe3 (PO4)2 8H2 O | ||
| Phtalocyanine blue | C32H16CuN8 | 20th | |
| Manganese blue | BaMnO4 | 20th |
6.3. Yellow
| Color | Composition | Date |
|---|---|---|
| Ochre | FeO(OH) goethite | Neolithic |
| Massicot | PbO | Antiquity |
| Orpiment & pararealgar | As2S3 - As4S4 | Antiquity? |
| Saffron | C20H24O4 crocetin | Antiquity |
| Ocre | Fe2O3 H2O + clays + SiO2 | Neolithic |
| Naples yellow Lead-antimony yellow | Pb2Sb2O7 pyrochlore | 1500 BC |
| Palmatine | [C21H18NO4]+ X− | Antiquity |
| Naples yellow Lead-tin yellow type I | Pb2SnO4 | Antiquity |
| Berberine | [C20H18NO4]+ | Antiquity |
| Silver yellow | Ag nanoparticules in glass matrix | 12th |
| Or mussif | SnS2 | 13th. |
| Indian yellow | MgC19H16O11 5H2O | 15th. |
| Naples yellow Lead-tin yellow type II | PbSn xSixO3 | 15th? |
| Strontium yellow | SrCrO4 | 1800 |
| Barium yellow | BaCrO4 | 19th. |
| Cadmium yellow | CdS (+CdSe) | 1829 (1845) |
| Chrome yellow/orange | PbCrO4 (+PbO) | 1809 (1820) |
| Aureolin Cambodia yellow | K3[CO(NO2)6] nH2O C38H44O8 et C29H36O6 | 1861 |
| Rutile yellow | (Ti,Ni,Nb)O2 rutile | 20th |
| Cassiterite yellow | (Sn,V)O2 cassiterite | 20th |
| Primrose yellow | 2Ni,3BaO,17TiO2 priderite | 20th |
| Zinc yellow | ZnCrO4 | 1809 (1850) |
| Curcuma | C21H20O6 | |
| Uranium yellow | PbUO4 | 19th |
| Stibine | Sb2O5 | |
| Malayite yellow | CaSnSiO5 sphene malayite | 20th |
| Praseodymium yellow | 20th |
6.4. Green
| Color | Composition | Date |
|---|---|---|
| Malachite | CuCO3 Cu(OH)2 | Antiquity |
| Atacamite | CuCl2 3Cu(OH)2 | Antiquity |
| Green earth | K[(Al3+, Fe3+)(Fe2+, Mg2+)],(AlSi3,Si4)O10(OH)2 | Antiquity |
| Vert-de-gris | Cu(CH3COO)2 | Middle age |
| [Cu(CH3COO)2]2 Cu(OH)2 5H2O | ||
| Cu(CH3COO)2 Cu(OH)2 | ||
| Green | Cr in glass | Ottoman |
| Scheele green | Cu(AsO2)2 | 1778 |
| Cobalt green | CoO nZnO | 1780, >1830 |
| Dark green | Cr2O3 haematite | 1800 |
| Emerald green | Cu[C2H3O2] 3Cu[AsO2]2 | 1814 |
| Viridian | Cr2O3 2H2O | 1838 (1859) |
| Victoria green | 3CaO, Cr2O3, 3SiO2 | 19th |
| Yellow green: + CaCl2 & CaF2 | ||
| Dark green: + CaF2 | ||
| Middle green: + LiF | ||
| Chromium Green | Light green: ZrO2, SiO2, Na2WO4, K2Cr2O7, NaCl Olive: ZrO2, SiO2, Na2WO4, NaCl | |
| Nickel green | Ni2SiO4 olivine | |
| Chromite green | CoCr2O4 spinel | |
| Cobalt green | Co2TiO4 spinel | |
| Yellow + blue | Yellow pigments in blue matrix | 18th |
6.5. Red
| Color | Composition | Date |
|---|---|---|
| Copper red | Co metal nanoparticles in glass matrix | Neolithic |
| Vermillion | HgS | Neolithic |
| Ochre & Earths | Fe2O3 + clay + SiO2 | Neolithic |
| Haematite | α-Fe2O3 | Neolithic |
| Purpurin | C14H18O5 | 3000 BC |
| Tyr purple | C16H10Br2N2O2 | 1400 BC |
| Litharge | PbO | Antiquity |
| Minium | Pb3O4 | Antiquity |
| Kermes | C16H10O8 | Antiquity |
| Realgar | As4S4 | Antiquity |
| Haematite/hercynite | Fe2O3+ SiO2 | Antiquity |
| Umbria earth | Fe2O3, MnO2 + clay + SiO2 | |
| Cassius’ purple Kinkel’ red | Au metal nanoparticles in glass matrix | Roman? |
| Madder | C14H8O4 | Middle-age |
| Carmine | C14H8O4 (alizarin) + purpurin | 16th. |
| Armenian Bole | Fe2O3 + clay + SiO2 | 14th |
| Thiviers sandstone | Fe2O3+ SiO2 | 17th |
| Mars red | Fe2O3 synthetic, nanosized | <18th. |
| Mendipite | Pb3O2Cl2 | |
| Cadmium red | CdS (+ CdSe) | 1907 |
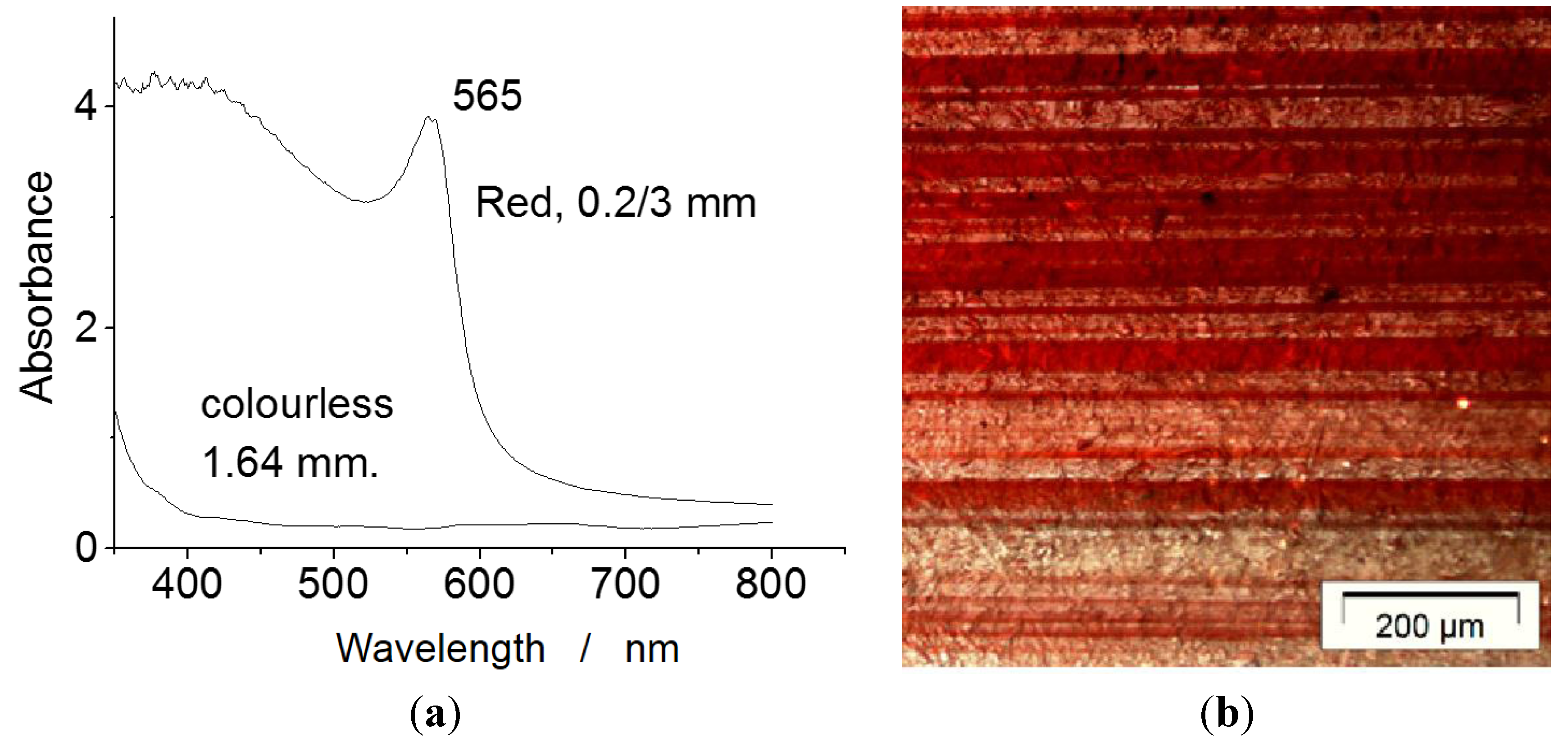
6.6. Black
| Color | Composition | Date |
|---|---|---|
