A Review of the Assessment Tools for the Efficiency of Nanolime Calcareous Stone Consolidant Products for Historic Structures
Abstract
1. Introduction
- Nanofillers, reducing the nanoporosity;
- Nano-reinforcements, increasing mechanical strength;
- Catalysts, due to the fact of their higher surface area/volume ratio.
2. Analysis and Discussion
2.1. Experimental Techniques Employed for the Characterization of Nanoparticles
2.1.1. Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM)/Energy-Dispersive Spectroscopy X-ray (EDX)
2.1.2. X-ray Diffraction (XRD)
2.1.3. UV-Vis
2.1.4. Dynamic Light Scattering (DLS)
2.1.5. X-ray Fluorescence
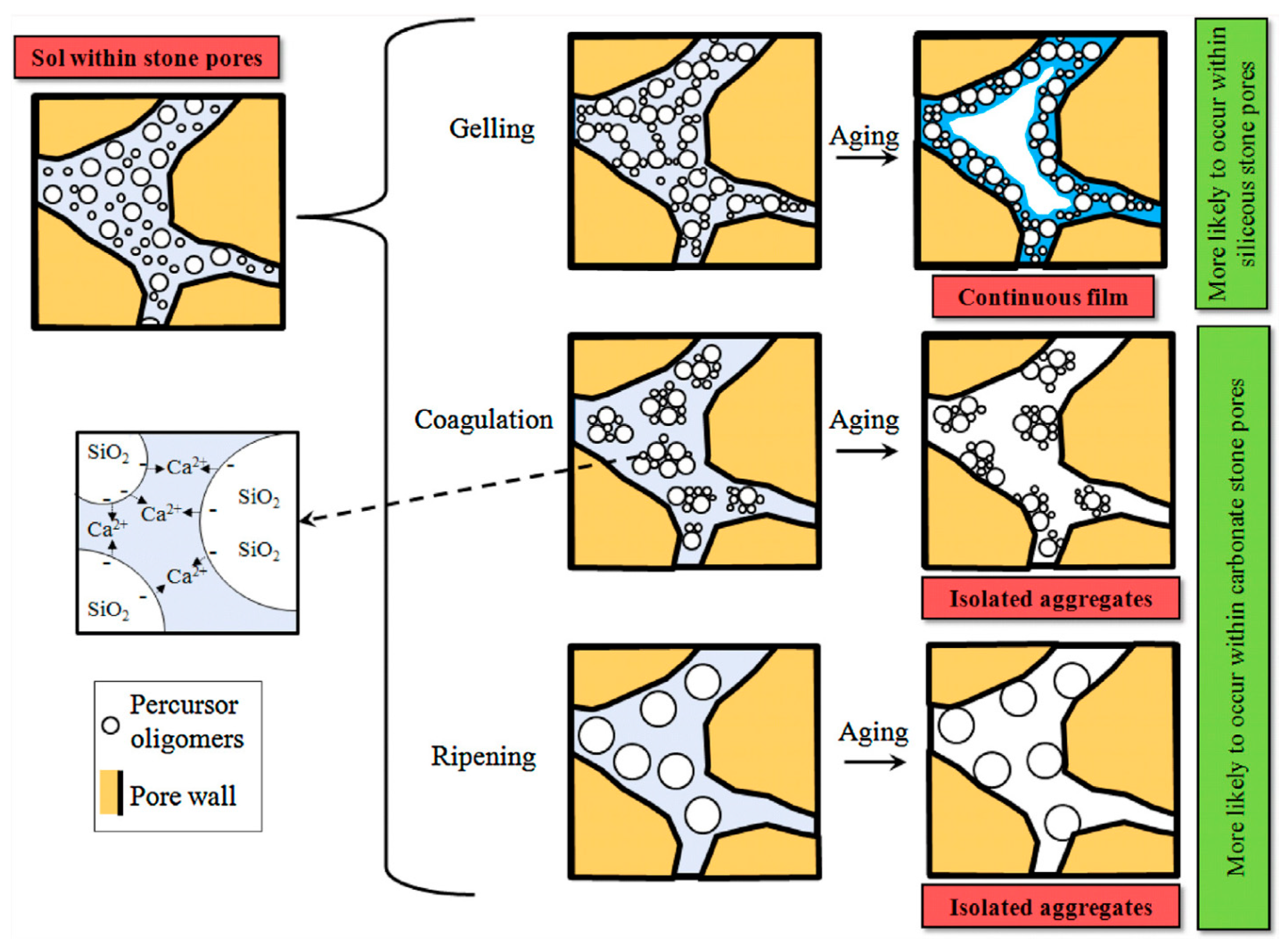
2.2. Results of the Characterization of Nanolime Dispersions
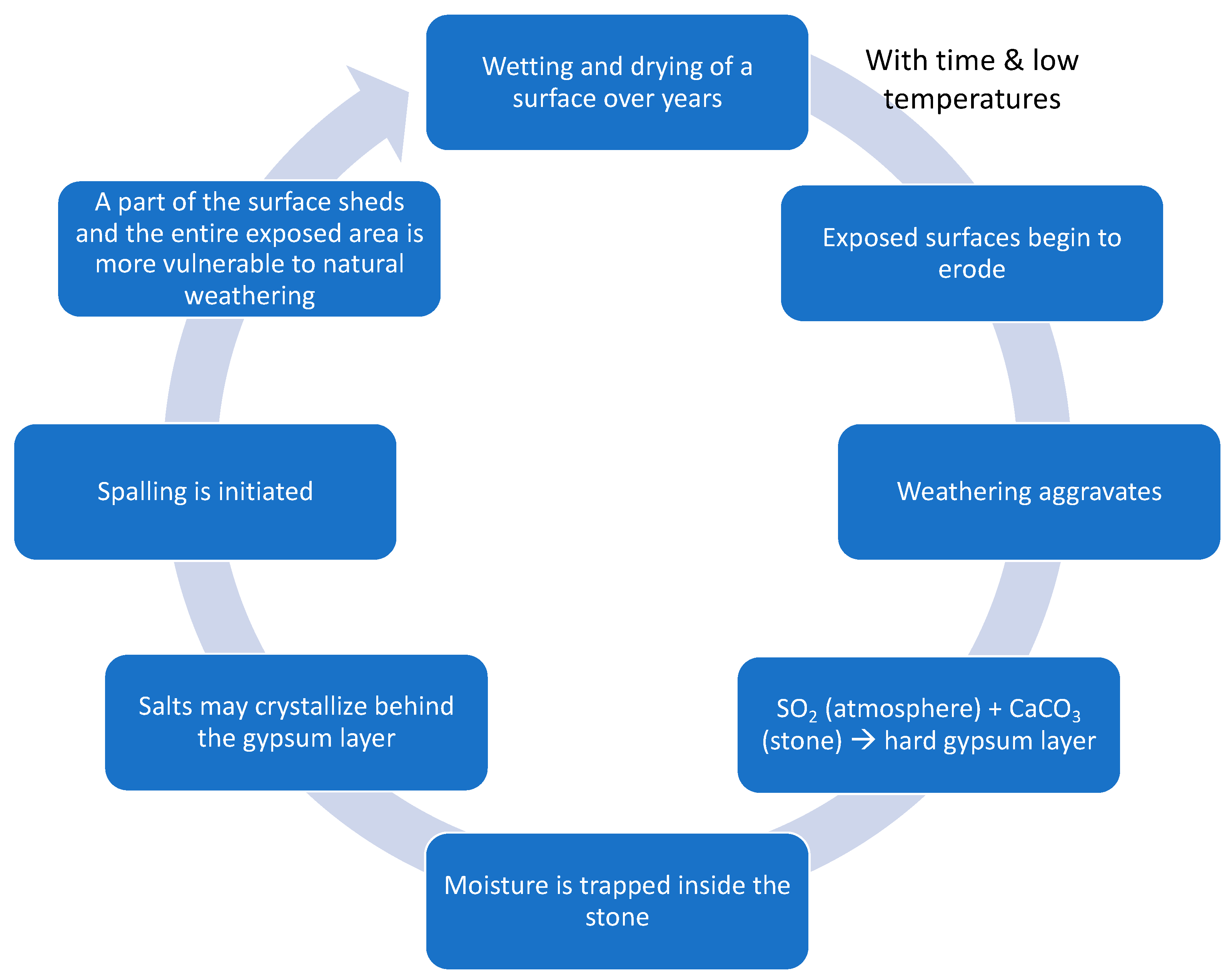
2.3. Artificial Aging Techniques
- Grinding and sieving of stones to selected particle sizes similar to that of sound stones;
- Mixing of grains with air lime powder;
- Application of the produced “mortar” at the top layer of the stone after pre-wetting of the surface;
- Curing for two weeks at 20 °C, 65% RH, and 0.5% CO2.
2.4. Assessment Tools for Nanolime-Treated Calcareous Stones
2.5. Other Aspects
2.6. Some Remarks on Additional Characterization Methods that can be Employed in Future Investigations
2.7. A Framework of Assessment Tools/Characterization Methods
3. Conclusions
- The term “effectiveness” was re-defined through a number of efficiency parameters/material properties that should be characterized;
- A significant number of characterization techniques were reviewed for both the characterization of nanoparticles and the effectiveness of the weathering and consolidation treatment (before and after weathering);
- Different applications of nanolimes have been described (brushing, drop-by-drop, immersion, contact capillary, spraying);
- Non-destructive techniques have been highlighted.
