Assessment of Biogenic Healing Capability, Mechanical Properties, and Freeze–Thaw Durability of Bacterial-Based Concrete Using Bacillus subtilis, Bacillus sphaericus, and Bacillus megaterium
Abstract
1. Introduction
2. Materials and Testing Methods
2.1. Concrete Materials
2.2. Bacterial Microorganisms
2.3. Nutrient Media
2.4. Spores Immobilization
2.5. Specimen Preparation
2.6. Specimens Pre-Cracking
2.7. Self-Healing Evaluation
2.7.1. Visual Assessment
2.7.2. UPV Assessment
2.8. Mechanical and Freeze–Thaw Durability Tests
2.9. Microstructural Characterization Tests
3. Results and Discussion
3.1. Evaluation of Self-Healing Efficiency
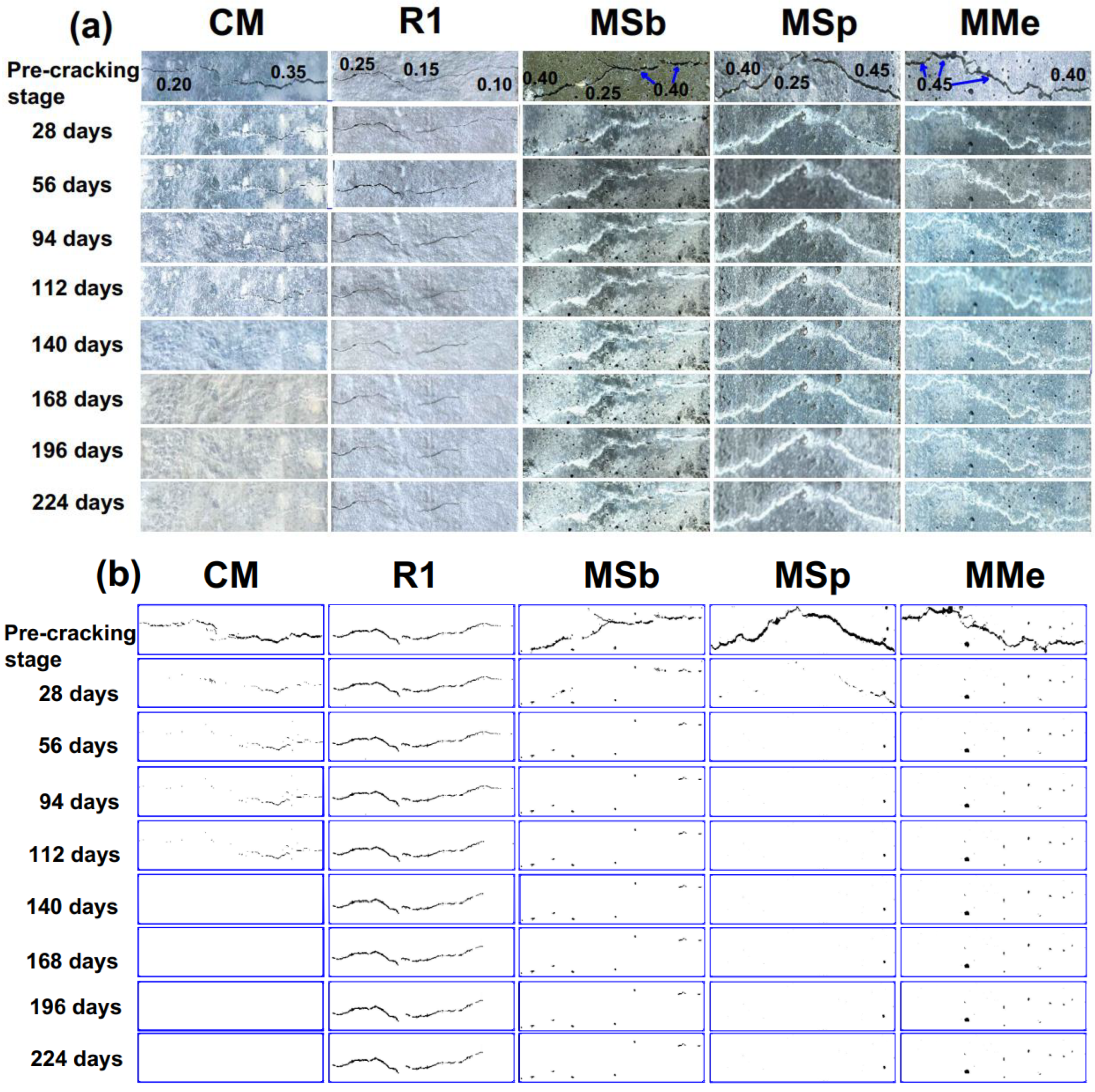
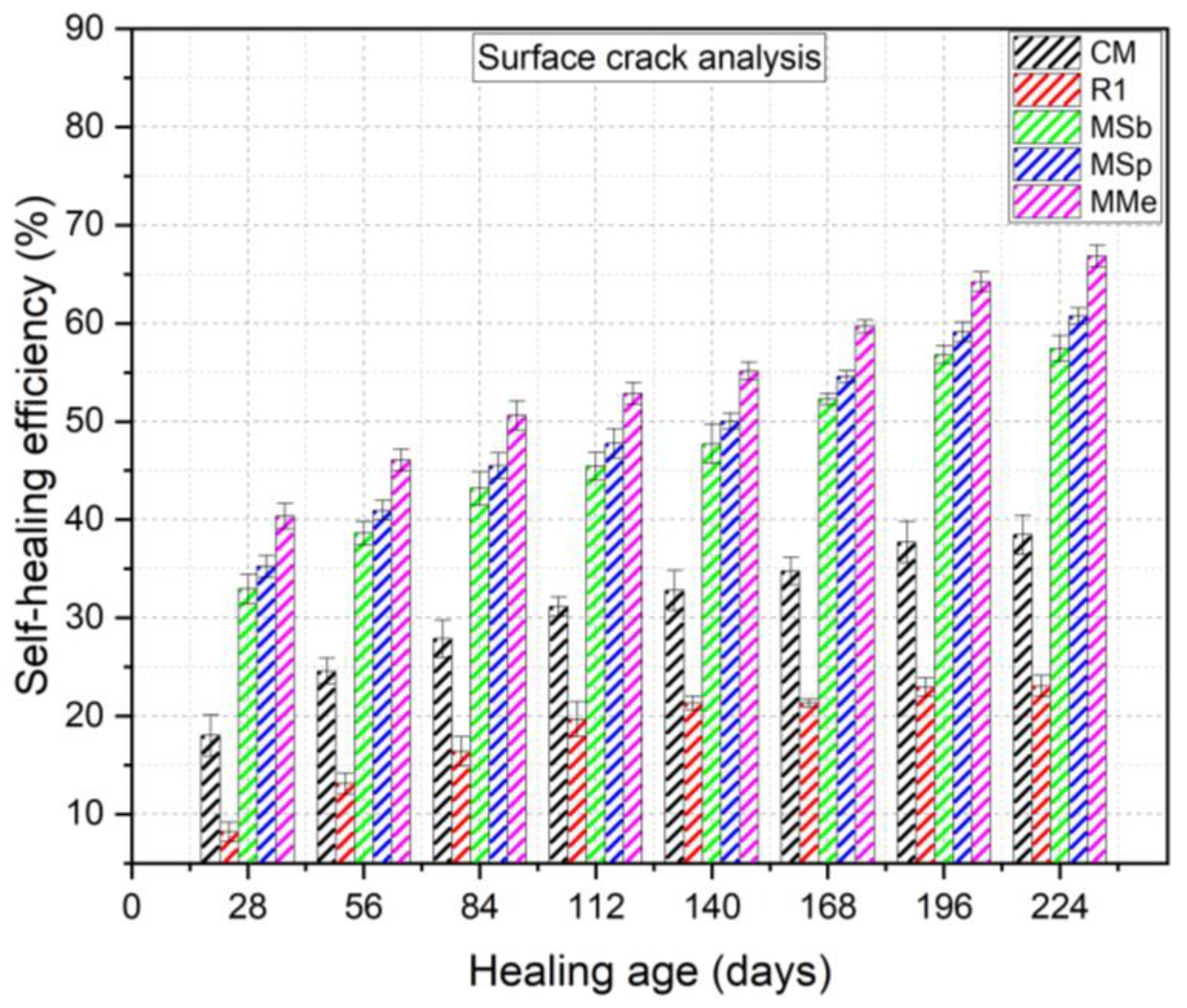
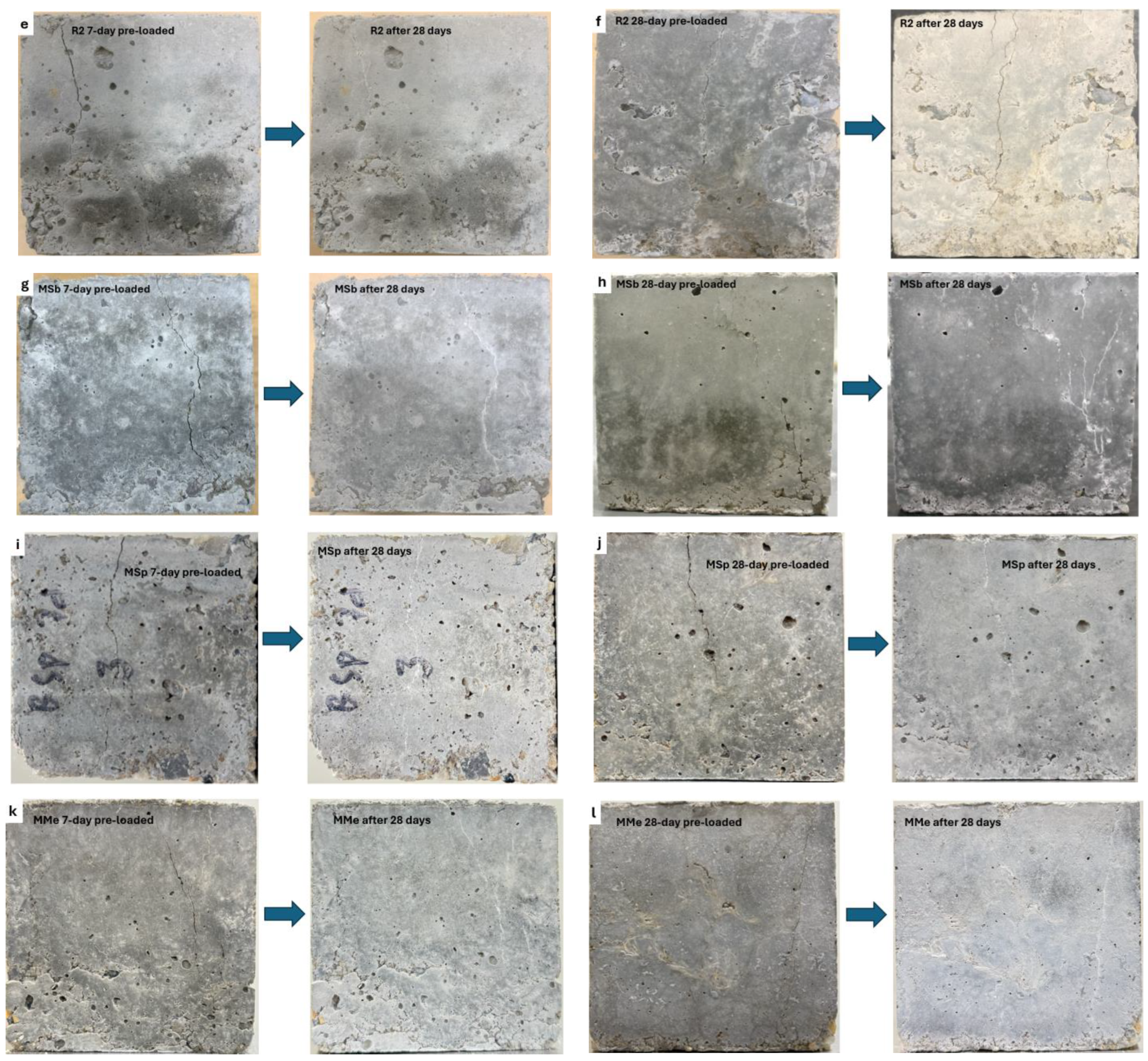
3.1.1. Visual Assessment and Analysis of Surface Cracks
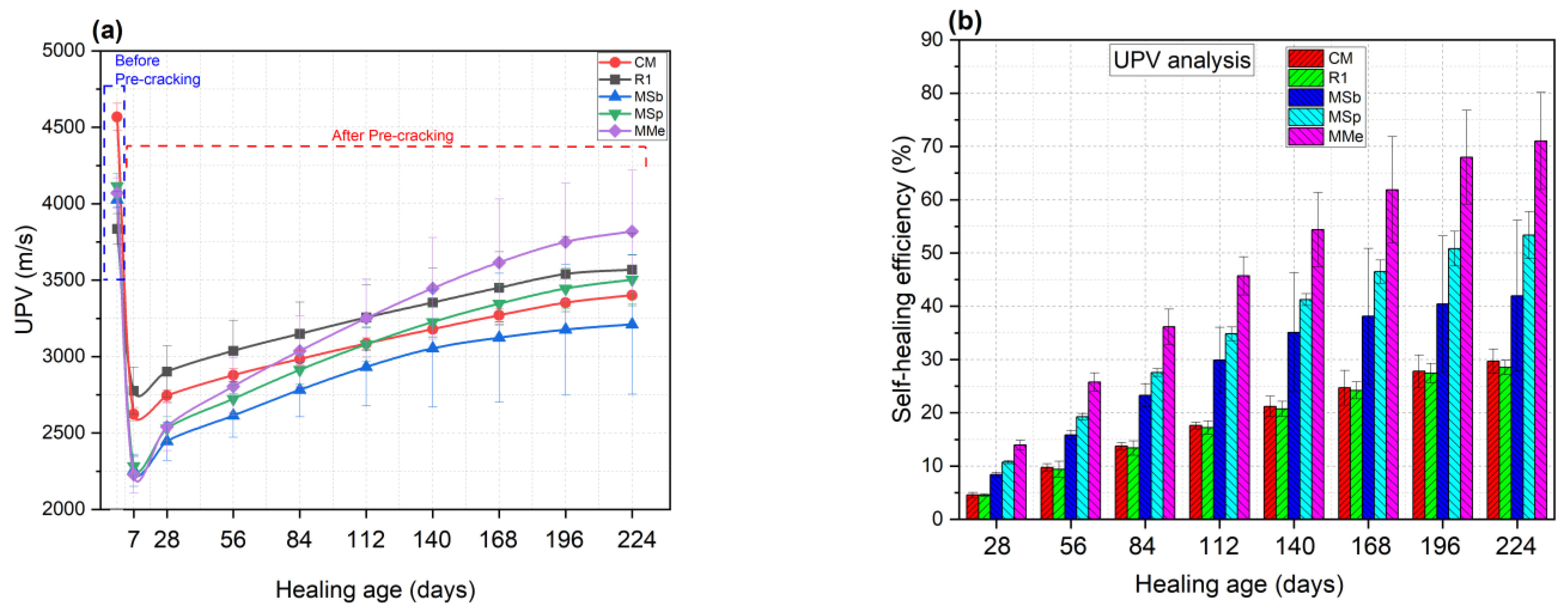
