Influence of Minor Additives on the Performance of Calcined Clay and Blast Furnace Slag Based One Part Alkali-Activated Mortars
Abstract
1. Introduction
1.1. Overview
1.2. Research Significance
2. Materials and Methods
2.1. Raw Material Characteristics
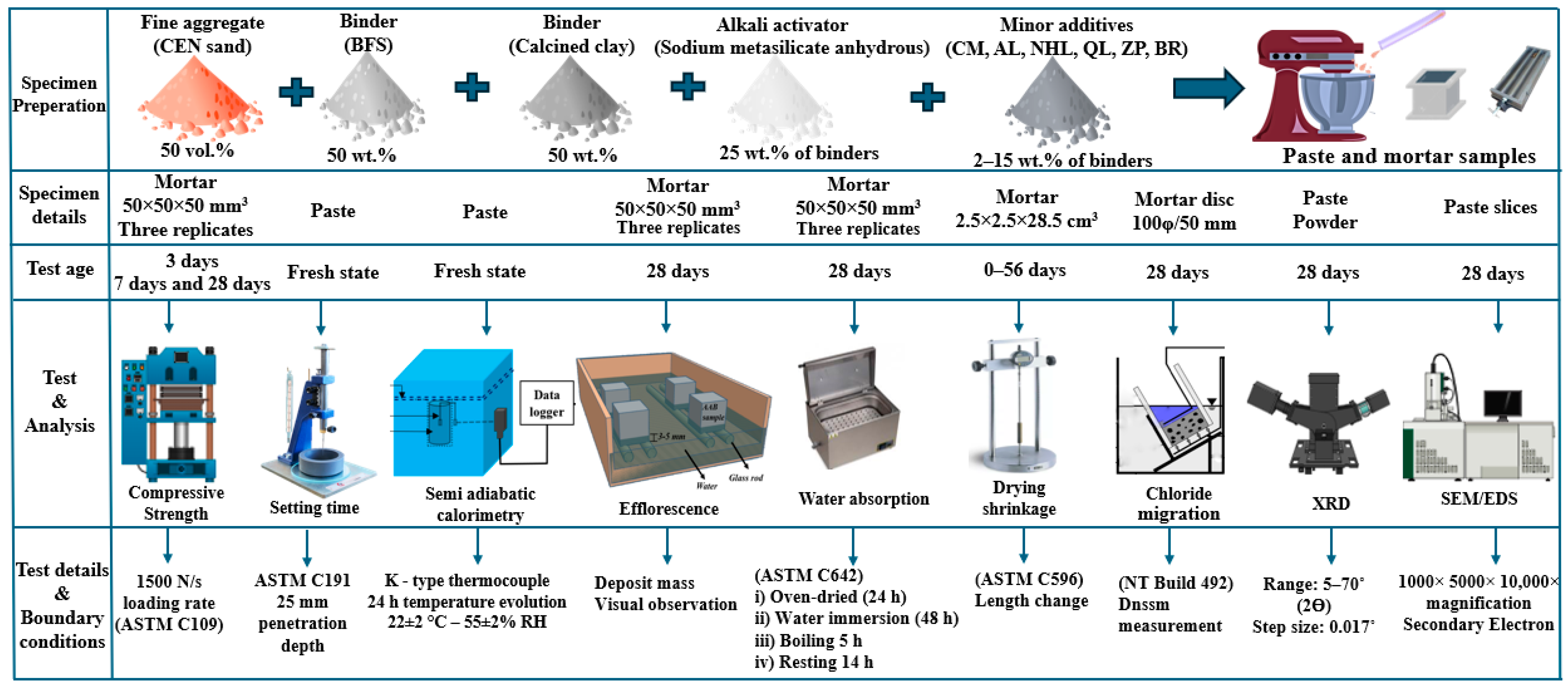
2.2. Mix Design and Specimen Preparation
2.3. Experimental Schedule
3. Results and Discussion
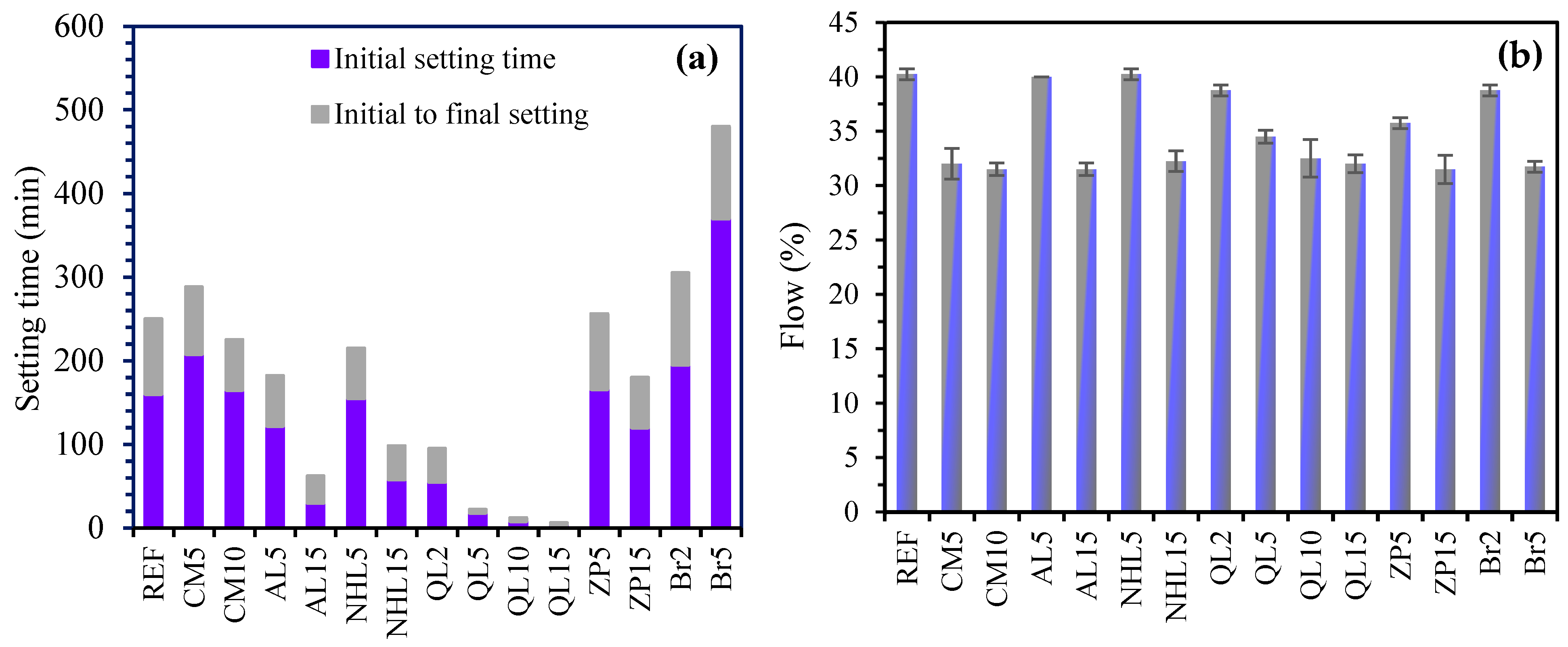
3.1. Setting Time and Flowability
3.2. Semi-Adiabatic Calorimetry
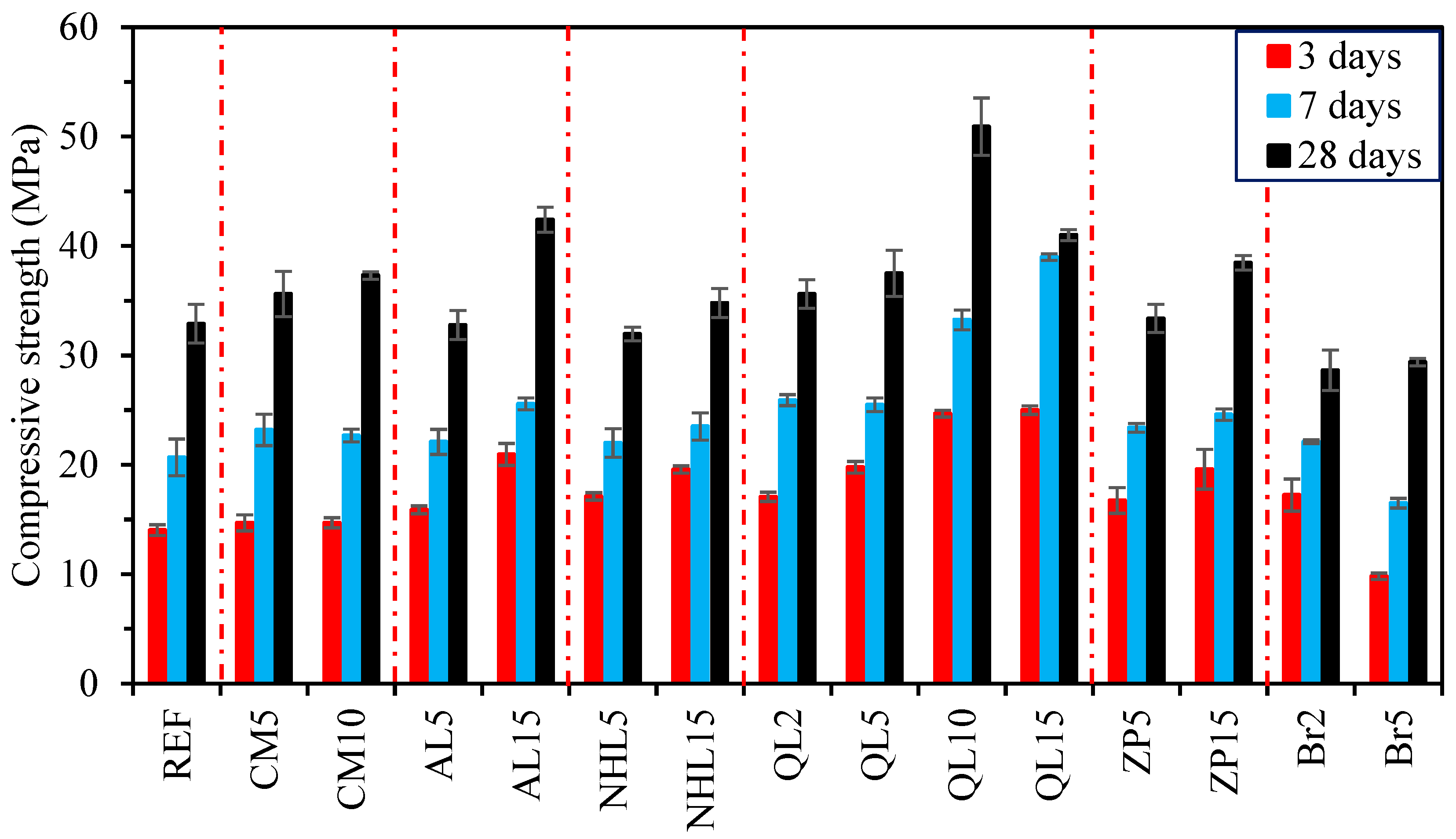
3.3. Compressive Strength
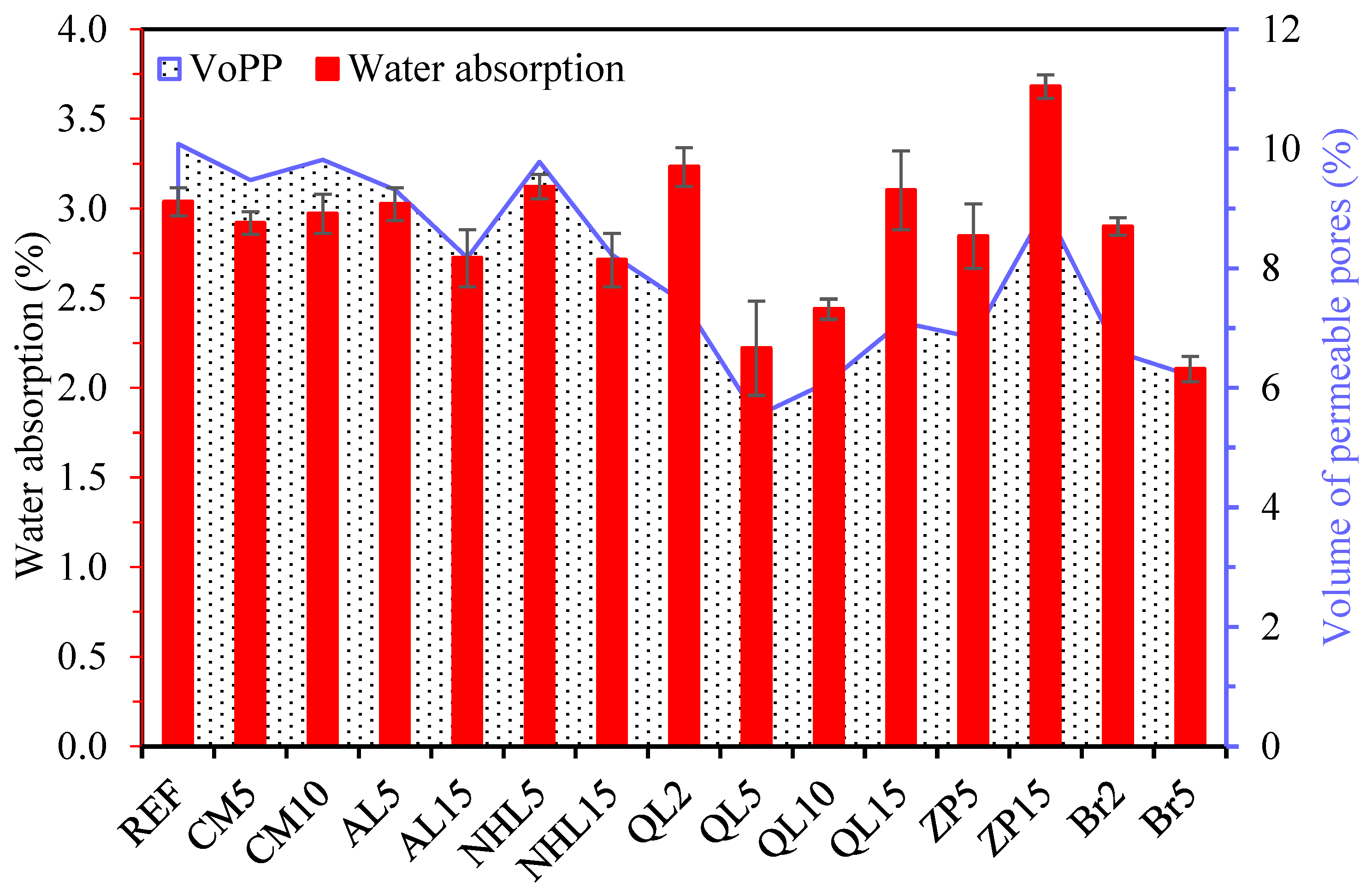
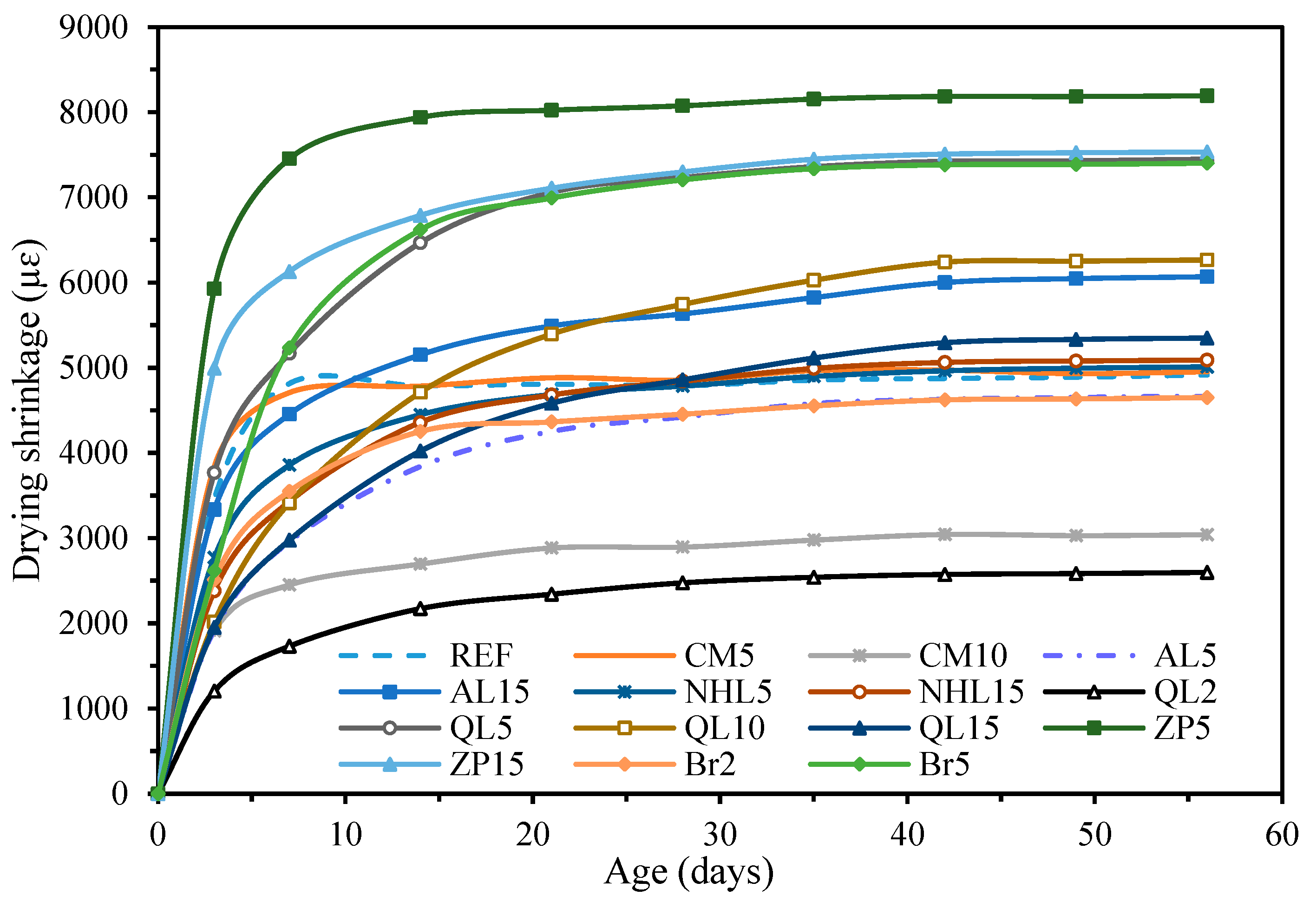
3.4. Physical Properties
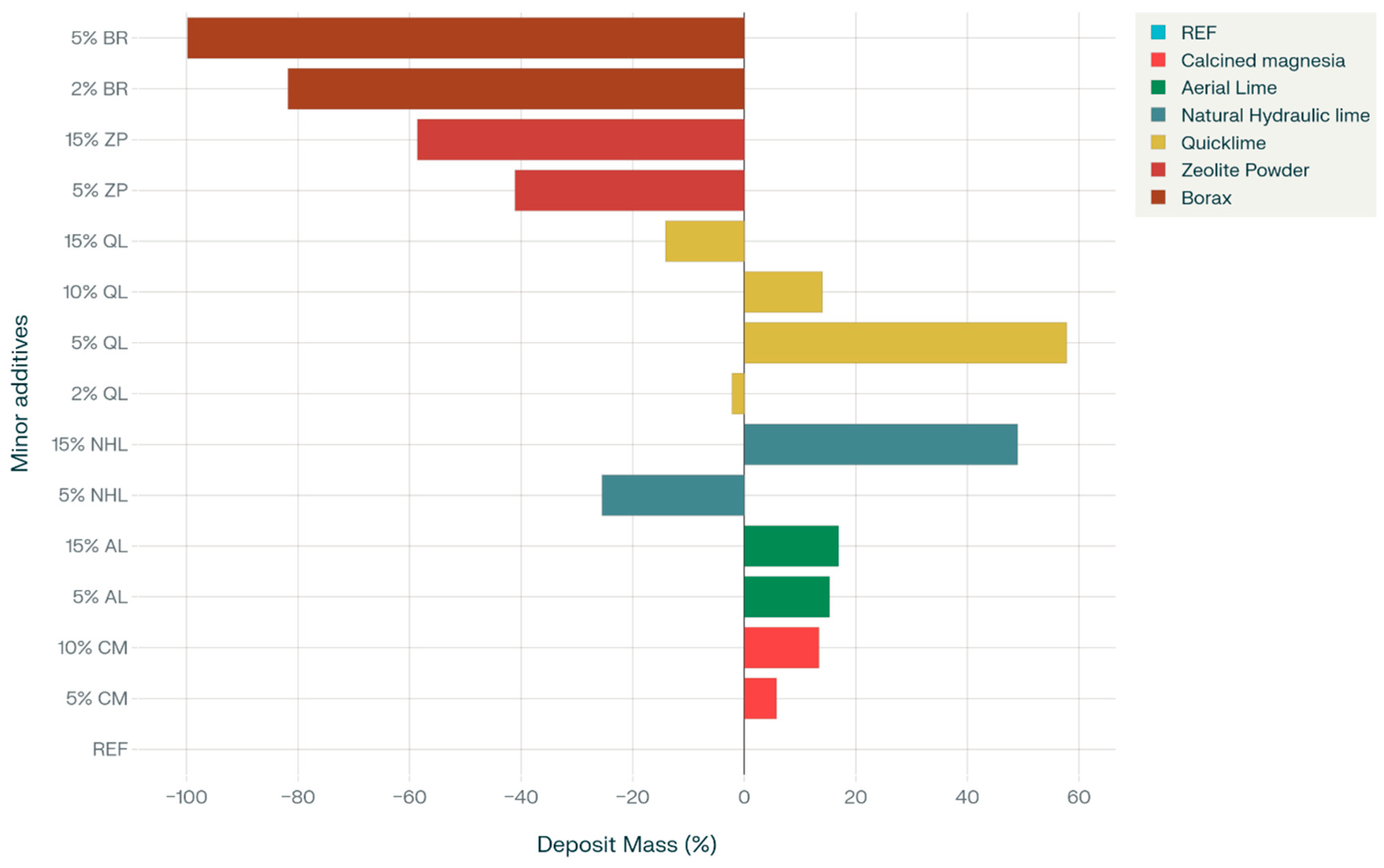
3.5. Efflorescence
3.6. Chloride Migration Resistance
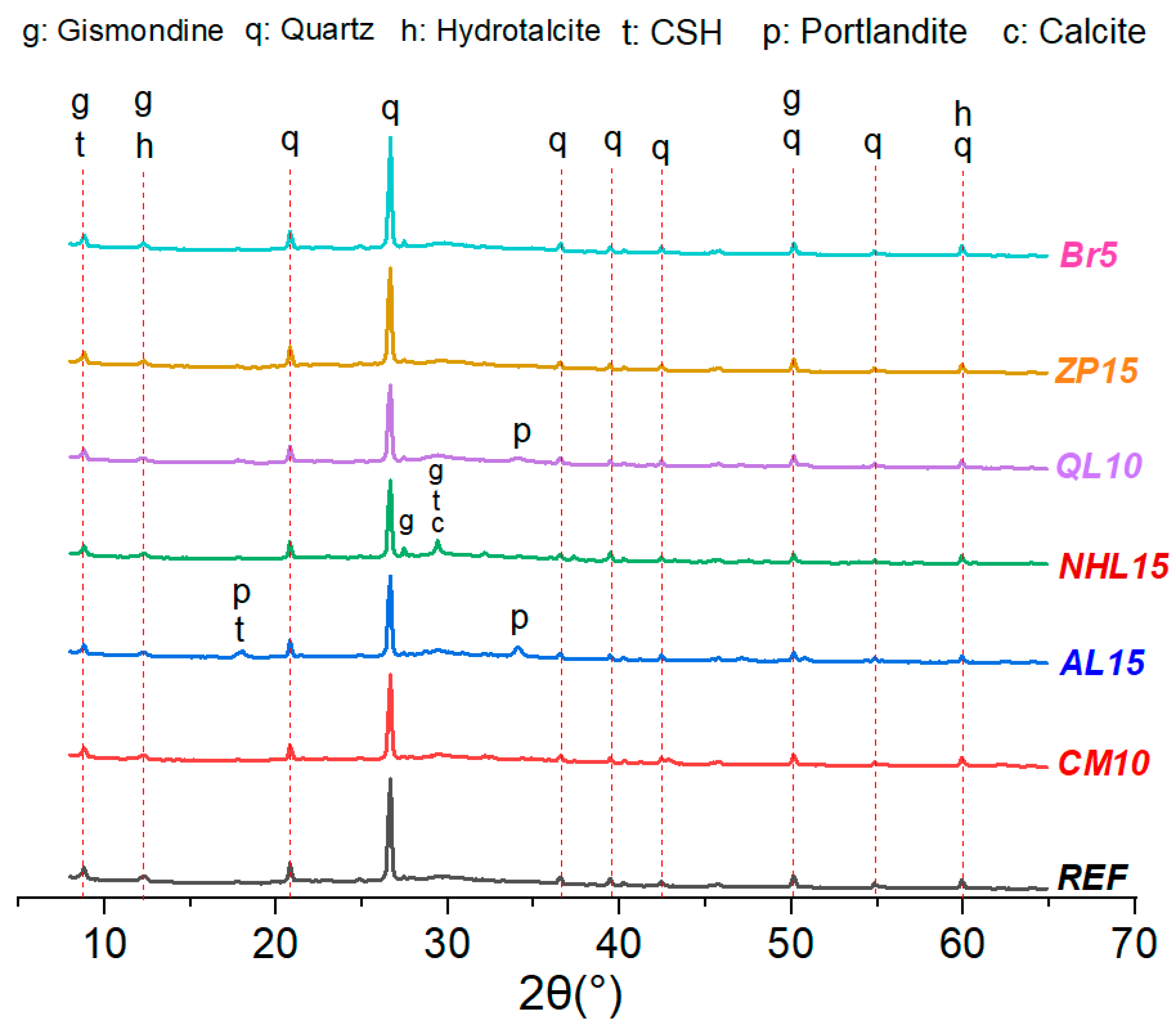
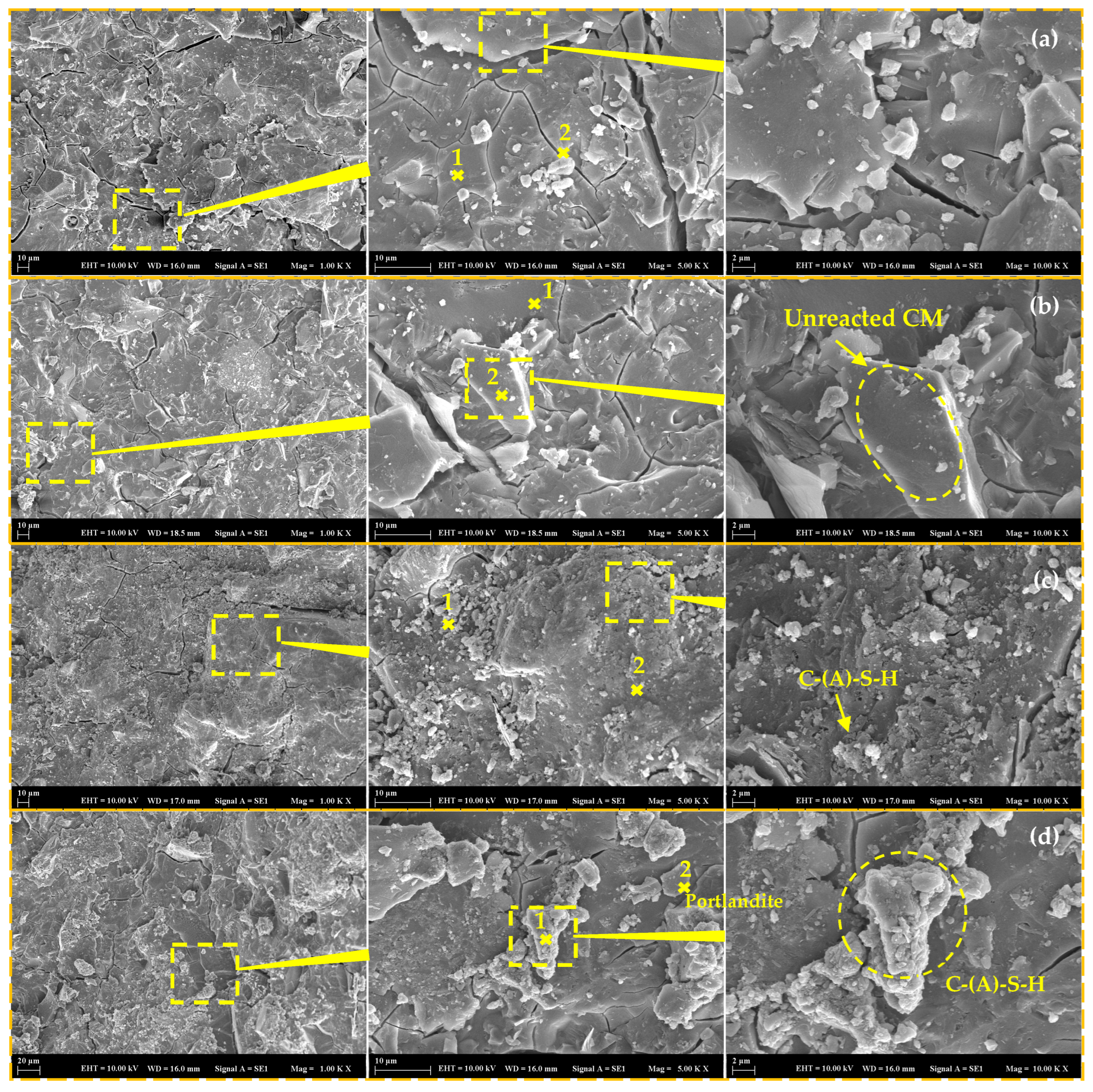
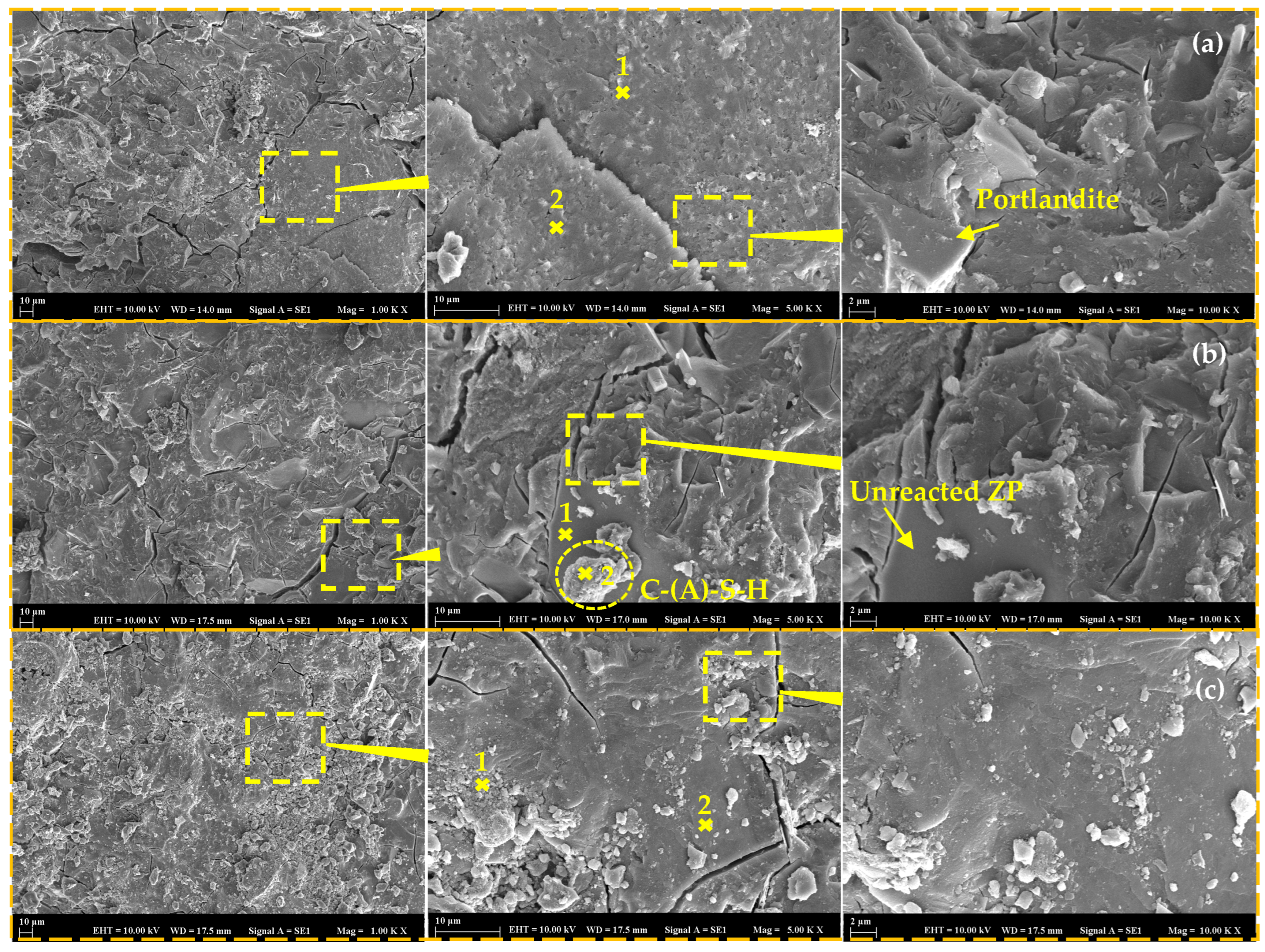
3.7. Microstructural Investigations
4. Conclusions
- The incorporation of CM resulted in the development of secondary voluminous reaction products like hydrotalcite, which consistently enhanced the compressive strength and chloride resistance of mortars, while simultaneously decreasing water absorption and drying shrinkage. On the other hand, it was found that CM had a negligible influence on the setting time and efflorescence characteristics of mortars;