Author Contributions
Funding
Conflicts of Interest
References
- Lubelli, B.; van Hees, R.P.J.; Nijland, T.G.; Bolhuis, J. A new method for making artificially weathered stone specimens for testing of conservation treatments. J. Cult. Herit. 2015, 16, 698–704. [Google Scholar] [CrossRef]
- Borsoi, G.; Lubelli, B.; Van Hees, R.; Veiga, R.; Silva, A.S.; Colla, L.; Fedele, L.; Tomasin, P. Effect of solvent on nanolime transport within limestone: How to improve in-depth deposition. Coll. Surf. A Physicochem. Eng. Asp. 2016, 497, 171–181. [Google Scholar] [CrossRef]
- Papatzani, S. Effect of nanosilica and montmorillonite nanoclay particles on cement hydration and microstructure. Mater. Sci. Technol. 2016, 32, 138–153. [Google Scholar] [CrossRef]
- Tomasin, P.; Mondin, G.; Zuena, M.; el Habra, N.; Nodari, L.; Moretto, L.M. Calcium alkoxides for stone consolidation: Investigating the carbonation process. Powder Technol. 2019, 344, 260–269. [Google Scholar] [CrossRef]
- Kapridaki, C.; Verganelaki, A.; Dimitriadou, P.; Maravelaki-Kalaitzaki, P. Conservation of Monuments by a Three-Layered Compatible Treatment of TEOS-Nano-Calcium Oxalate Consolidant and TEOS-PDMS-TiO2 Hydrophobic/Photoactive Hybrid Nanomaterials. Materials 2018, 11, 684. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Rabanal, M.E.; Fort, R. New nanomaterials for applications in conservation and restoration of stony materials: A review. Mater. Constr. 2017, 67, 107. [Google Scholar] [CrossRef]
- Da Fonseca, B.S.; Ferreira, M.J.; Taryba, M.G.; Piçarra, S.; Pinto, A.P.F.; de Fátima Montemor, M. Alkoxysilane-based sols for consolidation of carbonate stones: Impact of the carbonate medium in the sol-gel processes. J. Cult. Herit. 2019, 37, 63–72. [Google Scholar] [CrossRef]
- Papatzani, S.; Badogiannis, E.G.; Paine, K. The pozzolanic properties of inorganic and organomodified nano-montmorillonite dispersions. Constr. Build. Mater. 2018, 167, 299–316. [Google Scholar] [CrossRef]
- D’Armada, P.; Hirst, E. Nano-Lime for Consolidation of Plaster and Stone. J. Archit. Conserv. 2012, 18, 63–80. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Suzuki, A.; Ruiz-Agudo, E. Alcohol Dispersions of Calcium Hydroxide Nanoparticles for Stone Conservation. Langmuir 2013, 29, 11457–11470. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, C.; Elert, K.; Ševčík, R. Amorphous and crystalline calcium carbonate phases during carbonation of nanolimes: Implications in heritage conservation. CrystEngComm 2016, 18, 6594–6607. [Google Scholar] [CrossRef]
- Borsoi, G.; Lubelli, B.; van Hees, R.; Veiga, R.; Silva, A.S. Understanding the transport of nanolime consolidants within Maastricht limestone. J. Cult. Herit. 2016, 18, 242–249. [Google Scholar] [CrossRef]
- Daehne, A.; Herm, C. Calcium hydroxide nanosols for the consolidation of porous building materials—Results from EU-STONECORE. Herit. Sci. 2013, 1, 11. [Google Scholar] [CrossRef]
- Ylmén, R.; Jäglid, U.; Steenari, B.-M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Papatzani, S.; Grammatikos, S.; Adl-Zarrabi, B.; Paine, K. Pore-structure and microstructural investigation of organomodified/Inorganic nano-montmorillonite cementitious nanocomposites. Am. Inst. Phys. 2018, 1957, 030004. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Füllmann, T.; Gallucci, E.; Walenta, G.; Bermejo, E. Quantitative study of Portland cement hydration by X-ray diffraction/Rietveld analysis and independent methods. Cem. Concr. Res. 2004, 34, 1541–1547. [Google Scholar] [CrossRef]
- Soin, A.V.; Catalan, L.J.J.; Kinrade, S.D. A combined QXRD/TG method to quantify the phase composition of hydrated Portland cements. Cem. Concr. Res. 2013, 48, 17–24. [Google Scholar] [CrossRef]