3.1.2. Self-Healing Effectiveness Based on UPV Analysis
3.2. Compressive Strength
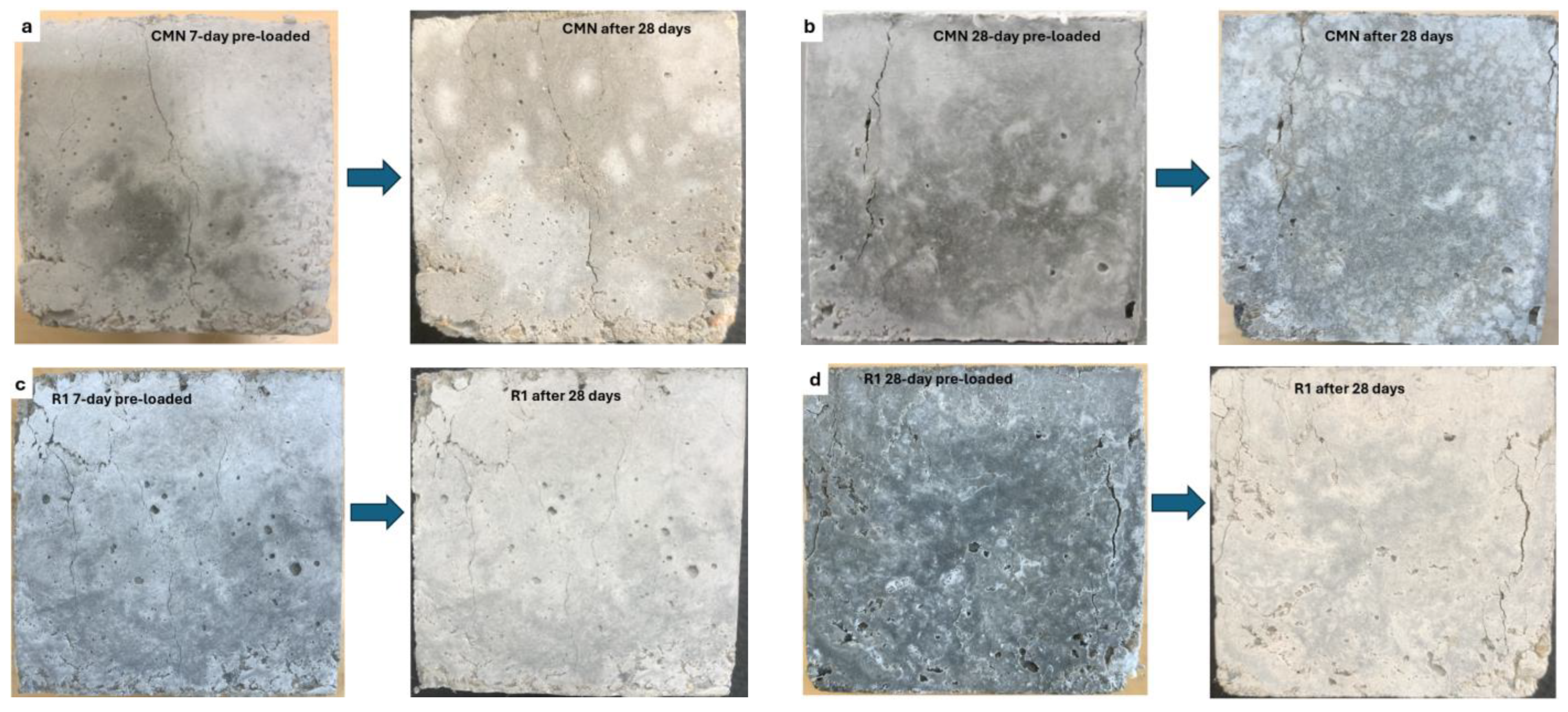
3.3. Restoration in Compression Strength
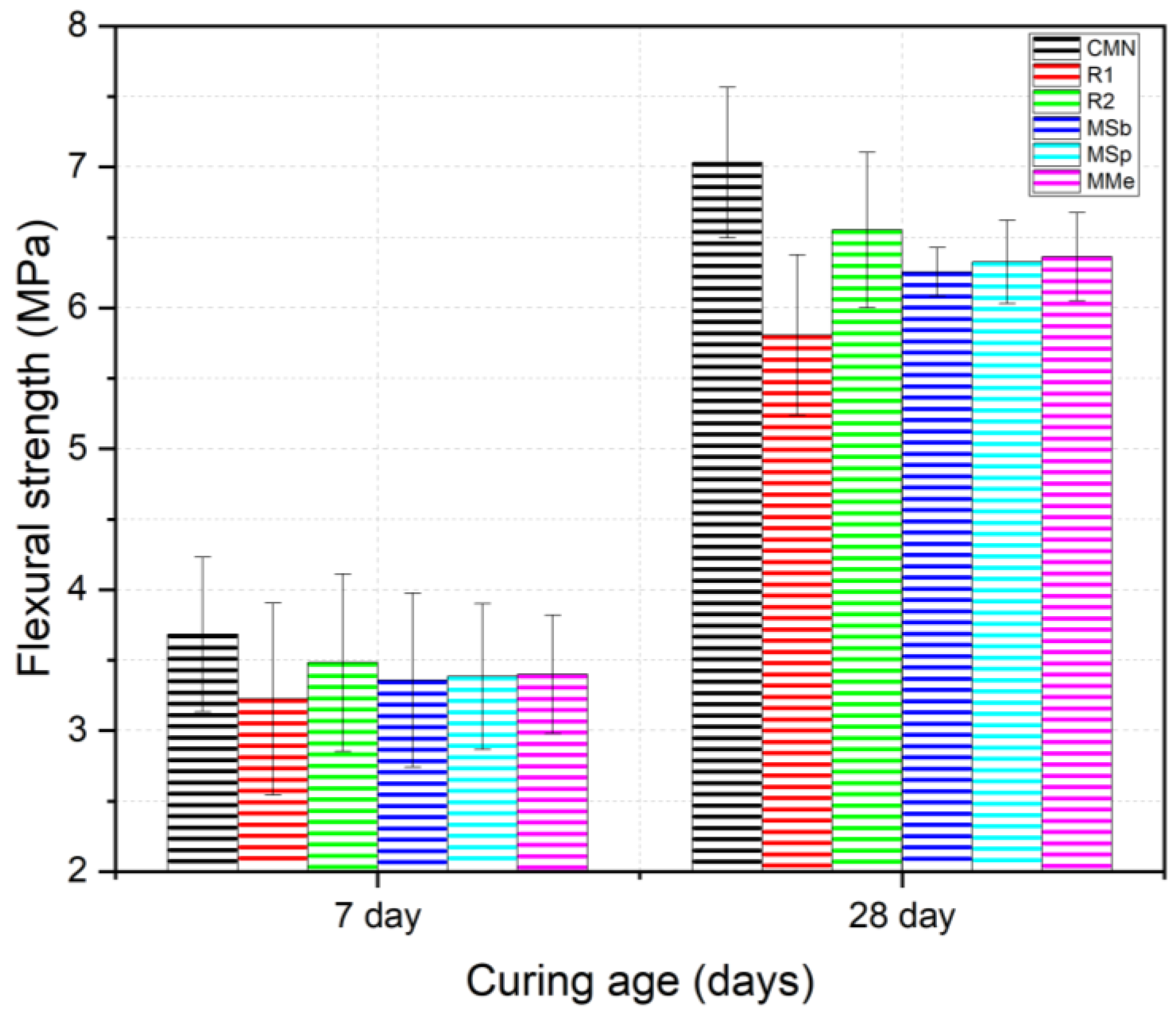
3.4. Flexural Strength
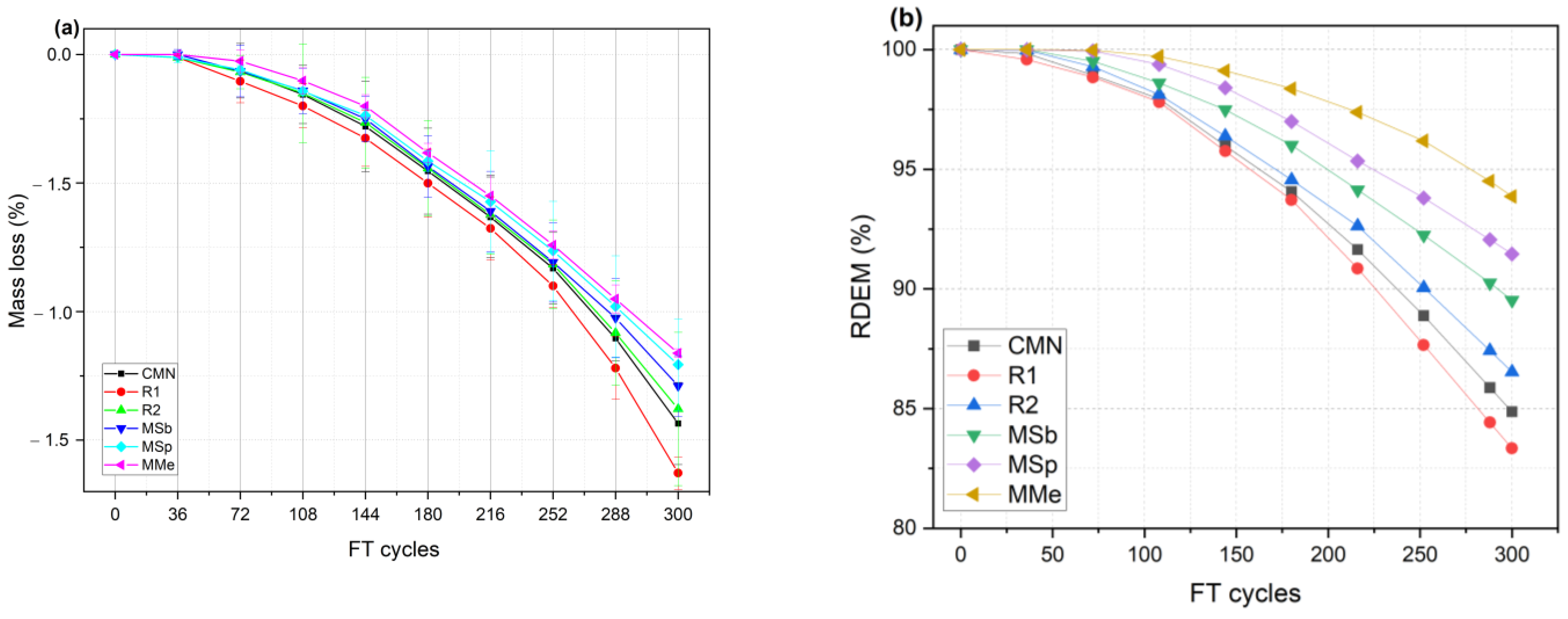
3.5. Freeze–Thaw Durability
3.6. Microstructural Analysis
4. Conclusions
- Crack healing results exhibited that LECA is a promising carrier material for microorganisms, sheltering them for up to 224 days due to its internal porous structure, which accommodated both the microorganisms and nutrients while shielding them from the highly alkaline environment within the concrete.
- Assessment of self-healing performance through UPV analysis demonstrated that MMe achieved the highest self-healing efficiency, with a value of 71.03%, followed by MSp (53.38%), MSb (41.96%), CM (29.70%), and R1 (28.53%) after 224 days. Additionally, the maximum