| Carbon black | C | Neolithic |
| Earths | MnO2, clays, Fe(OH)3 | Neolithic |
| Bismuth black | Bi | >15th |
| Spinel black | Cr2MnO4 spinel | 15th |
| Tin-massicot | Pb2SnO4 | |
| Copper black | CuO | |
| Cobalt black | CoO |
7. Lustre, Another Way to Master the Color
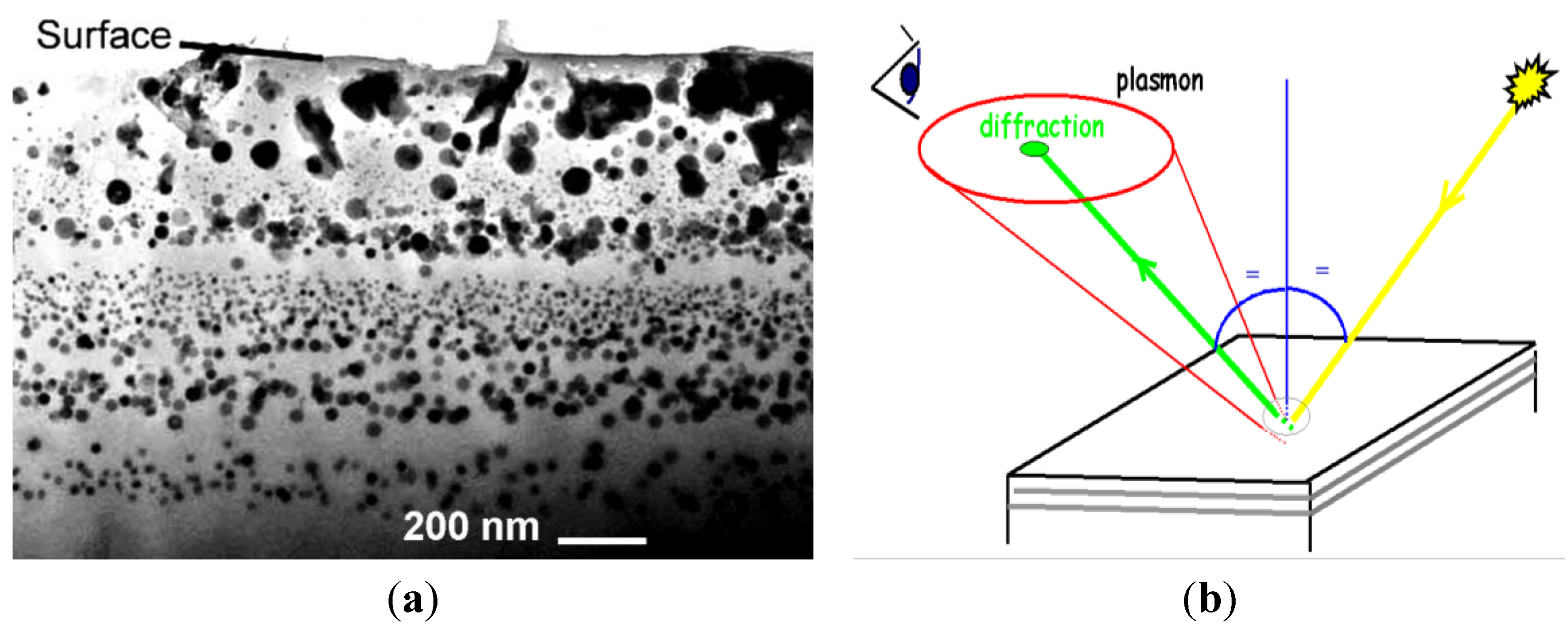
8. Conclusion: Master’s Secrets as Chronological/Technological Milestones
| Artifact | Period | Technology | Ref. |
|---|---|---|---|
| Begram glass Treasure | 1st century AD Roman Empire | Lapis lazuli | [198] |
| Ptolemaic faience pottery | 1st century AD Roman Empire | Lapis lazuli | [213] |
| Lübsov glass beaker | 2nd century AD Roman Empire | Lapis lazuli as blue & calcium antimonate as white pigments | [95] |
| Abbasid pottery | 9th c. | Cassiterite opacifier; Lustre | [57] |
| Lâjvadina pottery, Iran | 13th c. | Lapis lazuli and cobalt as blue glaze pigments | [189,190] |
| Enameled glass and Pottery, Frederic II Souabian Court, Apuglia | 13th–14th c. | Lapis lazuli as blue & calcium phosphate as white pigments | [195] |
| Mamluk mosque lamps | 13th–14th c. | Lapis lazuli as blue & calcium phosphate as white glaze pigments | [113,198] |
| Medici porcelain Florence | 1575–1587 | Calcium phosphate | [65] |
| Böttger porcelain Meissen | 1700–1708 | Lapis lazuli grains at the body-glaze interface. Bubble as opacifier. | [179] |
References and Notes
- T.A. Rickard. L’Homme et les Métaux. Paris, France: Editions Gallimard, 1938. [Google Scholar]
- R.J. Forbes. “Metallurgy.” In A History of Technology. Edited by C.H. Singer, E.J. Holmyard, A.R. Hall and T.J. Williams. Oxford, UK: Oxford University Press, 1975, Volume 1. [Google Scholar]
- B. Gilles. Histoire des Techniques. Paris, France: Encyclopédie de la Pléiade, Editions Gallimard, 1978. [Google Scholar]
- J. Rosmoduc. Une histoire de la Physique et de la Chimie. Paris, France: Editions du Seuil -Points-Sciences, 1985. [Google Scholar]
- B. Bensaude-Vincent, and I. Stengers. Histoire de la Chimie. Paris, France: La Découverte, 1992. [Google Scholar]
- A. Djebbar. Une histoire de la Science arabe – Entretiens avec J. Paris, France: Rosmorduc, Editions du Seuil - Points-Sciences, 2001. [Google Scholar]
- R. Rashed. Histoire des Sciences Arabes. Technologie, alchimie et sciences; Paris, France: Editions du Seuil, 1997, Volume 3. [Google Scholar]
- P. Lory. Jàbir ibn Hayyàan, Dix traités d’alchimie. Les dix premiers traités du livre des Soixantes-dix (Textes traduits et présentés). Paris, France: Sindbad, 1983. [Google Scholar]
- J. Gimpel. La révolution industrielle du Moyen Age. Paris, France: Editions du Seuil - Points-Histoire, 1975. [Google Scholar]
- D. Cosandey. Le Secret de l’Occident – Vers une Théorie Générale du Progrès Scientifique. Paris, France: Champs-Flammarion, 2007. [Google Scholar]
- Herodote. Historia (~450 BC, Athènes). translated and edited by Larcher and Charpentier: Paris, France; 1850. [Google Scholar]
- Theophraste. Recherches sur les Plantes—Le Livre des Pierres (Athènes, 300 B.C.). Paris, France: Les Belles Lettres, 2006, tome V. [Google Scholar]
- P. Dioscoride. De Materia Medica (Rome, 60). Paris, France: Henri Estienne, 1516. [Google Scholar]
- “Pline.” In Naturalis Historia. Vol XII-XXXVII (Rome, 77); Paris, France: French translation, Veuve Desaint, 1771.
- Strabon. Geôgraphiká (Rome, 20). Hamilton, H.C., Transl.; Falconer, W., Bohn, H.G.: London, UK; pp. 1854–1857.