- Papatzani, S.; Paine, K. Inorganic and organomodified nano-montmorillonite dispersions for use as supplementary cementitious materials—A novel theory based on nanostructural studies. Nanocomposites 2017, 3, 2–19. [Google Scholar] [CrossRef]
- Calabria-Holley, J.; Papatzani, S.; Naden, B.; Mitchels, J.; Paine, K. Tailored montmorillonite nanoparticles and their behaviour in the alkaline cement environment. Appl. Clay Sci. 2017, 143, 67–75. [Google Scholar] [CrossRef]
- Papatzani, S.; Paine, K. From Nanostructural Characterization of Nanoparticles to Performance Assessment of Low Clinker Fiber–Cement Nanohybrids. Appl. Sci. 2019, 9, 22. [Google Scholar] [CrossRef]
- Papatzani, S.; Paine, K. Polycarboxylate/nanosilica-modified quaternary cement formulations – enhancements and limitations. Adv. Cem. Res. 2018, 30, 256–269. [Google Scholar] [CrossRef]
- Papatzani, S.; Paine, K. Lowering cement clinker: A thorough, performance based study on the use of nanoparticles of SiO2 or montmorillonite in Portland limestone nanocomposites. Eur. Phys. J. Plus 2018, 133, 430. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar] [CrossRef]
- Pecora, R. Dynamic Light Scattering Measurement of Nanometer Particles in Liquids. J. Nanoparticle Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 60. [Google Scholar] [CrossRef]
- Daniele, V.; Taglieri, G.; Quaresima, R. The nanolimes in Cultural Heritage conservation: Characterisation and analysis of the carbonatation process. J. Cult. Herit. 2008, 9, 294–301. [Google Scholar] [CrossRef]
- López-Arce, P.; Gomez-Villalba, L.S.; Pinho, L.; Fernández-Valle, M.E.; de Buergo, M.Á.; Fort, R. Influence of porosity and relative humidity on consolidation of dolostone with calcium hydroxide nanoparticles: Effectiveness assessment with non-destructive techniques. Mater. Charact. 2010, 61, 168–184. [Google Scholar] [CrossRef]
- Daniele, V.; Taglieri, G. Nanolime suspensions applied on natural lithotypes: The influence of concentration and residual water content on carbonatation process and on treatment effectiveness. J. Cult. Herit. 2010, 11, 102–106. [Google Scholar] [CrossRef]
- Pesce, G.L.; Morgan, D.; Odgers, D.; Henry, A.; Allen, M.; Ball, R.J. Consolidation of weathered limestone using nanolime. Proc. Inst. Civ. Eng. Constr. Mater. 2013, 166, 213–228. [Google Scholar] [CrossRef]
- López-Arce, P.; Zornoza-Indart, A.; Gomez-Villalba, L.S.; Fort, R. Short- and Longer-Term Consolidation Effects of Portlandite (CaOH)2 Nanoparticles in Carbonate Stones. J. Mater. Civ. Eng. 2013, 25, 1655–1665. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Licchelli, M.; Malagodi, M.; Rovella, N.; la Russa, M. Consolidation of bio-calcarenite stone by treatment based on diammonium hydrogenphosphate and calcium hydroxide nanoparticles. Measurement 2018, 127, 396–405. [Google Scholar] [CrossRef]
- Taglieri, G.; Otero, J.; Daniele, V.; Gioia, G.; Macera, L.; Starinieri, V.; Charola, A.E. The biocalcarenite stone of Agrigento (Italy): Preliminary investigations of compatible nanolime treatments. J. Cult. Herit. 2018, 30, 92–99. [Google Scholar] [CrossRef]
- Daniele, V.; Taglieri, G.; Macera, L.; Rosatelli, G.; Otero, J.; Charola, A.E. Green approach for an eco-compatible consolidation of the Agrigento biocalcarenites surface. Constr. Build. Mater. 2018, 186, 1188–1199. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; La Russa, M.F.; Aloise, P.; Belfiore, C.M.; Macchia, A.; Pezzino, A.; Crisci, G.M. Efficacy of nanolime in restoration procedures of salt weathered limestone rock. Appl. Phys. A 2014, 114, 753–758. [Google Scholar] [CrossRef]
- British Standards Institution. BS EN 12370:1999 Natural Stone Test Methods: Determination of Resistance to Salt Crystallization; BSI: London, UK, 1999. [Google Scholar]