- The physicomechanical properties of AABs were generally positively influenced by AL and NHL, which promote the entry of additional alkalinity and calcium ions into the microstructure. Accordingly, the addition of AL or NHL resulted in a moderate enhancement of the compressive strength (up to 29%), water absorption, and chloride penetration properties of mortars, while also shortening the setting time by 75% and 61% at a 15% AL and NHL content, respectively;
- The test results indicate that the incorporation of QL emerged as the most effective among the minor additives regarding its influence on the characteristics of AAB mixtures. Even at 2% QL addition, the mixtures exhibited a remarkable reduction in setting time and drying shrinkage, achieving decreases of 62% and 47%, respectively. The optimal ratio identified was a 10% addition, which resulted in a 55% increase in strength development, an 82% decrease in the Dnssm coefficient, and a 20% decrease in water absorption. Incorporating over 10% QL resulted in an excessively rapid reaction of the mixture, and the elevated hydration heat subsequently impeded strength development at later ages;
- The incorporation of BR markedly enhanced water absorption and resistance to chloride penetration. The mixtures containing BR, which are capable of limiting sodium ions within their framework, showed efflorescence diminished to almost negligible levels. Similarly, ZP addition reduced the efflorescence formation on mortar surfaces due to its elevated cation exchange capacity;
- Overall, the results of this research indicate that the mechanical, physical, and durability characteristics of calcined clay and BFS-based AABs can be enhanced with the use of minor additives even at low ratios. Thus, employing QL, AL, NHL, and CM in suitable proportions generally enhances strength and durability characteristics, whereas ZP and BR effectively mitigate the intensity of efflorescence without compromising strength and physical properties. Nevertheless, it was noticed that the excessive usage of QL or BR led to quick and prolonged setting behaviors, respectively, which restrict their usage in specific civil engineering applications. QL, characterized by its outstanding early strength and quick setting capabilities, is particularly well-suited for applications requiring rapid repair. In contrast, the other minor additives analyzed are appropriate for civil engineering uses, including screed and plastering, owing to their well-balanced setting times and material characteristics. Subsequent investigations may explore the efficacy of calcined clay and BFS-based one-part AABs with QL as repair mortars, and preliminary trials could be carried out for various field applications involving each minor additive.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAB | Alkali-activated binder |