crack width completely healed by MMe was 0.45 mm within the first 28 days, while MSp and MSb achieved complete healing of crack widths of 0.45 mm and 0.40 mm within 56 days, respectively. These results indicated the rapid germination and sequential calcite precipitation capability of Bacillus megaterium as compared to Bacillus subtilis and Bacillus sphaericus.
- Compression and flexural strengths were reduced by the partial replacement of natural coarse aggregates with LECAs. However, this negative impact on strength behavior was slightly reduced with the inclusion of PVA fibers and bacteria, resulting in strength values higher than R1 but still lower than CMN. Furthermore, the slightly reduced strength behavior of bacterial mixes as compared to R2 was attributed to the impact of higher concentrations of calcium lactate.
- Bacterial mixes successfully restored both 7-day and 28-day compression strengths after 28 days of healing, underscoring the effectiveness of biomineralization in improving the bond strength and densifying the matrix.
- Freeze–thaw durability performance of bacterial mixes, particularly MMe, exhibited reduced mass loss and higher RDEM compared to control and reference mixes after 300 freeze–thaw cycles. The mass losses recorded at the end of 300 freeze–thaw cycles were 1.44%, 1.62%, 1.38%, 1.29%, 1.20%, and 1.16% for CMN, R1, R2, MSb, MSp, and MMe, respectively. Similarly, the RDEM values recorded at the end of 300 freeze–thaw cycles were 84.87%, 83.84%, 86.53%, 89.53%, 91.46%, and 93.87% for CMN, R1, R2, MSb, MSp, and MMe, respectively. These results indicated the contribution of calcite precipitation in the densification of the critical zones such as ITZs, impeding water ingress and thus reducing the freeze–thaw damage.