- British Standards Institution. BS EN 13755:2008 Natural Stone Test Methods: Determination of Water Absorption at Atmospheric Pressure; BSI: London, UK, 2008. [Google Scholar]
- Bonazza, A.; Vidorni, G.; Natali, I.; Ciantelli, C.; Giosuè, C.; Tittarelli, F. Durability assessment to environmental impact of nano-structured consolidants on Carrara marble by field exposure tests. Sci. Total Environ. 2017, 575, 23–32. [Google Scholar] [CrossRef]
- ASTM D5313. Standard Test Method for Evaluation of Durability of Rock for Erosion Control Under Wetting and Drying Conditions; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- UNE-EN 1936:2007. Conservation of Cultural Property-Test Methods-Determination of Water Absorption by Capillarity; AENOR: Madrid, Spain, 2010. [Google Scholar]
- Giorgi, R.; Dei, L.; Baglioni, P. A New Method for Consolidating Wall Paintings Based on Dispersions of Lime in Alcohol. Stud. Conserv. 2000, 45, 154–161. [Google Scholar] [CrossRef]
- UNE-EN. 1936:2007 Métodos de Ensayo Para Piedra Natural. Determinación de la Densidad Real y Aparente y de la Porosidad Abierta y Total; AENOR: Madrid, Spain, 2007. [Google Scholar]
- Papatzani, S.; Paine, K.; Calabria-Holley, J. A comprehensive review of the models on the nanostructure of calcium silicate hydrates. Constr. Build. Mater. 2015, 74, 219–234. [Google Scholar] [CrossRef]
- ASTM D3359/2017 Standard. Test Methods for Rating Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Borsoi, G.; Lubelli, B.; van Hees, R.; Veiga, R.; Silva, A.S. Optimization of nanolime solvent for the consolidation of coarse porous limestone. Appl. Phys. A 2016, 122, 846. [Google Scholar] [CrossRef]
- Borsoi, G.; Lubelli, B.; van Hees, R.; Veiga, R.; Silva, A.S. Evaluation of the effectiveness and compatibility of nanolime consolidants with improved properties. Constr. Build. Mater. 2017, 142, 385–394. [Google Scholar] [CrossRef]
- British Standards Institution. BS EN 14579:2004 - Natural Stone Test Methods: Determination of Sound Speed Propagation; BSI: London, UK, 2005. [Google Scholar]
- Niedoba, K.; Slížková, Z.; Frankeová, D.; Nunes, C.L.; Jandejsek, I. Modifying the consolidation depth of nanolime on Maastricht limestone. Constr. Build. Mater. 2017, 133, 51–56. [Google Scholar] [CrossRef]
- Otero, J.; Starinieri, V.; Charola, A.E. Influence of substrate pore structure and nanolime particle size on the effectiveness of nanolime treatments. Constr. Build. Mater. 2019, 209, 701–708. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Vettori, I.; Ruiz-Agudo, E. Kinetics and Mechanism of Calcium Hydroxide Conversion into Calcium Alkoxides: Implications in Heritage Conservation Using Nanolimes. Langmuir 2016, 32, 5183–5194. [Google Scholar] [CrossRef]
- Papatzani, S.; Grammatikos, S.; Paine, K. Permeable Nanomontmorillonite and Fibre Reinforced Cementitious Binders. Materials 2019, 12, 3245. [Google Scholar] [CrossRef]
- Hoo, C.M.; Starostin, N.; West, P.; Mecartney, M.L. A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanoparticle Res. 2008, 10, 89–96. [Google Scholar] [CrossRef]
- Calabria-Holley, J.; Paine, K.; Papatzani, S. Effects of nanosilica on the calcium silicate hydrates in Portland cement–fly ash systems. Adv. Cem. Res. 2015, 27, 187–200. [Google Scholar] [CrossRef]
- Wild, S. A discussion of the paper: Mercury porosimetry: An inappropriate method for the measurement of pore size distributions in cement-based materials. Author’s reply. Cem. Concr. Res. 2001, 31, 1653–1656. [Google Scholar] [CrossRef]
- Adamaki, V. Manufacturing and Characterisation of Ti-Suboxides for Sensing and Energy Applications. Ph.D. Thesis, University of Bath, Bath, UK, 2015. [Google Scholar]
- Diamond, S. Mercury porosimetry: An inappropriate method for the measurement of pore size distributions in cement-based materials. Cem. Concr. Res. 2000, 30, 1517–1525. [Google Scholar] [CrossRef]