| CM | Calcined magnesia |
| AL | Aerial lime |
| NHL | Natural hydraulic lime |
| QL | Quicklime |
| ZP | Zeolite powder |
| BR | Anhydrous borax |
| Dnssm | Non-steady state chloride migration coefficient |
| BFS | Blast furnace slag |
| FA | Fly ash |
| LC3 | Limestone calcined clay cement |
| MK | Metakaolin |
| C-A-S-H | Calcium aluminate silicate hydrate |
| N-A-S-H | Sodium aluminate silicate hydrate |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
| SEM | Scanning electron microscopy |
| EDS | Energy dispersive spectroscopy |
| SMS | Anhydrous sodium metasilicate |
| WA | Water absorption |
| VoPP | Volume of permeable pores |
| Na2CO3.xH2O | Sodium carbonate hydrates |
References
- Ascensão, G.; Bernardo, E.; Ferreira, V.M. An Investigation on the Synthesis of Alkali Activated Materials from Thermally Modified Clays. Appl. Sci. 2022, 12, 9085. [Google Scholar] [CrossRef]
- Nejad, B.M.; Enferadi, S.; Andrew, R. A comprehensive analysis of process-related CO2 emissions from Iran’s cement industry. Clean. Environ. Syst. 2025, 16, 100251. [Google Scholar] [CrossRef]