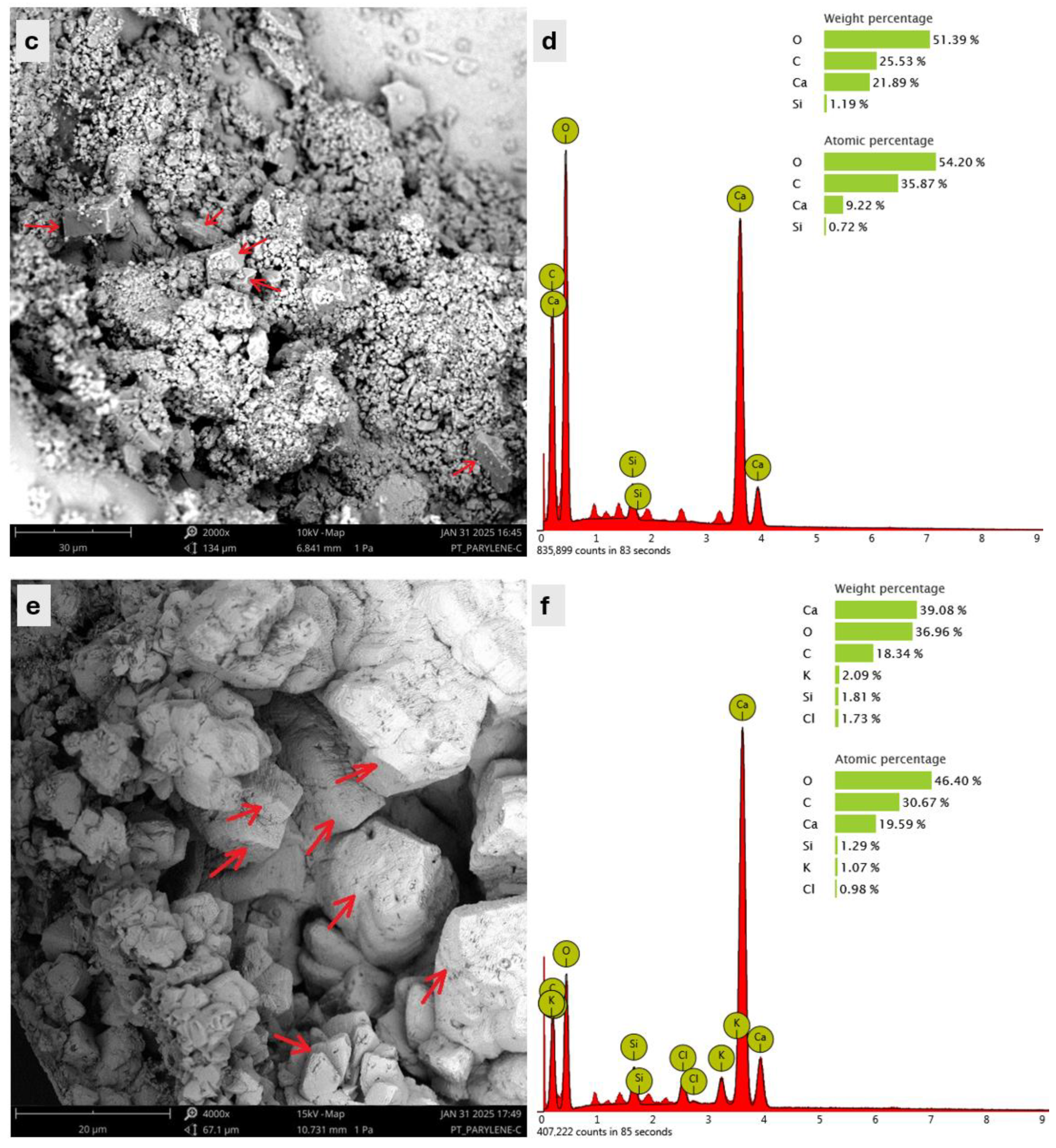
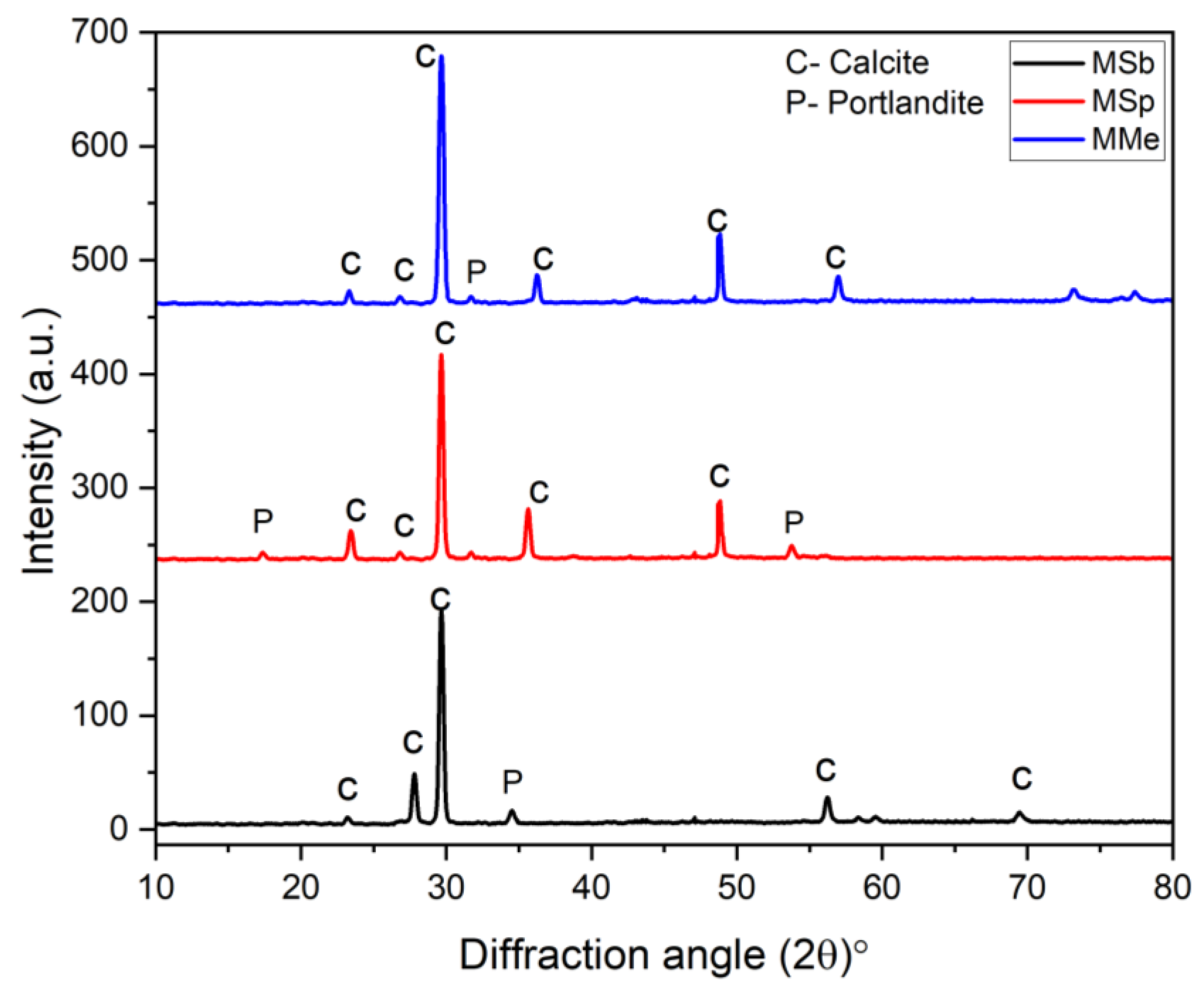
- SEM analysis verified the formation of rhombohedral crystals composed of calcium, carbon, and oxygen elements corresponding to the precipitation of calcite as a healing product. Furthermore, the EDS and XRD results demonstrated a higher content of calcite formation in the case of MMe as compared to MSp and MSb at the healing age of 28 days, proving the fast rate of biomineralization capability of Bacillus megaterium.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmad, I.; Shen, D.; Khan, K.A.; Jan, A.; Khubaib, M.; Ahmad, T.; Nasir, H. Effectiveness of limestone powder in controlling the shrinkage behavior of cement based system: A review. Silicon 2021, 14, 359–371. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Guan, J.; Zhou, X.; Guo, L. Fully coupled peridynamic model for analyzing the chemo-diffusion-mechanical behavior of sulfate attack in concrete. Constr. Build. Mater. 2023, 409, 133874. [Google Scholar] [CrossRef]
- Chen, J.; Jia, J.; Zhu, M. Development of admixtures on seawater sea sand concrete: A critical review on concrete hardening, chloride ion penetration and steel corrosion. Constr. Build. Mater. 2024, 411, 134219. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, W.; Li, Z.; Jia, G.; Zhang, Y.; Ma, G.; Gao, Y. Mechanical properties and frost resistance of self-healing concrete based on expanded perlite with different particle sizes as microbial carrier. Constr. Build. Mater. 2024, 422, 135450. [Google Scholar] [CrossRef]
- Mignon, A.; Snoeck, D.; Dubruel, P.; Van Vlierberghe, S.; De Belie, N. Crack mitigation in concrete: Superabsorbent polymers as key to success? Materials 2017, 10, 237. [Google Scholar] [CrossRef]
- Verma, D.; Sharma, M.; Goh, K.-L.; Jain, S.; Sharma, H. Sustainable Biopolymer Composites: Biocompatibility, Self-Healing, Modeling, Repair and Recyclability; Woodhead Publishing: Sawston, UK, 2021. [Google Scholar]
- Zhao, J.; Csetenyi, L.; Gadd, G.M. Fungal-induced CaCO3 and SrCO3 precipitation: A potential strategy for bioprotection of concrete. Sci. Total Environ. 2022, 816, 151501. [Google Scholar] [CrossRef]
- Khushnood, R.A.; Qureshi, Z.A.; Shaheen, N.; Ali, S. Bio-mineralized self-healing recycled aggregate concrete for sustainable infrastructure. Sci. Total Environ. 2020, 703, 135007. [Google Scholar] [CrossRef]
- Song, Y.; Chetty, K.; Garbe, U.; Wei, J.; Bu, H.; O’moore, L.; Li, X.; Yuan, Z.; McCarthy, T.; Jiang, G. A novel granular sludge-based and highly corrosion-resistant bio-concrete in sewers. Sci. Total Environ. 2021, 791, 148270. [Google Scholar] [CrossRef]
- Meera, C.; Subha, V. Strength and durability assessment of bacteria based self-healing concrete. IOSR J. Mech. Civ. Eng. 2016, 3, 1–7. [Google Scholar]
- Ahmad, I.; Shokouhian, M.; Jenkins, M.; McLemore, G.L. Factors influencing bacterial-based precipitation, assessment of crack inducing, durability and characterization methods: A comprehensive review. Innov. Infrastruct. Solut. 2025, 10, 107. [Google Scholar] [CrossRef]
- Ahmad, I.; Shokouhian, M.; Jenkins, M.; McLemore, G.L. Quantifying the self-healing efficiency of bioconcrete using Bacillus subtilis immobilized in polymer-coated lightweight expanded clay aggregates. Buildings 2024, 14, 3916. [Google Scholar] [CrossRef]
- Huynh, N.N.T.; Imamoto, K.-I.; Kiyohara, C. A study on biomineralization using Bacillus subtilis natto for repeatability of self-Healing concrete and strength improvement. J. Adv. Concr. Technol. 2019, 17, 700–714. [Google Scholar] [CrossRef]
- Ferral-Pérez, H.; Galicia-García, M. Bioprecipitation of calcium carbonate by Bacillus subtilis and its potential to self-healing in cement-based materials. J. Appl. Res. Technol. 2020, 18, 245–258. [Google Scholar]
- Nasser, A.A.; Sorour, N.M.; Saafan, M.A.; Abbas, R.N. Microbially-Induced-Calcite-Precipitation (MICP): A biotechnological approach to enhance the durability of concrete using Bacillus pasteurii and Bacillus sphaericus. Heliyon 2022, 8, e09879. [Google Scholar] [CrossRef]
- Wang, J.-Y.; De Belie, N.; Verstraete, W. Diatomaceous earth as a protective vehicle for bacteria applied for self-healing concrete. J. Ind. Microbiol. Biotechnol. 2012, 39, 567–577. [Google Scholar] [CrossRef]
- Wang, J.; Van Tittelboom, K.; De Belie, N.; Verstraete, W. Use of silica gel or polyurethane immobilized bacteria for self-healing concrete. Constr. Build. Mater. 2012, 26, 532–540. [Google Scholar] [CrossRef]