- Raneri, S.; Barone, G.; Mazzoleni, P.; Alfieri, I.; Bergamonti, L.; De Kock, T.; Cnudde, V.; Lottici, P.P.; Lorenzi, A.; Predieri, G.; et al. Efficiency assessment of hybrid coatings for natural building stones: Advanced and multi-scale laboratory investigation. Constr. Build. Mater. 2018, 180, 412–424. [Google Scholar] [CrossRef]
- Raneri, S.; Barone, G.; Mazzoleni, P.; Alfieri, I.; Bergamonti, L.; De Kock, T.; Cnudde, V.; Lottici, P.P.; Lorenzi, A.; Predieri, G.; et al. Multi-scale laboratory routine in the efficacy assessment of conservative products for natural stones. MethodsX 2018, 5, 1095–1101. [Google Scholar] [CrossRef]





| Particle | Ionic Radius (nm = 10−9 m) |
|---|---|
| Calcium ion | 0.1 |
| Hydroxyl ion | 0.14 |
| Nanolime | 50–300 |
| Conventional lime hydrate | ~103–105 |
| Pore space | ~103–104 |
| Nanolime Used and Calcareous Stone | Experimental Techniques Employed | Main Results | Reference |
|---|---|---|---|
| Nanoparticles and baking soda solution applied to Estoril and Pietra Serena lithotypes with brush | Laboratory tests Nanoparticles: XRD, TEM, and SEM Treated stones: Scotch Tape Test (STT), porosity and water absorption tests and SEM to attest penetration depth | Consolidation was confirmed with by tests, but further research must be undertaken to avoid efflorescence phenomena | [26] |
| Nanoparticles of slacked lime (Nanorestore®) applied to calcareous substrates drop by drop | Laboratory tests Nanoparticles: XRD, TEM Treated stones: Environmental scanning electron microscope (ESEM), mobile optical surface roughness (OSR), ultrasound velocity, capillarity, water absorption under vacuum NMR MRI | Non-destructive techniques are reliable. 75% relative humidity enhanced consolidation by filling the pores and the grain contacts | [27] |
| Nanoparticles CaCl2 and NaOH applied to 6 different lithotypes by brush | Laboratory tests Nanoparticles: XRD Treated stones: Scotch Tape test, capillarity tests and MIP to attest pore filling capacity of different concentrations | The effect of different suspension concentrations and of the residual water of the suspension was studied | [28] |
| CaLoSiL® E25, applied using a continuous feed on three different unweathered UK limestones: Weldon, Ketton, and Clipsham | Phenolphthalein | Maximum depth of deposition at 4–5.5 cm | [9] |
| CaLoSiL® E25 and IP25 were used for two different lithotypes | Laboratory tests Nanoparticles: TEM Treated stones: Karsten tube penetration test, the Scotch Tape test, optical microscopy, ESM-EDX, drilling resistance measurements (DRM), and mercury intrusion porosimetry | The DRM test can successfully measure the enhancement in the mechanical properties with respect to the depth of the treatment. Other tests were sensitive to the nanolime treatment | [29] |
| Nanoparticles of slacked lime (Nanorestore®) applied to calcareous substrates, drop by drop | Laboratory tests Nanoparticles: XRD, ESEM Treated stones: Non-destructive techniques (NDTs) (ESEM, spectrophotometry, water absorption under vacuum and capillarity, ultrasonic velocity, and optical surface roughness analyses) were compared with destructive techniques (MIP and micro-drilling resistance measurement) | Different RH exposure conditions (33% and 75% RH). NDTs corroborated well with the destructive techniques | [30] |
| Nanorestore® for artificially degraded stones by salt crystallization | Laboratory tests Treated stones: Peeling test (STT), point load test, spectrophotometry, and MIP | STT and the point load tests are complementary. MIP can be processed to give crystallization pore pressures. An increase in the crystallization pressure within the pores can possibly lead to stone decay | [34] |