- S. Gougguenheim. Aristote au Mont Saint-Michel – Les Racines Grecques de l’Europe Chrétienne. Paris, France: Editions du Seuil – L’Univers Historique, 2008. [Google Scholar]
- A. Lambert. Bibliothèque de Physique et d’Histoire Naturelle. Paris, France: Veuve David Jeune, 1758, Volume 5. [Google Scholar]
- J.A. (Abbé) Nollet. Leçons de Physique Expérimentale. Paris, France: Les Frères Guerin, 1769, Volume 6. [Google Scholar]
- M. Macquer. Elemens de Chymie-Pratique. Edited by J.-T. Herissant. Paris, 1751, Dictionnaire de Chymie, Didot, Paris, France, 1766–1777; Volume 2. [Google Scholar]
- Buffon (Georges Leclerc Comte de). Histoire Naturelle des Minéraux. Paris, France: Imprimerie Royale, 1787, Volume 7. [Google Scholar]
- Théophilus(11th–12th c.). Schedula Diversarum Artium. Translated by C.R. Dodwell. the various arts, London, UK: Theophilus, 1961. [Google Scholar]
- Anonymous. “El Lapidario del Rey Alphonso X; translation in Spanish by King Alfonso in the year 1279, see ibidem in N. Heaton.” In J. British Society of Master Glass-Painters. 1947, Volume 48, pp. 9–24. [Google Scholar]
- Cl. Lautier, and D. Sandron. Antoine de Pise – L’art du vitrail vers 1400. Paris, France: Comité des travaux historiques et scientifiques (CTHS, Coll. Corpus Vitrearum – France, série « Études », 2008, vol. VIII. [Google Scholar]
- al-Qâsem Kâshâni Abu. Arâyes al-javâher. Edited by I. Afshâr. Teheran, 1966, see also ibidem J.W. Allan Iran, 1973, 11,111–140. [Google Scholar]
- Y. Porter. Les Techniques du lustre Métallique - Jowhar-Nâme-ye-Nezâm (1196), Actes du VIIe Congrès International sur la Céramique Médiévale en Méditerranée, Thessaloniki, 11–16 October 1999; Athènes, Grece: Caisse des Recettes Archéologiques, 2003, pp. 427–436.
- A.-F. Cannela. Gemmes, verre coloré, fausses pierres précieuses au Moyen Âge, Le quatrième livre du «Trésorier de Philosophie naturelle des pierres précieuses » de Jean d’Outremeuse. Bibliothèque de la Faculté de Philosophie et Lettres de l’Université de Liège-Fascicule CCLXXXVIII; Genève, Suisse: Librairie Droz S.A., 2006. [Google Scholar]
- C. Picolpasso. Li Tre Libri dell’Arte del Vasaio. 1557, first translated and edited by C. Popelin, Les trois Livres de l’Art du Potier, (Paris, 1881). See also The Three Book of the Potter’s Art, La Revue de La Céramique et du Verre (Fac-Similé Edition), Vendin-le-Vieil, France 2007. [Google Scholar]
- G. Bauer alias Agricola. De Re Metallica, (Fac-similé Edition) ed. Basel: Froben, 1556, Klopp, G., Transl.; Klopp Editeur-Imprimeur; Thionville, 1987. [Google Scholar]
- B. Perez de Vargas. De Re Metallica. Madrid, Spain, 1568, idem Traité Singulier de Métallique, Prault Père: Paris, France, 1743. [Google Scholar]
- G. Rosetti. Plictho de l’arte de Tentori(1548). Translated by S.M. Edelstein, and H.C. Borghetty. Cambridge, MA, USA: The MIT Press, 1969. [Google Scholar]
- G.P. Lomazzo. Trattato Dell’arte Della Pittura. Milan, Italy: Paolo Gottardo Ponto, 1590. [Google Scholar]
- C. Merrett. The World’s Most Famous Book on Glassmaking ‘The Art of Glass’ by Antonio Neri. Edited by M. Cable. Sheffield, England, 1662, see, The Society of Glass and Technology Reprint, 2003 (1rst published in 1612). [Google Scholar]
- P. Brunet. “Les premiers linéaments de la Science Géologique: Agricola, Palissy, G. Owen.” Rev. Hist. Sci. Appl 3 (1950): 67–79. [Google Scholar]
- J.-L. Alléon-Dulac. Mémoire pour servir à l’histoire naturelle des provinces de Lyonnais, Forez et Beaujolais. imprimé chez Claude Cizeron; Lyon, France, 1765. [Google Scholar]
- M.P. Merrifield. Medieval and Renaissance Treatises on the Arts of Painting (1849). reprint; New York, NY, USA: Merified, 1967. [Google Scholar]
- M. Berthelot. Introduction à la Chimie des Anciens. Paris, France: Steinheil, 1889. [Google Scholar]
- Ferchault de Réaumur, R.A. Observations sur la matière qui colore des perles fausses et sur quelques autres matières animales d’une semblable couleur, à l’occasion de quoi on essaie d’expliquer la formation des écailles de poissons, Mémoires Académie des Sciences, Paris, France, 1716. Idée générale des différentes manières dont on peut faire la Porcelaine et quelles sont les véritables matières de celle de la Chine, ibidem, 1727. Second mémoire sur la porcelaine ou suite des principes qui doivent conduire dans la composition des porcelaines de différents genres et qui établissent les caractères des matières fondantes qu’on ne peut choisir pour tenir lieu de celle qu’on employe à la Chine, ibidem, 1729. Mémoire sur l’art de faire une nouvelle espèce de Porcelaine par des moyens extrêmement simples et faciles ou de transformer le verre en porcelaine, ibidem, 1739.
- W. Lewis. “Glass and Enamel by Preparations of Gold.” In Commercium Philosophico-Technicum; or, The Philosophical Commerce of Arts: Designed as an Attempt to Improve Arts, Trades, and Manufactures. London, UK, 1763, p. 170. [Google Scholar]
- A. d’Albis. “Steps in the Manufacture of the Soft Paste Porcelain of Vincennes, According to the Book of Hellot.” In Ancient Technology to Modern Science. Edited by W.D. Kingery. Ceramic and Civilization Serie; Columbus, USA: The American Ceramic SocIety, 1984, Volume 1. [Google Scholar]
- A. Brongniart. Mémoire sur la Peinture sur Verre. Paris, France: Imprimerie Sellingue, 1829, see also Mezzadri, B.; Revue de la Céramique et du Verre, 1986, 26, 5–10. [Google Scholar]
- A. Brongniart. Traité des Arts Céramiques ou des Poteries Considérées dans leur Histoire, leur Pratique et leur Théorie, 3rd ed. with Notes et Additions by Salvetat, A., Asselin, P.; Paris, France: Libraire de la Faculté de Médecine, 1877, Volume 2. [Google Scholar]
- L.A. Salvetat. Leçons de Céramiques professées à l’Ecole Centrale des Arts et Manufactures. Paris, France: Malet-Bachelier, 1857. [Google Scholar]
- G. Bontemps. Guide du Verrier-Traité Historique et Pratique de la Fabrication des Verres, Cristaux, Vitraux. Paris, France: Librairie du Dictionnaire des Arts Manufacturés, 1868. [Google Scholar]
- Th. Deck. La Faïenc. Paris, France: Maison Quantin, 1887. [Google Scholar]
- F. Bastenaire-Daudenart. L’Art de fabriquer la faïence. Paris, France: La Librairie Scientifique et Industrielle, De Mahler et Cie, 1827. [Google Scholar]
- A. Jacquemart. Histoire de la Céramique. Paris, France: Librairie Hachette et Cie, 1875. [Google Scholar]
- E. Cooper. Ten Thousand Years of Pottery. London, UK: British Museum Press, 2000. [Google Scholar]
- P.B. Vandiver, O. Soffer, B. Klina, and J. Svoboda. “The origin of ceramic technology at Dolni-Vestonice Czechoslovakia.” Science 246 (1989): 1002–1008. [Google Scholar]
- Begouen (Comte de), and C.R. La Grotte préhistorique. Séances Acad. Inscription Belles Lett. 56 (1912): 532–538. [CrossRef]
- P. McCray. Prehistory and History of Glassmaking Technology. Westerville, OH, USA: The American Ceramic Society, 1998, Ceramics and Civilization Series; Volume VIII. [Google Scholar]
- W.D. Kingery. Ancient Technology to Modern Science. Westerville, OH, USA: The American Ceramic Society, 1984, Ceramic and Civilization; Volume I. [Google Scholar]