- Justo-Reinoso, I.; Reeksting, B.J.; Hamley-Bennett, C.; Heath, A.; Gebhard, S.; Paine, K. Air-entraining admixtures as a protection method for bacterial spores in self-healing cementitious composites: Healing evaluation of early and later-age cracks. Constr. Build. Mater. 2022, 327, 126877. [Google Scholar] [CrossRef]
- Ivaškė, A.; Gribniak, V.; Jakubovskis, R.; Urbonavičius, J. Bacterial viability in self-healing concrete: A case study of non-ureolytic Bacillus species. Microorganisms 2023, 11, 2402. [Google Scholar] [CrossRef]
- Bifathima, S.; Matcha, B.N. Self healing concrete by adding Bacillus megaterium MTCC with glass & steel fibers. Civ. Environ. Eng. 2020, 16, 184–197. [Google Scholar]
- Su, Y.; Zheng, T.; Qian, C. Application potential of Bacillus megaterium encapsulated by low alkaline sulphoaluminate cement in self-healing concrete. Constr. Build. Mater. 2021, 273, 121740. [Google Scholar] [CrossRef]
- Wani, S.; Gęca, M.J.; Selvaraj, T.; Priya, T.S. Assessing the influence of Bacillus megaterium and Bacillus sphaericus in cementitious materials: Promoting sustainability towards strength, durability and crack repair. Ain Shams Eng. J. 2024, 15, 102748. [Google Scholar] [CrossRef]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzym. Microb. Technol. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Sarda, D.; Choonia, H.S.; Sarode, D.; Lele, S. Biocalcification by Bacillus pasteurii urease: A novel application. J. Ind. Microbiol. Biotechnol. 2009, 36, 1111–1115. [Google Scholar] [CrossRef]
- Omar, O.; Noorvand, H.; Hassan, M.; Subedi, S.; Milla, J.; Rupnow, T. Effect of using calcium lactate with Bacillus pseudofirmus bacteria on self-healing efficiency of bacterial concrete. Tran-SET 2022, 2022, 67–76. [Google Scholar]
- Elgendy, I.M.; Elkaliny, N.E.; Saleh, H.M.; Darwish, G.O.; Almostafa, M.M.; Metwally, K.; Yahya, G.; Mahmoud, Y.A. Bacteria-powered self-healing concrete: Breakthroughs, challenges, and future prospects. J. Ind. Microbiol. Biotechnol. 2024, 52, kuae051. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, B.; Sun, W.; Wang, Y. Microbial induced calcium carbonate precipitation study using Bacillus subtilis with application to self-healing concrete preparation and characterization. Constr. Build. Mater. 2021, 280, 122460. [Google Scholar] [CrossRef]
- Elmahdy, M.A.; ELShami, A.; Yousry, E.-S.M.; Ahmad, S.S. Self-healing mortar using different types, content, and concentrations of bacteria to repair cracks. Frat. Integrita Strutt. 2022, 59, 486–513. [Google Scholar]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Feng, T.; Zhou, M.; Zhao, L.; Zhou, A.; Li, Z. Immobilizing bacteria in expanded perlite for the crack self-healing in concrete. Constr. Build. Mater. 2017, 148, 610–617. [Google Scholar] [CrossRef]
- Mostofinejad, D.; Nosouhian, F.; Tayebani, B. Evaluation of mechanical properties of bacteria-containing mortar in seawater environment. ACI Mater. J. 2021, 118, 35–43. [Google Scholar]
- Ahmed, S.O.; Nasser, A.A.; Abbas, R.N.; Kamal, M.M.; Zahran, M.A.; Sorour, N.M. Production of bioconcrete with improved durability properties using Alkaliphilic Egyptian bacteria. 3 Biotech 2021, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Chaerun, S.K.; Syarif, R.; Wattimena, R.K. Bacteria incorporated with calcium lactate pentahydrate to improve the mortar properties and self-healing occurrence. Sci. Rep. 2020, 10, 17873. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Özyurt, N. Improved strength and durability of fly ash-amended concrete by microbial calcite precipitation. Ecol. Eng. 2011, 37, 554–559. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, C. A new type capsule-based healing agent for concrete and its protective function of spores. Smart Mater. Struct. 2020, 29, 105035. [Google Scholar] [CrossRef]
- Wang, J.; Soens, H.; Verstraete, W.; De Belie, N. Self-healing concrete by use of microencapsulated bacterial spores. Cem. Concr. Res. 2014, 56, 139–152. [Google Scholar] [CrossRef]
- Wang, J.; Snoeck, D.; Van Vlierberghe, S.; Verstraete, W.; De Belie, N. Application of hydrogel encapsulated carbonate precipitating bacteria for approaching a realistic self-healing in concrete. Constr. Build. Mater. 2014, 68, 110–119. [Google Scholar] [CrossRef]