- Supriya; Chaudhury, R.; Sharma, U.; Thapliyal, P.; Singh, L. Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Mostafa, S.A.; Fathy, I.N.; Mahmoud, A.A.; Abouelnour, M.A.; Mahmoud, K.; Shaaban, S.M.; Elhameed, S.A.; Nabil, I.M. Optimization of UHPC with basil plant ash: Impacts on strength, durability, and gamma-ray attenuation. Ann. Nucl. Energy 2026, 226, 111825. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; El-Sayed, A.A.; Aboraya, A.M.; Fathy, I.N.; Abouelnour, M.A.; Elfakharany, M.E.; Fattouh, M.S.; Alahmer, A.E.; Nabil, I.M. Influence of elevated temperature exposure on the residual compressive strength and radiation shielding efficiency of ordinary concrete incorporating granodiorite and ceramic powders. Sci. Rep. 2025, 15, 3572. [Google Scholar] [CrossRef]
- Cakmak, T.; Ustabas, I. Investigating experimentally the potency of divergent sodium hydroxide and sodium silicate molar proportions on silica fume and obsidian-based geopolymer mortars. Struct. Concr. 2025, 26, 1962–1987. [Google Scholar] [CrossRef]
- Tao, Y.; Rahul, A.; Mohan, M.K.; De Schutter, G.; Van Tittelboom, K. Recent progress and technical challenges in using calcium sulfoaluminate (CSA) cement. Cem. Concr. Compos. 2022, 137, 104908. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Nepal, J.; Das, B.B. Properties, compatibility, environmental benefits and future directions of limestone calcined clay cement (LC3) concrete: A review. J. Build. Eng. 2023, 79, 107794. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, X.; Shi, Y.; Yang, K.; Zhou, J.; Umar, H.A.; Long, G.; Xie, Y. A comparative study on mechanical properties and environmental impact of UHPC with belite cement and portland cement. J. Clean. Prod. 2022, 380, 135003. [Google Scholar] [CrossRef]
- Soares, E.G.; Castro-Gomes, J. Carbonation curing influencing factors of carbonated reactive magnesia cements (CRMC)—A review. J. Clean. Prod. 2021, 305, 127210. [Google Scholar]
- Wang, L.; Chen, L.; Poon, C.S.; Wang, C.-H.; Ok, Y.S.; Mechtcherine, V.; Tsang, D.C.W. Roles of biochar and CO2 curing in sustainable magnesia cement-based composites. ACS Sustain. Chem. Eng. 2021, 9, 8603–8610. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Severson, K.; Grandgeorge, P.; Roumeli, E. Closed-loop optimization using machine learning for the accelerated design of sustainable cements incorporating algal biomatter. Matter 2025, 8, 102267. [Google Scholar] [CrossRef]
- Borrachero, M.V.; Payá, J.; Brito, S.; Segura, Y.P.; Soriano, L.; Tashima, M.M.; Monzó, J.M. Reusing construction and demolition waste to prepare alkali-activated cement. Materials 2022, 15, 3437. [Google Scholar] [CrossRef]
- Hossain, S.S.; Roy, P.; Bae, C.-J. Utilization of waste rice husk ash for sustainable geopolymer: A review. Constr. Build. Mater. 2021, 310, 125218. [Google Scholar] [CrossRef]