- W.D. Kingery. Technology and Style. Westerville, OH, USA: The American Ceramic Society, 1986, Ceramic and Civilization; Volume II. [Google Scholar]
- W.D. Kingery. High Technology Ceramics –Past, Present, and Future. The Nature of Innovation and Change in Ceramic Technology. Westerville, OH, USA: The American Ceramic Society, 1986, Ceramic and Civilization; Volume III. [Google Scholar]
- P.E. McGovern, M.D. Notis, and W.D. Kingery. Cross-craft and Cross-Cultural Interactions in Ceramics. Westerville, OH, USA: The American Ceramic Society, 1989, Ceramic and Civilization; Volume IV. [Google Scholar]
- J. Li. “The Evolution of Chinese Pottery and Porcelain Technology.” In Ancient Technology to Modern Science. Edited by W.D. Kingery. Westerville, OH, USA: The American Ceramic Society, 1984, Ceramic and Civilization; Volume I, pp. 135–162. [Google Scholar]
- Y. Leon, C. Lofrumento, A. Zoppi, R. Carles, E.M. Castelluci, and P. Sciau. “Micro-Raman investigation of terra sigillata slip: A comparison study of central Italian and southern Gaul productions.” J. Raman Spectrosc. 41 (2010): 1550–1555. [Google Scholar] [CrossRef]
- P. Colomban, and C. Truong. “A Non-destructive Raman Study of the Glazing Technique in Lustre Potteries and Faiences (9th-14th centuries): Silver ions, Nanoclusters, Microstructure and Processing.” J. Raman Spectrosc. 35 (2004): 195–207. [Google Scholar] [CrossRef]
- P. Colomban, G. Sagon, A. Louhichi, H. Binous, and N. Ayed. “Identification par Microscopie Raman des Tessons et Pigments de Glaçures de l’Ifryqiya (Dougga: XI-XVIIIe siècles).” Revue d’Archéomètrie 25 (2001): 101–112. [Google Scholar]
- F. Rosi, V. Manuali, T. Grygar, P. Bezdicka, B.G. Brunetti, A. Sgamellotti, L. Burgio, C. Seccaroni, and C. Miliani. “Raman scattering features of lead pyroantimonate compounds: Implication for the non-invasive identification of yellow pigments on ancient ceramics. Part II. In situ characterisation of Renaissance plates by portable micro-Raman and XRF studies.” J. Raman Spectrosc. 42 (2011): 407–414. [Google Scholar] [CrossRef]
- C. Sandalinas, S. Ruiz-Moreno, A. López-Gil, and J. Miralles. “Experimental confirmation by Raman spectroscopy of a Pb-Sn-Sb triple oxide yellow pigment in sixteenth-century Italian pottery.” J. Raman Spectrosc. 37 (2006): 1146–1153. [Google Scholar] [CrossRef]
- K. Sakellariou, C. Miliani, A. Morresi, and M. Ombelli. “Spectroscopic investigation of yellow majolica glaze.” J. Raman Spectrosc. 35 (2004): 61–67. [Google Scholar] [CrossRef]
- P. Colomban, V. Milande, and L. Le Bihan. “On-site Raman Analysis of Iznik pottery glazes and pigments.” J. Raman Spectrosc. 35 (2004): 527–535. [Google Scholar] [CrossRef]
- P. Colomban, R. de Laveaucoupet, and V. Milande. “On Site Raman Analysis of Kütahya fritwares.” J. Raman Spectrosc. 36 (2005): 857–863. [Google Scholar] [CrossRef]
- G. Simsek, and E. Geckinli. “An assessment study of tiles from Topkapı Palace Museum with energy-dispersive X-ray and Raman spectrometers.” J. Raman Spectrosc. 43 (2012): 917–927. [Google Scholar] [CrossRef]
- P. Colomban, V. Milande, and H. Lucas. “On-site Raman analysis of medici porcelain.” J. Raman Spectrosc. 35 (2004): 68–72. [Google Scholar] [CrossRef]
- P. Colomban. “Recent case studies in the raman analysis of ancient ceramics: Glaze opacification in abbasid pottery, medici and 18th century french porcelains, iznik and kütahya ottoman fritwares and unexpected lapis lazuli pigment in lajvardina wares.” MRS Fall Meet. Proc. 852 (2005): 153–160. [Google Scholar]
- P. Colomban, I. Robert, C. Roche, G. Sagon, and V. Milande. “Identification des porcelaines tendres du 18ème siècle par spectroscopie Raman: Saint-Cloud, Chantilly, Mennecy et Vincennes/Sèvres.” Revue d’Archéomètrie 27 (2004): 153–167. [Google Scholar]
- P. Colomban, and F. Treppoz. “Identification and differentiation of ancient and modern european porcelains by raman macro- and microspectroscopy.” J. Raman Spectrosc. 32 (2001): 93–102. [Google Scholar] [CrossRef]
- X. Comte. “de Chavagnac, Marquis de Grollier.” In Histoire des Manufactures Françaises de Porcelaine. Paris, France: A. Picard & Fils, 1906. [Google Scholar]
- H. Mostaghaci. Advanced Ceramic Materials. Zuerich-Uetikon, Switzerland: Trans Tech Publications, 1996, Key Engineering Materials Volume 122–124. [Google Scholar]
- P. Ricciardi, P. Colomban, B. Fabbri, and V. Milande. “Towards the establishment of a Raman database of early European porcelain.” e-Preserv. Sci. 6 (2009): 22–26. [Google Scholar]
- Phase Diagrams for Ceramists. Westerville, OH, USA: American Ceramic Society, 1971, Volume 10.
- W.M. Carty, and U. Senapati. “Porcelain-raw materials.” J. Am. Ceram. Soc. 81 (1998): 3–20. [Google Scholar] [CrossRef]
- C.B. Carter, and M.G. Norton. Ceramic Materials—Science and Engineering. New York, NY, USA: Springer, 2007. [Google Scholar]
- P. Colomban, G. Sagon, and X. Faurel. “Differentiation of antique ceramics from the Raman spectra of their coloured glazes and paintings.” J. Raman Spectrosc. 32 (2001): 351–360. [Google Scholar] [CrossRef]
- R. Eppler, and D. Eppler. Glazes and Glass Coatings. Westerville, OH, USA: The American Ceramic Society, 2000. [Google Scholar]
- L. Meneret. Couleurs Céramiques. ENSCI Report; Sèvres, France: ENSCI, 1975. [Google Scholar]
- P. Colomban. “Secrets retrouvés du Lustre Abbasside.” La Revue de la Céramique et du Verre 139 (2004): 13–21. Available online: http://www.ladir.cnrs.fr/pages/colomban/Lustreceramique.pdf (accessed on 8th July 2013). [Google Scholar]
- P. Colomban. “The use of metal nanoparticles to produce yellow, red and iridescent colour, from Bronze Age to Present Times in Lustre pottery and glass: Solid state chemistry, spectroscopy and nanostructure.” J. Nano Res. 8 (2009): 109–132. [Google Scholar] [CrossRef]
- P. Sciau. “Nanoparticle in Ancient Materials, the Metallic Lustre of Medieval Ceramics.” In The Delivery of Nanoparticles, Chapter: 25. Winchester, UK: InTech, 2012. [Google Scholar] [CrossRef]
- S. Baroni. Restauration et Conservation des Tableaux—Manuel Pratique. Paris, France: CELIC, 1992. [Google Scholar]
- E. Kendix, G. Moscardi, R. Mazzeo, P. Baraldi, S. Prati, E. Joseph, and S. Capelli. “Far infrared and Raman spectroscopy analysis of inorganic pigments.” J. Raman Spectrosc. 39 (2008): 1104–1112. [Google Scholar] [CrossRef]
- G. Artioli, I. Angelini, and A. Polla. “Crystals and phase transitions in protohistoric glass materials.” Phase Trans. 81 (2008): 233–252. [Google Scholar] [CrossRef]
- P. Vandiver, and W.D. Kingery. “Egyptian Faience: The first High-Tech Ceramic.” In High Technology Ceramics –Past, Present, and Future. The Nature of Innovation and Change in Ceramic Technology. Edited by W.D. Kingery. Westerville, OH, USA: The American Ceramic Society, 1986, Ceramic and Civilization; Volume III, pp. 19–34. [Google Scholar]
- L. Ellis, and R. Newman. “The analyzis of glazed quartzite sculpture from Kerma, Capital of ancient Kush (Sudan).” MRS Fall Mee. Proc. 852 (2005): OO7.3–OO7.10. [Google Scholar] [CrossRef]
- I. Angelini, G. Artioli, P. Bellintani, V. Diella, M. Gemmi, A. Polla, and A. Rossi. “Chemical analyses of bronze age glasses from Frattesina di Rovigo, northern Italy.” J. Archaeol. Sci. 31 (2004): 1175–1184. [Google Scholar] [CrossRef]