- Wang, J.; Mignon, A.; Trenson, G.; Van Vlierberghe, S.; Boon, N.; De Belie, N. A chitosan based pH-responsive hydrogel for encapsulation of bacteria for self-sealing concrete. Cem. Concr. Compos. 2018, 93, 309–322. [Google Scholar] [CrossRef]
- Palin, D.; Wiktor, V.; Jonkers, H. A bacteria-based bead for possible self-healing marine concrete applications. Smart Mater. Struct. 2016, 25, 084008. [Google Scholar] [CrossRef]
- Shahid, S.; Aslam, M.A.; Ali, S.; Zameer, M.; Faisal, M. Self-healing of cracks in concrete using Bacillus strains encapsulated in sodium alginate beads. ChemistrySelect 2020, 5, 312–323. [Google Scholar] [CrossRef]
- Yan, Y.; Jia, G.; Zhang, Y.; Gao, Y.; Li, Z. The influence of expanded perlite as a bio-carrier on the freeze–thaw properties of self-healing concrete. Constr. Build. Mater. 2023, 409, 133891. [Google Scholar] [CrossRef]
- Prasad, P.; Neeraja, D.; Ravitheja, A. Effect of LECA on mechanical properties of self-curing concrete. Mater. Today Proc. 2019, 19, 484–488. [Google Scholar]
- Shivanshi, S.; Chakraborti, G.; Upadhyaya, K.S.; Kannan, N. A study on bacterial self-healing concrete encapsulated in lightweight expanded clay aggregates. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Yan, Y.; Tian, H.; Liu, W.; Jia, G.; Li, Z.; Gao, Y.; Zhang, Y.; Ma, G. Study on frost resistance and life prediction of microbial self-healing concrete based on expanded perlite as carrier. J. Build. Eng. 2024, 91, 109693. [Google Scholar] [CrossRef]
- Liu, Z.; Chin, C.S.; Xia, J. Novel method for enhancing freeze–thaw resistance of recycled coarse aggregate concrete via two-stage introduction of denitrifying bacteria. J. Clean. Prod. 2022, 346, 131159. [Google Scholar] [CrossRef]
- ASTM C150/C150M–19a; Standard Specification for Portland Cement. American Society for Testing and Materials (ASTM) International: West Conshohocken, PA, USA, 2019.
- ASTM C127–15; Standard Test Method for Relative Density (Specific Gravity) and Absorption of Coarse Aggregate. ASTM International: West Conshohocken, PA, USA, 2015.
- Khaliq, W.; Ehsan, M.B. Crack healing in concrete using various bio influenced self-healing techniques. Constr. Build. Mater. 2016, 102, 349–357. [Google Scholar] [CrossRef]
- Pachaivannan, P.; Hariharasudhan, C.; Mohanasundram, M.; Bhavani, M.A. Experimental anaylsis of self healing properties of bacterial concrete. Mater. Today Proc. 2020, 33, 3148–3154. [Google Scholar] [CrossRef]
- Hossain, M.R.; Sultana, R.; Patwary, M.M.; Khunga, N.; Sharma, P.; Shaker, S.J. Self-healing concrete for sustainable buildings. A review. Environ. Chem. Lett. 2022, 20, 1265–1273. [Google Scholar] [CrossRef]
- Ducasse-Lapeyrusse, J.; Gagné, R.; Lors, C.; Damidot, D. Effect of calcium gluconate, calcium lactate, and urea on the kinetics of self-healing in mortars. Constr. Build. Mater. 2017, 157, 489–497. [Google Scholar] [CrossRef]
- Abo Sabah, S.H.; Anneza, L.H.; Juki, M.I.; Alabduljabbar, H.; Othman, N.; Al-Gheethi, A.A.; Al-Shalif, A.F. The use of calcium lactate to enhance the durability and engineering properties of bioconcrete. Sustainability 2021, 13, 9269. [Google Scholar] [CrossRef]
- Lucas, S.S.; Moxham, C.; Tziviloglou, E.; Jonkers, H. Study of self-healing properties in concrete with bacteria encapsulated in expanded clay. Sci. Technol. Mater. 2018, 30, 93–98. [Google Scholar] [CrossRef]
- Xu, J.; Du, Y.; Jiang, Z.; She, A. Effects of calcium source on biochemical properties of microbial CaCO3 precipitation. Front. Microbiol. 2015, 6, 1366. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Wei, G.; Ma, G.; Zhang, Y.; Zheng, X.; Zhang, L.; Xu, R. Influence of bacterial concentration on crack self-healing of cement-based materials. Constr. Build. Mater. 2020, 244, 118372. [Google Scholar] [CrossRef]
- Rauf, M.; Khaliq, W.; Khushnood, R.A.; Ahmed, I. Comparative performance of different bacteria immobilized in natural fibers for self-healing in concrete. Constr. Build. Mater. 2020, 258, 119578. [Google Scholar] [CrossRef]
- Alghamri, R.; Kanellopoulos, A.; Al-Tabbaa, A. Impregnation and encapsulation of lightweight aggregates for self-healing concrete. Constr. Build. Mater. 2016, 124, 910–921. [Google Scholar] [CrossRef]
- Kanellopoulos, A.; Qureshi, T.; Al-Tabbaa, A. Glass encapsulated minerals for self-healing in cement based composites. Constr. Build. Mater. 2015, 98, 780–791. [Google Scholar] [CrossRef]
- ASTM C666; Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing. ASTM International: West Conshohocken, PA, USA, 2015.