| CaLoSiL® E25 | Laboratory tests Treated stones: Capillary absorption test, drying test, phenolphthalein test, stereomicroscopy, and SEM-EDX | Nanolime particles penetrate to depths up to 40 mm but partly back-migrate towards the drying surface and, thus, accumulation beneath the treated surface occurs | [12] |
| Comparison of synthetized nanolimes in ethanol, isopropanol, butanol, and water and applied by capillary absorption | Laboratory tests Nanoparticles: dynamic light scattering and SEM-EDX, UV-Vis spectroscopy, XRD Treated stones: Capillary absorption test, drying test, phenolphthalein test, SEM-EDX, and optical microscopy | Ethanol and isopropanol solvents gave the most stable dispersions. Butanol and water solvents did not show significant back migration | [2] |
| Comparison of synthetized Nanolimes dispersed with ethanol-based solvents with different percentages of water and applied by capillary absorption | Laboratory tests Nanoparticles: UV-Vis spectroscopy Treated stones: phenolphthalein test, SEM-EDX, and optical microscopy | Optimal results were provided for the 95% ethanol and 5% water content | [45] |
| Synthetized nanolimes dispersed with pure ethanol solvents and applied by nebulization on Maastricht limestone | Laboratory tests Treated stones: DRM, capillary absorption tests, photography, and digital microscopy | The synthesized nanolime dispersion can reach up to 16 mm in depth, maximizing its effect in the outer 5–6 mm | [46] |
| Synthetized nanolimes (NANOMATCH1) and CaLoSiL® | Field exposure and laboratory tests optical microscopy, ESM-EDX, STT, capillary water absorption, ultrasonic pulse velocity tests | Both materials did not penetrate deep into the substrate. They both remained on the surface as sacrifice material or protective coating rather than functioning as a consolidant. Heavy rain was found to be the most detrimental of all environmental parameters | [38] |
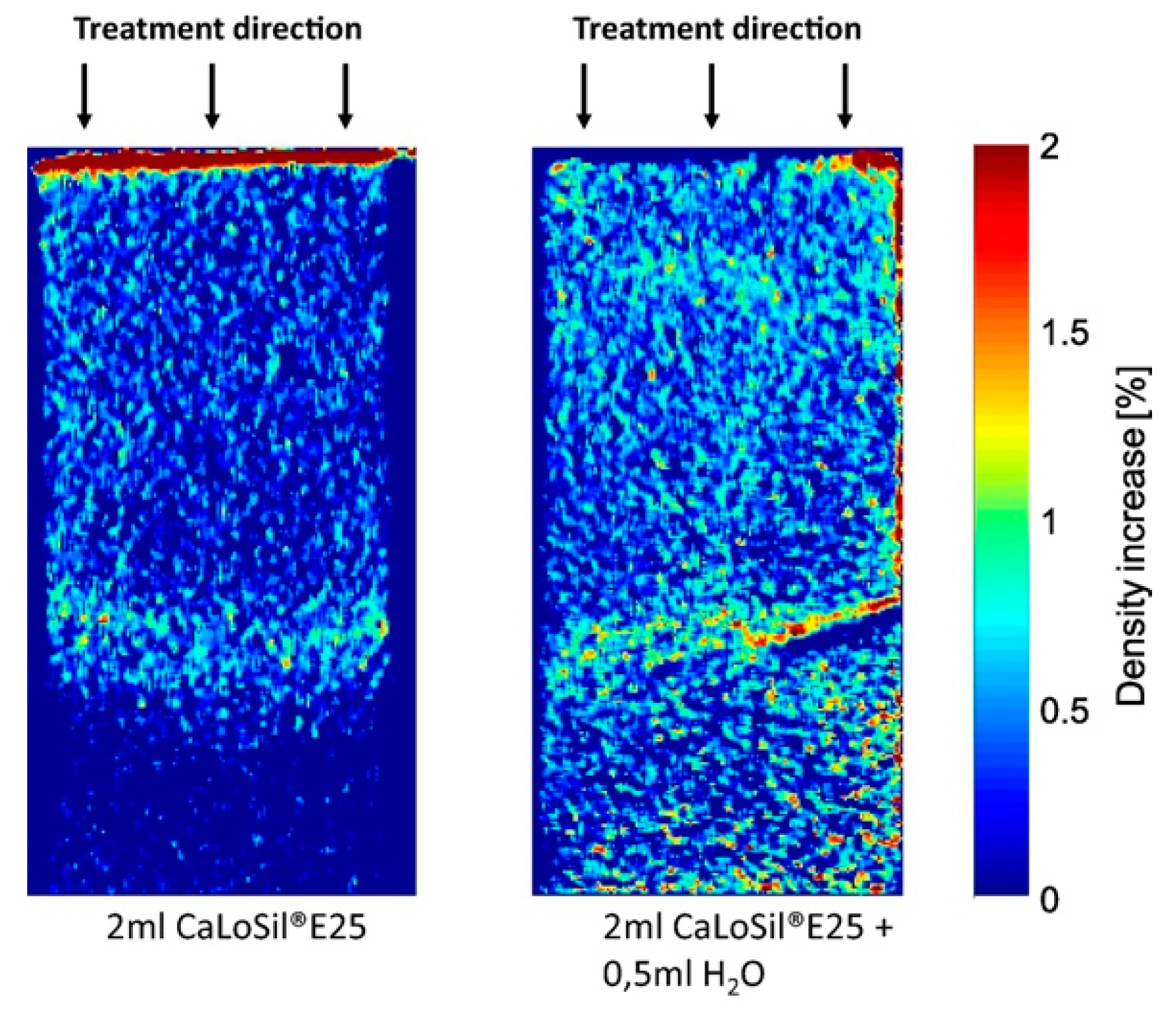
| CaLoSil® E25, CaLoSil® E50, and nanoparticles dispersed in water, lime water, barium water, and ethanol | Differential X-ray transmission radiography and DRM | Applying water over samples treated with CaLoSil® prevented the formation of a high-density layer at the surface of the stone and promoted homogeneous dispersion of nanolimes | [48] |
| Nanolime in isopropanol applied by brush on Lecce stone and then DAP applied by brush | Laboratory tests Nanoparticles: DLS, SEM, and XRD In situ mineralization Treated stones: Water capillary absorption and permeability, spectrophotometry, XRD, SEM, SEM-EDX, MIP, Scotch Tape test and resistance to salt crystallization | The nanolime + DAP application enhanced hydric properties, surface cohesion, and strength of the treated stone to the extent that it can be suggested for in situ applications | [31] |
| Superficial consolidation of bio – calcarenites by brushing (i) water/isopropanol of 5 g/L; (ii) water/isopropanol of 10 g/L; and (iii) water/1-butanol dispersion of 5 g/L | Laboratory tests Nanoparticles: XRD, TEM, UV/Vis Treated stones: Water absorption by capillarity, STT, DRM, MIP, spectrophotometry | Best results: the alcoholic suspension 10 g/L after three treatments: 60% reduction in surface material removal and 50% reduction in water adsorption by capillary | [32] |
| Nanolime in varied dispersion medium; alcohol, water and mixtures of both applied by spraying or brushing | Laboratory tests Nanoparticles: XRD, UV/Vis Treated stones: Water absorption by capillarity, STT, DRM, MIP, stereomicroscopy | Best results: spraying and aqueous dispersion—90% reduction of materials removed from the surface for a 1 cm depth | [33] |
| Nanorestore Plus Propanol 5® and synthetized nanolime at 5 g/l dispersed in 50% water and 50% isopropanol and applied by brushing | Laboratory tests Treated stones: Phenolphthalein solution, MIP, the capillarity water absorption, STT, DRM, and spectrophotometry | Nanolime consolidants with smaller particle size close both smaller and larger pores equally | [49] |