- Bature, A.; Khorami, M.; Ganjian, E.; Tyrer, M. Influence of alkali activator type and proportion on strength performance of calcined clay geopolymer mortar. Constr. Build. Mater. 2021, 267, 120446. [Google Scholar] [CrossRef]
- Rahman, M.M.; Law, D.W.; Patnaikuni, I.; Gunasekara, C.; Yamchelou, M.T. Low-grade clay as an alkali-activated material. Appl. Sci. 2021, 11, 1648. [Google Scholar] [CrossRef]
- Elzeadani, M.; Bompa, D.; Elghazouli, A. One part alkali activated materials: A state-of-the-art review. J. Build. Eng. 2022, 57, 104871. [Google Scholar]
- Okoye, F.; Durgaprasad, J.; Singh, N. Mechanical properties of alkali activated flyash/Kaolin based geopolymer concrete. Constr. Build. Mater. 2015, 98, 685–691. [Google Scholar] [CrossRef]
- Zheng, D.; Liang, X.; Cui, H.; Tang, W.; Liu, W.; Zhou, D. Study of performances and microstructures of mortar with calcined low-grade clay. Constr. Build. Mater. 2022, 327, 126963. [Google Scholar] [CrossRef]
- Bakil, S.N.A.; Kristály, F.; Mucsi, G. Preliminary study of low-grade clay as secondary raw material for geopolymer. Geosci. Eng. 2023, 11, 68–84. [Google Scholar] [CrossRef]
- Hattaf, R.; Aboulayt, A.; Samdi, A.; Lahlou, N.; Touhami, M.O.; Gomina, M.; Moussa, R. Metakaolin and fly ash-based matrices for geopolymer materials: Setting kinetics and compressive strength. Silicon 2021, 14, 6993–7004. [Google Scholar] [CrossRef]