- Zou, D.; Wang, Z.; Shen, M.; Liu, T.; Zhou, A. Improvement in freeze-thaw durability of recycled aggregate permeable concrete with silane modification. Constr. Build. Mater. 2021, 268, 121097. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, J.; Fu, M. Effect of molding methods on free-thaw durability of pervious concrete. Proc. J. Phys. Conf. Ser. 2023, 2424, 012015. [Google Scholar] [CrossRef]
- Chen, S.; Ren, J.; Ren, X.; Li, Y. Deterioration laws of concrete durability under the coupling action of salt erosion and drying–wetting cycles. Front. Mater. 2022, 9, 1003945. [Google Scholar] [CrossRef]
- Lanzilli, M.; Donadio, G.; Addevico, R.; Saggese, A.; Cangiano, G.; Baccigalupi, L.; Christie, G.; Ricca, E.; Isticato, R. The exosporium of Bacillus megaterium QM B1551 is permeable to the red fluorescence protein of the coral Discosoma sp. Front. Microbiol. 2016, 7, 1752. [Google Scholar] [CrossRef]
- Stewart, G.C. The exosporium layer of bacterial spores: A connection to the environment and the infected host. Microbiol. Mol. Biol. Rev. 2015, 79, 437–457. [Google Scholar] [CrossRef]
- Checinska, A.; Paszczynski, A.; Burbank, M. Bacillus and other spore-forming genera: Variations in responses and mechanisms for survival. Annu. Rev. Food Sci. Technol. 2015, 6, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Uttatree, S.; Kobtrakool, K.; Ketsuk, A.; Kaenngam, W.; Thakolprajak, P.; Charoenpanich, J. A novel metal-tolerant, solvent and surfactant stable protease from a new strain of Bacillus megaterium. Biocatal. Agric. Biotechnol. 2017, 12, 228–235. [Google Scholar] [CrossRef]
- Grage, K.; McDermott, P.; Rehm, B.H. Engineering Bacillus megaterium for production of functional intracellular materials. Microb. Cell Factories 2017, 16, 211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Al-Dossary, A.; Hussain, M.; Setlow, P.; Li, J. Applications of Bacillus subtilis spores in biotechnology and advanced materials. Appl. Environ. Microbiol. 2020, 86, e01096-20. [Google Scholar] [CrossRef]
- Obidi, O.; Wenner, S.; Halverson, L. Molecular-based analysis of the bacterial community structure of spoilt latex paints in Nigeria. Egypt. Soc. Exp. Biol. 2017, 13, 145–149. [Google Scholar] [CrossRef]
- Enyedi, N.T.; Makk, J.; Kótai, L.; Berényi, B.; Klébert, S.; Sebestyén, Z.; Molnár, Z.; Borsodi, A.K.; Leél-Őssy, S.; Demény, A. Cave bacteria-induced amorphous calcium carbonate formation. Sci. Rep. 2020, 10, 8696. [Google Scholar] [CrossRef]
- Lian, B.; Hu, Q.; Chen, J.; Ji, J.; Teng, H.H. Carbonate biomineralization induced by soil bacterium Bacillus megaterium. Geochim. Cosmochim. Acta 2006, 70, 5522–5535. [Google Scholar] [CrossRef]
- Tziviloglou, E.; Wiktor, V.; Jonkers, H.; Schlangen, E. Bacteria-based self-healing concrete to increase liquid tightness of cracks. Constr. Build. Mater. 2016, 122, 118–125. [Google Scholar] [CrossRef]
- Vijay, K.; Murmu, M. Effect of calcium lactate on compressive strength and self-healing of cracks in microbial concrete. Front. Struct. Civ. Eng. 2019, 13, 515–525. [Google Scholar] [CrossRef]
- Bashaveni, B.; Pannem, R.M.R. Development of self-healing property in self compacting concrete. Case Stud. Constr. Mater. 2024, 20, e02942. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Wang, Z.; Yao, W. Use of recycled concrete aggregates as carriers for self-healing of concrete cracks by bacteria with high urease activity. Constr. Build. Mater. 2022, 337, 127581. [Google Scholar] [CrossRef]
- Elmenshawy, Y.; Elmahdy, M.A.; Moawad, M.; Elshami, A.A.; Ahmad, S.S.; Nagai, K. Investigating the bacterial sustainable self-healing capabilities of cracks in structural concrete at different temperatures. Case Stud. Constr. Mater. 2024, 20, e03188. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Pang, S.D. Healing cement mortar by immobilization of bacteria in biochar: An integrated approach of self-healing and carbon sequestration. Cem. Concr. Compos. 2018, 86, 238–254. [Google Scholar] [CrossRef]
- Nam, J.; Kim, G.; Lee, B.; Hasegawa, R.; Hama, Y. Frost resistance of polyvinyl alcohol fiber and polypropylene fiber reinforced cementitious composites under freeze thaw cycling. Compos. Part B Eng. 2016, 90, 241–250. [Google Scholar] [CrossRef]
- He, R.; Lu, N.L. Air void system and freezing-thawing resistance of concrete composite with the incorporation of thermo-expansive polymeric microspheres. Constr. Build. Mater. 2024, 419, 135535. [Google Scholar] [CrossRef]
- Cappellesso, V.G.; Van Mullem, T.; Gruyaert, E.; Van Tittelboom, K.; De Belie, N. Bacteria-based self-healing concrete exposed to frost salt scaling. Cem. Concr. Compos. 2023, 139, 105016. [Google Scholar] [CrossRef]
- Ahmad, I.; Shokouhian, M.; Cheng, H.; Radlińska, A. Enhancement of mechanical properties and freeze–thaw durability of recycled aggregate concrete using aggregate pretreatment. Iran. J. Sci. Technol. Trans. Civ. Eng. 2024, 1–25. [Google Scholar] [CrossRef]
- Ivaškė, A.; Jakubovskis, R.; Boris, R.; Urbonavičius, J. Effects of low temperature, freeze–thaw cycles, and healing conditions on viability of non-ureolytic bacteria in biological self-healing concrete. Materials 2024, 17, 5797. [Google Scholar] [CrossRef]
- Seifan, M.; Sarmah, A.K.; Ebrahiminezhad, A.; Ghasemi, Y.; Samani, A.K.; Berenjian, A. Bio-reinforced self-healing concrete using magnetic iron oxide nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 2167–2178. [Google Scholar] [CrossRef]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]




| Materials (per m3 Concrete) | Units | CM | CMN | R1 | R2 | MSb | MSp | MMe |
|---|---|---|---|---|---|---|---|---|
| Cement | kg | 483 | 483 | 483 | 483 | 483 | 483 | 483 |
| Natural coarse aggregate | kg | 755 | 755 | 604 | 604 | 604 | 604 | 604 |
| Fine aggregate | kg | 933 | 933 | 933 | 933 | 933 | 933 | 933 |
| LECA | kg | 0 | 0 | 151 | 151 | 151 | 151 | 151 |
| w/c ratio | - | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| Amount of spores (per gram of LECAs) | µL | 20 | - | - | - | 20 | 20 | 20 |
| Air-entraining admixture | L | - | - | - | 1.45 | 1.45 | 1.45 | 1.45 |
| PVA fibers | % | - | - | - | 1 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, I.; Shokouhian, M.; Owolabi, D.; Jenkins, M.; McLemore, G.L. Assessment of Biogenic Healing Capability, Mechanical Properties, and Freeze–Thaw Durability of Bacterial-Based Concrete Using Bacillus subtilis, Bacillus sphaericus, and Bacillus megaterium. Buildings 2025, 15, 943. https://doi.org/10.3390/buildings15060943
Ahmad I, Shokouhian M, Owolabi D, Jenkins M, McLemore GL. Assessment of Biogenic Healing Capability, Mechanical Properties, and Freeze–Thaw Durability of Bacterial-Based Concrete Using Bacillus subtilis, Bacillus sphaericus, and Bacillus megaterium. Buildings. 2025; 15(6):943. https://doi.org/10.3390/buildings15060943
Chicago/Turabian StyleAhmad, Izhar, Mehdi Shokouhian, David Owolabi, Marshell Jenkins, and Gabrielle Lynn McLemore. 2025. "Assessment of Biogenic Healing Capability, Mechanical Properties, and Freeze–Thaw Durability of Bacterial-Based Concrete Using Bacillus subtilis, Bacillus sphaericus, and Bacillus megaterium" Buildings 15, no. 6: 943. https://doi.org/10.3390/buildings15060943
APA StyleAhmad, I., Shokouhian, M., Owolabi, D., Jenkins, M., & McLemore, G. L. (2025). Assessment of Biogenic Healing Capability, Mechanical Properties, and Freeze–Thaw Durability of Bacterial-Based Concrete Using Bacillus subtilis, Bacillus sphaericus, and Bacillus megaterium. Buildings, 15(6), 943. https://doi.org/10.3390/buildings15060943