- N. Brun, L. Mazerolles, and M. Pernot. “Microstructure of opaque red glass containing copper.” J. Mater. Sci. Lett. 10 (1991): 1418–1420. [Google Scholar] [CrossRef]
- P.T. Nicholson. “Glass-making and glass-working at Amarna: Some new work.” J. Glass Stud. 37 (1995): 11–19. [Google Scholar]
- A.J. Shortland, and M.S. Tite. “The Interdependence of Glass and Vitreous Faience Production at Amarna.” In Prehistory and History of Glassmaking Technology. Edited by P. McCray. Westerville, OH, USA: The American Ceramic Society, 1998, Ceramics and Civilization Series; Volume VIII, pp. 251–265. [Google Scholar]
- B. Gratuze, I. Soulier, J.N. Barrandon, and D. Foy. “De l’origine du cobalt dans les verres.” Revue d’Archéomètrie 16 (1992): 97–108. [Google Scholar]
- B. Gratuze, I. Soulier, M. Blet, and L. Vallauri. “De l’origine du cobalt: Du verre à la céramique.” Revue d’Archéomètrie 20 (1996): 77–94. [Google Scholar]
- P. Sciau, S. Relaix, Y. Kihn, and C. Roucau. “The role of microstructure, nanostructure and composition in the brilliant red slip of Roman terra sigillata pottery from southern Gaul.” MRS Fall Meet. Proc. 852 (2005): OO6.4. [Google Scholar]
- R.H. Brill. Chemical Analyses of Early Glasses. New York, NY, USA: The Corning Museum of glass, 1999. [Google Scholar]
- P. Colomban, Th. Calligaro, Cl. Vibert-Guigue, N.Q. Liem, and H.G.M. Edwards. “Accrochage des dorures sur les céramiques et tesselles anciennes.” Revue d’Archéométrie-Archeosciences 29 (2006): 7–20. [Google Scholar]
- S. Greiff, and J. Schuster. “Technological study of enamelling on Roman glass: the nature of opacyfing, decolourising and fining agents used with the glass beakers from Lübsow (Lubieszewo, Poland).” J. Cult. Herit. 9 (2008): e27–e32. [Google Scholar] [CrossRef]
- E. West FitzHugh, and L.A. Zycherman. “A purple Baryum copper silicate pigment from early China.” J. Conserv. Stud. 37 (1992): 145–154. [Google Scholar] [CrossRef]
- X. Cheng, X. Yin, Y. Ma, and Y. Lei. “Three fabricated pigments (Han purple, indigo and emerald green) in ancient Chinese artifacts studied by Raman microscopy, energy-dispersive X-ray spectrometry and polarized light microscopy.” J. Raman Spectrosc. 38 (2007): 1274–1280. [Google Scholar] [CrossRef]
- S. Bouherour, H. Berke, and H.G. Wiedemann. “Ancient man-made copper silicate pigments studied by Raman microscopy.” Chimia 55 (2001): 942–951, Berke, H.; Wiedemann, H.G. The chemistry and fabrication of anthropogenic pigments Chinese blue and purple in ancient China. EASTM 2000, 94–120. [Google Scholar]
- P. Bianchetti, F. Talarico, M.G. Vigliano, and M. Fuad Ali. “Production and characterization of Egyptian Blue and green frit.” J. Cult. Herit. 1 (2000): 179–188. [Google Scholar] [CrossRef]
- L. Burgio, and R.J.H. Clark. “Comparative pigment analysis of six modern Egyptian papyri and an authentic one of the 13th century BC by Raman microscopy and other techniques.” J. Raman Spectrosc. 31 (2000): 395–401. [Google Scholar] [CrossRef]
- J. Guy. “Early ninth century Chinese export ceramics and the Persian Gulf connection: the Belitung shipwreck evidence, in Chine-Méditerranée, Routes et échanges de la céramique avant le XVIe siècle.” Taoci,(Editions SFECO-Findalkly,Suilly-la-Tour, France) 4 (2005): 145–152. [Google Scholar]
- Z. Fukang. “The Origin and Development of Traditional Chinese Glazes and Decorative Ceramic Colors.” In Ancient Technology to Modern Science. Edited by W.D. Kingery. Westerville, OH, USA: The American Ceramic Society, 1984, Ceramic and Civilization; Volume I. [Google Scholar]
- N. Wood. Chinese Glazes: Their Origins, Chemistry and Recreation. London, UK: A & C Black Publishers Lt , 1999. [Google Scholar]
- J. Soustiel. La Céramique Islamique-Le Guide du Connaisseur. Paris, France: Office du Livre, 1985. [Google Scholar]
- M. Tite, T. Pradell, and A. Shortland. “Discovery, production and use of tin-based opacifiers in glasses, enamels and glazes from the late iron age onwards: A reassement.” Archaeometry 50 (2008): 67–84. [Google Scholar]
- F. Rubio, S. Pérez-Villar, M.A. Garrido, J. Rubio, and J.L. Oteo. “Application of gradient and confocal raman spectroscopy to analyze silver nanoparticle diffusion in medieval glasses.” J. Nano Res. 8 (2009): 89–97. [Google Scholar] [CrossRef]
- P. Colomban, M.-P. Etcheverry, M. Asquier, M. Bounichou, and A. Tournié. “Raman identification of ancient stained glasses and their degree of deterioration.” J. Raman Spectrosc. 37 (2006): 614–626. [Google Scholar] [CrossRef]
- P. Colomban, A. Tournié, and P. Ricciardi. “Raman spectroscopy of copper nanoparticles-containing glass matrix: The ancient red stained-glass windows.” J. Raman Spectrosc. 40 (2009): 1949–1955. [Google Scholar] [CrossRef]
- B. Kirmizi, P. Colomban, and M. Blanc. “On-site Analysis of Limoges enamels from 16th to 19th century.” J. Raman Spectrosc. 41 (2010): 1240–1247. [Google Scholar] [CrossRef]
- M. Blanc. Emaux peints de Limoges, XVe-XVIIIe siècles – La collection du Musée des Arts décoratifs. Paris, France: Les arts décoratifs, 2011. [Google Scholar]
- B. Kirmizi, P. Colomban, and B. Quette. “On-site analysis of Chinese cloisonné enamels from 15th to 19th century.” J. Raman Spectrosc. 41 (2010): 780–790. [Google Scholar]
- P. Ricciardi, P. Colomban, A. Tournié, and V. Milande. “Non-destructive on-site identification of ancient glasses: Genuine artefacts, embellished pieces or forgeries? ” J. Raman Spectrosc. 40 (2009): 604–617. [Google Scholar] [CrossRef]
- P. Colomban, A. Tournié, M.C. Caggiani, and C. Paris. “Pigments and enamelling/gilding technology of Mamluk mosque lamps and bottle.” J. Raman Spectrosc. 43 (2012): 1975–1984. [Google Scholar] [CrossRef]
- B.H. Berrie, and L.C. Matthew. Material Innovation and Artistic Invention: New Materials and New Colors in Renaissance Venetian Paintings, in Scientific Examination of Art – Modern Techniques in Conservation and Analysis. Washington, DC, USA: The National Academies Press, 2005, pp. 12–26. [Google Scholar]
- N. Atasoy, and J. Raby. Iznik, the Pottery of Ottoman Turkey. Edited by Y. Petsoupoulos. London, UK: Alexandria Press, 1989, pp. 50–73. [Google Scholar]
- W. Goder, W. Schulle, O. Wagenbreth, and H. Walter. “Mise au Point Technique du grès de Böttger et de la Porcelaine de Böttger.” In Meissen, La Découverte de la Porcelaine Européenne en Saxe – J.F. Böttger 1709–1736. Paris, France: Pygmalion-Gérard Watelet, 1984, pp. 106–154. [Google Scholar]
- P. Colomban, and G. Gouadec. “The ideal ceramic fiber/oxide matrix composite: How to conciliate antagonist physical and chemical requirements? ” Ann. Chim. Sci. Matériaux 30 (2005): 673–688. [Google Scholar] [CrossRef]
- H. Bertran. Nouveau Manuel Complet de la Peinture sur Verre, sur Porcelaine et sur Email. Encyclopédie-Roret. Edited by L. Mulo. Paris, France, 1913. [Google Scholar]
- P. Kruger. Principles of Activation Analysis. New York, NY, USA: Wiley Interscience, 1971. [Google Scholar]
- A.M. Pollard, and C. Heron. Archaeological Chemistr. Cambridge, UK: Royal Society Chemistry Paperback, 1996. [Google Scholar]