| Nanolime Properties | Ambiental Factors | Other Parameters |
|---|---|---|
| Concentration | RH | Nature and porosity of substrate |
| Particle size | Temperature | Amount of consolidant deposited (surface or mass consolidation) |
| Solvent type | Exposure time | Storage conditions–duration |
| Morphology and surface area of particles | Available CO2 | Application method and number of applications |
| Kinetic stability and reactivity | Duration and intensity of rainfall | Time allowed for consolidation and weathering to take place |
| Consolidation Efficiency Parameters/Material Properties | Options on Characterization Techniques |
|---|---|
| Distribution of consolidants mapping | Differential X-ray radiography imaging (with micro CT-3D representation of pore structure) Environmental scanning electron microscopy Marking agents (phenolphthalein method) Neutron imaging Nuclear magnetic resonance (NMR) magnetic resonance imaging (MRI) (to measure the distribution of nanoproducts inside the pores) |
| Consolidation depth | Differential X-ray radiography imaging Marking agents (phenolphthalein) Drilling resistance measurements X-Ray radiography micro CT Nuclear magnetic resonance Small angle neutron imaging |
| Color changes (aesthetic compatibility) | Spectrophotometry Stereomicroscopy (or digital microscopy) |
| Pore structure | Mercury intrusion porosimetry (pore size distribution and open porosity) X-ray radiography micro CT Small angle neutron imaging Ultrasonic pulse velocity tests (EN 14579:2004 to assess durability affected by porosity, the extent of penetration) |
| Surface consolidation, cohesion, roughness | Scotch Tape test ASTM D3359 Stereomicroscopy (for surface texture and structure variations) Drilling resistance measurements Scanning electron microscopy Optical surface roughness (for 3D topography maps) |
| Strength | Resistance to salt crystallization Scotch Tape test Drilling resistance measurements Point load test Thermal gravimetric analysis |
| Crystals present, phases formed | X-ray diffraction Thermal gravimetric analysis |
| Hydric properties | Capillary absorption test for absorbed water (water absorption coefficient is determined by EN15801: 2010) Water absorption under vacuum (UNE-EN 1936:1999) Water vapor permeability Karsten tube penetration test (to determine water absorption) Neutron imaging (to monitor water movement) Nuclear magnetic resonance (NMR) magnetic resonance imaging (MRI) (to measure the distribution of water inside the pores) |
| Durability in terms of salt crystallization | Spanish standard UNE-EN: 12370 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papatzani, S.; Dimitrakakis, E. A Review of the Assessment Tools for the Efficiency of Nanolime Calcareous Stone Consolidant Products for Historic Structures. Buildings 2019, 9, 235. https://doi.org/10.3390/buildings9110235
Papatzani S, Dimitrakakis E. A Review of the Assessment Tools for the Efficiency of Nanolime Calcareous Stone Consolidant Products for Historic Structures. Buildings. 2019; 9(11):235. https://doi.org/10.3390/buildings9110235
Chicago/Turabian StylePapatzani, Styliani, and Emmanouil Dimitrakakis. 2019. "A Review of the Assessment Tools for the Efficiency of Nanolime Calcareous Stone Consolidant Products for Historic Structures" Buildings 9, no. 11: 235. https://doi.org/10.3390/buildings9110235
APA StylePapatzani, S., & Dimitrakakis, E. (2019). A Review of the Assessment Tools for the Efficiency of Nanolime Calcareous Stone Consolidant Products for Historic Structures. Buildings, 9(11), 235. https://doi.org/10.3390/buildings9110235