- K. Janssens. Modern Methods for Analysing Archaeological and Historical Glass, 1st ed. Chichester, UK: John Wiley & Sons Ltd, 2012, Volume 2. [Google Scholar]
- A. Fisher, P. Goodall, M.W. Hinds, S.N. Nelms, and D.M. Penny. “Atomic spectrometry update. Industrial analysis: Metals, chemicals and advanced materials.” J. Anal. Atom. Spectrosc. 18 (2003): 1497–1528. [Google Scholar] [CrossRef]
- R. Falcone, A. Renier, and M. Verita. “Wavelength-dispersive x-ray fluorescence analysis of ancient glasses.” Archaeometry 44 (2002): 531–542. [Google Scholar] [CrossRef]
- H.-J. Mucha, H.-G. Bartel, and J. Dolata. “Effects of Data Transformation on Cluster Analysis of Archaeometric Data.” In Data Analysis, Machine Learning and Applications Studies in Classification, Data Analysis, and Knowledge Organization. Berlin, Germany: Springer, 2008, Book Series: Studies in Classification Data Analysis and Knowledge Organization, XI; pp. 681–688. [Google Scholar]
- K. Michelaki, and R.G.V. Hancock. “Chemistry versus Data dispersion: Is there a better way to assess and interpret archaeometric data? ” Archaeometry 53 (2011): 1259–1279. [Google Scholar] [CrossRef]
- D. Agha-Alidol, P. Oliaiy, M. Mohsenian, M. Lamehi-Rachti, and F. Shokouhi. “Provenance study of ancient Iranian luster pottery using PIXE multivariate statistical analysis.” J. Cult. Herit. 10 (2009): 497–492. [Google Scholar]
- G. Padeletti, and P. Fermo. “A scientific approach to the attribution problem of renaissance ceramic productions based on chemical and mineralogical markers.” Appl. Phys. A 100 (2010): 771–784. [Google Scholar] [CrossRef]
- B. Giussani, D. Monticelli, and L. Rampazzi. “Role of laser ablation-inductively coupled plasma-mass spectrometry in cultural heritage research: A review.” Anal. Chim. Acta 635 (2009): 6–21. [Google Scholar] [CrossRef]
- G. Zachariadis, E. Dimitrakoudi, A. Anthemidis, and J. Stratis. “Optimized microwave-assisted decomposition method for multi-element analysis of glass standard reference material and ancient glass specimens by inductively coupled plasma atomic emission spectrometry.” Talanta 68 (2006): 1448–1456. [Google Scholar] [CrossRef]
- A. Giakoumaki, K. Melessanaki, and D. Anglos. “Laser-induced breakdown spectroscopy (LIBS) in archaeological science-applications and prospects.” Anal. Bioanal. Chem. 387 (2007): 749–760. [Google Scholar] [CrossRef]
- Y. Yoon, T. Kim, M. Yang, K. Lee, and G. Lee. “Quantitative analysis of pottery glaze by laser induced breakdown spectroscopy.” Microchem. J. 68 (2001): 251–256. [Google Scholar] [CrossRef]
- R.J.H. Clark. “Pigment identification by spectroscopic means: An arts/science interface.” Compte- Rendus Chim. 5 (2002): 7–20. [Google Scholar] [CrossRef]
- F. Colao, R. Fantoni, V. Lazic, and V. Spizzichino. “Laser-induced breakdown spectroscopy for semi-quantitative and quantitative analyses of artworks—Application on multi-layered ceramics and copper based alloys.” Spectrochim. Acta B 57 (2002): 1219–1234. [Google Scholar] [CrossRef]
- J.C Dran, J. Salomon, T. Calligaro, and P. Walter. “Review of accelerator gadgets for art and archaeology.” Nuclear Instr. Methods Phys. Res. Sect. B Phys. Inter. Mater. Atom. 226 (2004): 29–37. [Google Scholar] [CrossRef]
- O. Enguita, M.T. Fernandez-Jimenez, G. Garcia, A. Climent-Font, T. Calderon, and G.W. Grime. “The new external microbeam facility at the 5 MV Tandetron accelerator laboratory in Madrid: Beam characterisation and first results.” Nuclear Instr. Methods Phys. Res. Sect. B Phys. Inter. Mater. Atom. 219 (2004): 384–388. [Google Scholar]
- A. Denker, O. Hahn, B. Kanngiesser, W. Malzer, S. Merchel, M. Radtke, and S. Rohrs. “Chemistry of arts—Non-destructive analysis of artistic and cultural heritage objects.” MaterialPrufung 45 (2003): 485–503. [Google Scholar]
- P.A. Mando, M.E. Fedi, and N. Grassi. “The present role of small particle accelerators for the study of Cultural Heritage.” Eur. Phys. J. Plus 126 (2011). [Google Scholar]
- N. Salvado, S. Buti, M.J. Tobin, E. Pantos, A.J.N.W. Prag, and T. Pradell. “Advantages of the use of SR-FT-IR microspectroscopy: Applications to cultural heritage.” Anal. Chem. 77 (2005): 3444–3451. [Google Scholar] [CrossRef]
- K. Janssens, G. Vittiglio, I. Deraedt, A. Aerts, B. Vekemans, L. Vincze, F. Wei, I. Deryck, O. Schalm, F. Adams, and et al. “Use of microscopic XRF for non-destructive analysis in art and archaeometry.” X-ray Spectrom. 29 (2000): 73–91. [Google Scholar] [CrossRef]
- D.C. Creagh. “The characterization of artefacts of cultural heritage significance using physical techniques.” Radiat. Phys. Chem. 74 (2005): 426–442. [Google Scholar] [CrossRef]
- P. Moioli, and C. Seccaroni. “Analysis of art objects using a portable x-ray fluorescence spectrometer.” X-ray Spectrom. 29 (2000): 48–52. [Google Scholar] [CrossRef]
- D.N. Papadopoulou, G.A. Zachariadis, A.N. Anthemidis, N.C. Tsirliganis, and J.A. Stratis. “Development and optimisation of a portable micro-XRF method for in situ multi-element analysis of ancient ceramics.” Talanta 68 (2006): 1692–1699. [Google Scholar] [CrossRef]
- M.J. Nuevo, and A.M. Sanchez. “Application of XRF spectrometry to the study of pigments in glazed ceramic pots.” Appl. Rad. Isotopes 69 (2011): 574–579. [Google Scholar] [CrossRef]
- P. Colomban, A. Tournié, M. Maucuer, and P. Meynard. “On-site Raman and XRF analysis of Japanese/Chinese Bronze/Brass Patina—The search of specific Raman signatures.” J. Raman Spectrosc. 43 (2012): 799–808. [Google Scholar] [CrossRef]
- M. Ferretti, G. Cristoforetti, S. Legnaloll, V. Palleschi, A. Salvetti, E. Togrioni, E. Console, and P. Palaia. “In situ study of the Porticello Bronzes by portable X-Ray fluorescence and laser induced breakdown spectroscopy.” Spectrochim. Acta B Atom. Spectrosc. 62 (2007): 1512–1518. [Google Scholar] [CrossRef]
- M. Brai, G. Gennaro, T. Schillaci, and L. Tranchina. “Double pulse laser induced breakdown spectroscop applied to natural and artificial materials from cultural heritage – A comparison with micro-X-Ray fluorescence analyses.” Spectrochim. Acta B Atom. Spectrosc. 64 (2009): 1119–1127. [Google Scholar] [CrossRef]
- G. Turrell, and J. Corset. Raman Microscopy-Developments and Applications. San-Diego, CA, USA: Academic Press Ltd, 1976. [Google Scholar]
- D.A. Long. Raman Spectroscopy. New-York, NY, USA: McGraw-Hill International Book Cy, 1977. [Google Scholar]
- G. Gouadec, and P. Colomban. “Raman study of Nanomaterials: How spectra related to disorder, particle size and mechanical properties.” Progr. Cryst. Growth Charact. Mater. 53 (2007): 1–56. [Google Scholar] [CrossRef]
- L. Brillouin. “Diffusion of light and x-rays by a transparent homogeneous body. The influence of thermal agitation.” Ann. Phys. 17 (1922): 88–95, ibidem, Regarding the propagation of light in a dispersive medium. Comptes-Rendus Acad. Sci. 1921, 173, 1167–1170. [Google Scholar]
- A. Smekal. “The quantum, theory of dispersion.” Naturwissenschaften 11 (1923): 873–878, ibidem, Contribution to my work "Remarks on the quantisation of non determined periodic system. Zeitschrift fur Physick 1923, 15, 58–60. [Google Scholar] [CrossRef]
- J. Cabannes. “New optical phenomenon; pulsations produced when anisotropic molecules in rotation and vibration diffuse visible and ultra-violet light.” Comptes-Rendus Acad. Sci. 185 (1927): 1026–1028. [Google Scholar]
- Y. Rocard. “New diffused radiations.” Comptes-Rendus de l’Acad. Sci. 186 (1928): 1107–1115. [Google Scholar]
- G. Landsberg, and L. Mandelstam. “A novel effect of light scattering in crystals.” Naturwissenschaften 16 (1928): 557–558, ibidem, Light scattering in crystals, Zeitschrift fur Physik 1928, 50, 769–780. Pringsheim, P.; Rosen, B.; About the Raman Effect, Zeitschrift fur Physik 1928, 50, 741–755. [Google Scholar] [CrossRef]
- C.V. Raman, and K.S. Krishnan. “A new type of secondary radiation.” Nature 121 (1928): 501–516. [Google Scholar] [CrossRef]
- P. Colomban, and A. Tournié. “On-site raman identification and dating of ancient/modern stained glasses at the sainte-chapelle.” J. Cult. Herit. 8 (2007): 242–256. [Google Scholar] [CrossRef]
- D. Mancini, A. Tournié, M.-C. Caggiani, and P. Colomban. “Testing of Raman spectroscopy as a non-invasive tool for the investigation of glass-protected miniature portraits.” J. Raman Spectrosc. 43 (2012): 294–302. [Google Scholar] [CrossRef]
- P. Colomban, and M.-C. Caggiani. “Testing of Raman spectroscopy as a non-invasive tool for the investigation of glass-protected pastels.” J. Raman Spectrosc. 42 (2011): 790–798. [Google Scholar] [CrossRef]
- A. Tournié, L.C. Prinsloo, C. Paris, P. Colomban, and B. Smith. “The first in-situ Raman spectroscopic study of Bushman/San rock art in South Africa; procedures and preliminary results.” J. Raman Spectrosc. 42 (2011): 399–406. [Google Scholar] [CrossRef]
- P. Colomban. “Polymerisation degree and raman identification of ancient glasses used for jewellery, ceramics enamels and mosaics.” J. Non-Crystall. Solids 323 (2003): 180–187. [Google Scholar] [CrossRef]
- P. Colomban, and O. Paulsen. “Raman determination of the structure and composition of glazes.” J. Am. Ceram. Soc. 88 (2005): 390–395. [Google Scholar] [CrossRef]
- P. Colomban, A. Tournié, and L. Bellot-Gurlet. “Raman Identification of glassy silicates used in ceramic, glass and jewellry: A tentative differentiation guide.” J. Raman Spectrosc. 37 (2006): 841–852. [Google Scholar] [CrossRef]
- P. Colomban. “On-site Raman identification and dating of ancient glasses: Procedures and tools.” J. Cult. Herit. 9 (2008): e55–e60. [Google Scholar] [CrossRef]
- P. Colomban, and L. Prinsloo. “Optical Spectroscopy of Silicates and Glasses.” In Spectroscopic Properties of Inorganic and Organometallic Compounds. Edited by J. Yarwood, R. Douthwaite and S.B. Duckett. Cambridge, UK, The Royal Society of Chemistry: RSC Publishing, 2009, pp. 128–149. [Google Scholar]
- I.M. Bell, R.J.H. Clark, and P.J. Gibbs. “Raman spectroscopic library of natural an synthetic pigments (pre-~1850 AD).” Spectrochim. Acta Part A 53 (1997): 2159–2179. [Google Scholar] [CrossRef]
- W.P. Griffith. “Raman Spectroscopy of Terrestrial Minerals.” In Infrared and Raman Spectroscopy of Lunar and Terrestrial Minerals. Edited by C. Karr. New York, NY, USA: Academic Press, 1975, Chapter 12. [Google Scholar]
- K. Nakamoto. Infrared & Raman Spectra of Inorganic and Coordination Compounds: Theory and Application in Inorganic Chemistry. Chichester, UK: J. Wiley & Sons, 1997. [Google Scholar]
- R. Maestrati. Contribution à l’Edification du Catalogue Raman des Gemmes. Université de Nantes, Nantes, France: Diplôme de Gemmologie, 1989. [Google Scholar]
- M. Pinet, D.C. Smith, and B. Lasnier. “Utilité de la microsonde Raman pour l’identification non-destructive des gemmes, La Microsonde Raman en Géologie.” N° Hors-Série, Revue de Gemmologie, Paris, UK, 1992, Juin. 11–30. [Google Scholar]
- Information on minerals. Available online: http://minerals.gps.caltech.edu/files/raman (accessed on 4th February 2012); http://www.ens-lyon.fr/LST/Raman/index.php (accessed on 4th February 2012); http://www.aist.go.jp/RIODB/rasmin/E_index.htm; http://rruff.geo.arizona.edu/rruff/ (accessed on 4th February 2012); http://www.irug.org/ed2k/search.asp; http://www.fis.unipr.it/phevix/ramandb.html (accessed on 4th February 2012); Information on pigments. Available online: http://srv.chim.unifi.it/raman/ (accessed on 4th February 2012); http://www.chem.ucl.ac.uk/resources/raman/index.html (accessed on 4th February 2012).
- R.J.H. Clark. “The scientific investigation of artwork and archaeological artefacts: Raman microscopy as a structural, analytical and forensic tool.” Appl. Phys. A – Mater. Sci. & Process. 89 (2007): 833–840. [Google Scholar]
- L. Burgio, and R.J.H. Clark. “Library of FT-Raman spectra of pigments, minerals, pigment media and varnishes, and supplement to existing library of Raman spectra of pigments with visible excitation.” Spectrochim. Acta Part A 57 (2001): 1491–1521. [Google Scholar] [CrossRef]
- R.S. Williams. “On-site non-destructive ID-IR spectroscopy of plastic in museum objects using a portable FT-IR Spectrometer with fibre-optic probe, materials issues in art & archaeology.” MRS Proc. 462 (1997): 25–30. [Google Scholar] [CrossRef]
- L.L. Logan, G.R. Hunt, and J.W. Salisbury. “The Use of Mid-infrared Spectroscopy in Remote Sensing of Space Targets.” In Infrared and Raman Spectroscopy of Lunar and Terrestrial Minerals. Edited by C. Karr Jr. New York, NY, USA: Academic Press, 1975, pp. 117–142. [Google Scholar]
- J.B. Adams. “Interpretation of Visible and Near Infrared Diffuse Reflectance Spectra of Pyroxenes and other Rock Forming Minerals.” In Infrared and Raman Spectroscopy of Lunar and Terrestrial Minerals. Edited by C. Karr Jr. New York, NY, USA: Academic Press, 1975, pp. 91–116. [Google Scholar]
- P. Munier. Technologie des Faïences. Paris, France: Gauthier-Villars, 1957. [Google Scholar]
- N.Q. Liem, P. Colomban, G. Sagon, H.X. Tinh, and T.B. Hoanh. “Microstructure, composition and processing of the 15th century vietnamese porcelains and celadons.” J. Cult. Herit. 4 (2003): 187–197. [Google Scholar]
- N.Q. Liem, N.T. Thanh, and P. Colomban. “Reliability of raman microspectrometry in analysis of ancient ceramics: The case of ancient vietnamese porcelains and celadon glazes.” J. Raman Spectrosc. 33 (2002): 287–294. [Google Scholar] [CrossRef]
- P. Colomban, and V. Milande. “On Site Analysis of the earliest known Meissen Porcelain and Stoneware.” J. Raman Spectrosc. 37 (2006): 606–613. [Google Scholar] [CrossRef]
- P. Ricciardi, P. Colomban, A. Tournié, M. Macchiarola, and N. Ayed. “A non-invasive study of Roman Age mosaic glass tesserae by means of Raman spectroscopy.” J. Archaeol. Sci. 36 (2009): 2551–2559. [Google Scholar] [CrossRef]
- L.C. Prinsloo, A. Tournié, and P. Colomban. “A Raman spectroscopic study of the glass trade beads excavated at Mapungubwe hill and K2, two important archaeological sites in southern Africa, raises questions about the last occupation date of the hill.” J. Archaeol. Sci. 38 (2011): 3264–3277. [Google Scholar] [CrossRef]
- L.C. Prinsloo, A. Tournié, and P. Colomban. “Raman classification of the glass beads excavated on Mapungubwe hill and K2, two archaeological sites in South Africa.” J. Raman Spectrosc. 44 (2012): 532–542. [Google Scholar]
- K.S. Andrikopoulos, S. Daniilia, B. Roussel, and K. Janssens. “In vitro validation of a mobile Raman-XRF microanalytical instrument’s capabilities on the diagnosis of Byzantine icons.” J. Raman Spectrosc. 37 (2006): 1026–1034. [Google Scholar] [CrossRef]
- P. Baraldi, C. Fagnano, and P. Bensi. “Raman study of a “Tabula Colorum Physiologica” in a 1686 peinted journal.” J. Raman Spectrosc. 37 (2006): 1104–1110. [Google Scholar] [CrossRef]
- M.-J. Benquerenca, N.F.C. Mendes, E. Castelluci, V.M.F. Gaspar, and F.P.S.C. Gil. “Micro-Raman spectroscopy analysis of 16th century Portuguese Ferreirim Masters oil paintings.” J. Raman Spectrosc. 40 (2009): 2135–2143. [Google Scholar] [CrossRef]
- D. De Waal. “Micro-Raman and portable Raman spectroscopic investigation of blue pigments in selected Delft plates (17–20th Century).” J. Raman Spectrosc. 40 (2009): 2162–2170. [Google Scholar] [CrossRef]
- M. Bouchard, and A. Gambardella. “Raman microscopy study of synthetic cobalt blue spinels used in the field of art.” J. Raman Spectrosc. 41 (2009): 1477–1485. [Google Scholar] [CrossRef]
- P. Colomban, G. Sagon, L.Q. Huy, N.Q. Liem, and L. Mazerolles. “Vietnames (15th century) Blue-and-White Tam Tai and Lustre Porcelains/Stonewares: Glaze composition and decoration techniques.” Archaeometry 46 (2004): 125–136. [Google Scholar] [CrossRef]
- P. Colomban. “Les Routes du Lapis lazuli.” Taoci (Editions SFECO-Findalkly, Suilly-la-Tour, France) 4 (2005): 145–154. [Google Scholar]
- P. Colomban. “Lapis lazuli as unexpected pigment in Iranian Lâjvardina ceramics.” J. Raman Spectrosc. 34 (2003): 420–423. [Google Scholar] [CrossRef]
- Buffon (Comte de). Histoire Naturelle des Minéraux, Tome Septième. Paris, France: Imprimerie Royale, 1787, pp. 251–259. [Google Scholar]
- A. Lo Giudice, A. Re, S. Calusi, L. Giuntini, M. Massi, P. Olivero, G. Pratesi, M. Albonico, and E. Conz. “Multitechnique characterization of lapis lazuli for provenance study.” Anal. Bioanal. Chem. 395 (2009): 2211–2217. [Google Scholar] [CrossRef]
- R.J.H. Clark, M.L. Curri, and C. Laganara. “Raman microscopy: The identification of lapis lazuli on medieval pottery fragments from the south of Italy.” Spectrochim. Acta Part A 53 (1997): 597–603. [Google Scholar] [CrossRef]
- I.M. Catalano, A. Genga, C. Laganara, R. Laviano, A. Mangone, D. Marano, and A. Traini. “Lapis lazuli usage for blue decoration of polychrome painted glazed pottery: A recurrent technology during the Middle Ages in Apulia (Southern Italy).” J. Archaeol. Sci. 34 (2007): 503–511. [Google Scholar] [CrossRef]
- A. Mangone, M.C. Caggiani, L.C. Giannossa, R. Laviano, S. Mutino, L. Sabbatini, and A. Traini. “Islamic Gilded and Enamelled Glasses from Melfi (Southern Italy): An Archaeometric Study.” In Proceedings of the 5th International Congress “Science and Technology for the Safeguard of Cultural Heritage in the Mediterranean Basin”, Istanbul, Turkey, 22–25 November 2011.
- R. Ward. Gilded and Enamelled Glass from the Middle East. London, UK: British Museum Press, 1998. [Google Scholar]
- I.C. Freestone, and C.P. Stapleton. “Composition and Technology of Islamic enamelled glass of the 13–14th centuries (ch 24).” In Gilded and Enamelled Glass from the Middle East. Edited by R. Ward. London, UK: British Museum Press, 1998. [Google Scholar]
- M.C. Caggiani, P. Colomban, A. Mangone, and P. Cambon. “Mobile Raman spectroscopy analysis of ancient enamelled glass masterpieces.” Analyst. submitted.
- R.J.H. Clark. “Raman microscopy as structural and analytical tool in the field of art and archaeology.” J. Mol. Struct. 834 (2007): 74–80. [Google Scholar] [CrossRef]
- M. Perreira, T. de Lacerda-Aroso, M.J.M. Gomes, A. Mata, L.C. Alves, and P. Colomban. “Ancient portuguese ceramic wall tiles (« Ajulejos »): Characterization of the glaze and ceramic pigments.” J. Nano Res. 8 (2009): 79–88. [Google Scholar] [CrossRef]
- P. Colomban, and H. Schreiber. “Raman signature modification induced by copper nanoparticles in silicate glass.” J. Raman Spectrosc. 36 (2005): 884–890. [Google Scholar] [CrossRef]
- P. Colomban. “Gel technology in ceramics, glass-ceramics and ceramic-ceramic composites.” Ceramics Int. 15 (1989): 23–50. [Google Scholar] [CrossRef]
- P. Colomban, G. March, L. Mazerolles, T. Karmous, N. Ayed, A. Ennabli, and H. Slim. “Raman identification of materials used for jewelry and mosaic in ifriqiya.” J. Raman Spectrosc. 34 (2003): 205–215. [Google Scholar] [CrossRef]
- J. Bénard, and B. Dragesco. Bernard Perrot et les verreries royales du Duché d’Orléans, 1662–1754. Orléans, France: Editions des amis du musée d’Orléans, 1989, pp. 55–66. [Google Scholar]
- J. Barrelet. “Le Verre en France.” Cahiers de la Céramique, du Verre et des Arts du feu 36 (1964): 254–270. [Google Scholar]
- A. Vaughan. “Raman nanotechnology—The lycurgus cup.” IEE Electr. Insul. Mag. 24 (2008): 4–8. [Google Scholar] [CrossRef]
- I. Freestone, N. Meeks, M. Sax, and C. Higgitt. “The lycurgus cup—A Roman nanotechnology.” Gold Bull. 40 (2007): 270–277. [Google Scholar] [CrossRef]
- M.C. De Lucas, F. Moncada, and J. Rosen. “Micro-Raman study of red decorations in French faiences of the 18th and 19th centuries.” J. Raman Spectrosc. 37 (2006): 1154–1159. [Google Scholar] [CrossRef]
- M.C. Caggiani, and P. Colomban. “Raman identification of strongly absorbing phases: The ceramic black pigments.” J. Raman Spectrosc. 42 (2011): 839–843. [Google Scholar] [CrossRef]
- J. Girel. “Les noirs des Song: hypothèses et expériences.” Lett. SFECO 6 (2002): 31–35. Available online: http://www.ladir.cnrs.fr/pages/colomban/SF6imagine.pdf (accessed on 4th February 2013). [Google Scholar]
- J.M. Perez, and R. Esteve-Tebar. “Pigment identification in Greek pottery by Raman microspectroscopy.” Archaeometry 46 (2004): 607–614. [Google Scholar] [CrossRef]
- P. Mirti, M. Gulmini, A. Perardi, P. Davit, and D. Elia. “Technology of production of red figure pottery from Attic and southern Italian workshops.” Anal. Bioanal. Chem. 380 (2004): 712–718. [Google Scholar] [CrossRef]
- A. Mangone, G.E. de Benedetto, D. Fico, L.C. Giannossa, R. Laviano, L. Sabbatini, I.D. van der Werf, and A. Traini. “A multianalytical study of archaeological faience from the Vesuvian area as a valid tool to investigate provenance and technological features.” New J. Chem. 35 (2011): 2860–2868. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Colomban, P. The Destructive/Non-Destructive Identification of Enameled Pottery, Glass Artifacts and Associated Pigments—A Brief Overview. Arts 2013, 2, 77-110. https://doi.org/10.3390/arts2030077
Colomban P. The Destructive/Non-Destructive Identification of Enameled Pottery, Glass Artifacts and Associated Pigments—A Brief Overview. Arts. 2013; 2(3):77-110. https://doi.org/10.3390/arts2030077
Chicago/Turabian StyleColomban, Philippe. 2013. "The Destructive/Non-Destructive Identification of Enameled Pottery, Glass Artifacts and Associated Pigments—A Brief Overview" Arts 2, no. 3: 77-110. https://doi.org/10.3390/arts2030077
APA StyleColomban, P. (2013). The Destructive/Non-Destructive Identification of Enameled Pottery, Glass Artifacts and Associated Pigments—A Brief Overview. Arts, 2(3), 77-110. https://doi.org/10.3390/arts2030077