- Hasnaoui, A.; Ghorbel, E.; Wardeh, G. Optimization approach of granulated blast furnace slag and metakaolin based geopolymer mortars. Constr. Build. Mater. 2019, 198, 10–26. [Google Scholar] [CrossRef]
- Alcamand, H.A.; Borges, P.H.; Silva, F.A.; Trindade, A.C.C. The effect of matrix composition and calcium content on the sulfate durability of metakaolin and metakaolin/slag alkali-activated mortars. Ceram. Int. 2018, 44, 5037–5044. [Google Scholar] [CrossRef]
- Boakye, K.; Khorami, M. Impact of low-reactivity calcined clay on the performance of fly ash-based geopolymer mortar. Sustainability 2023, 15, 13556. [Google Scholar] [CrossRef]
- Cardinaud, G.; Rozière, E.; Martinage, O.; Loukili, A.; Barnes-Davin, L.; Paris, M.; Deneele, D. Calcined clay–Limestone cements: Hydration processes with high and low-grade kaolinite clays. Constr. Build. Mater. 2021, 277, 122271. [Google Scholar] [CrossRef]
- Khan, R.; Iqbal, S.; Soliyeva, M.; Ali, A.; Elboughdiri, N. Advanced clay-based geopolymer: Influence of structural and material parameters on its performance and applications. RSC Adv. 2025, 15, 12443–12471. [Google Scholar] [CrossRef] [PubMed]
- Karozou, A.; Konopisi, S.; Pavlidou, E.; Stefanidou, M. Long-term behavior and durability of alkali-activated clay mortars. Materials 2020, 13, 3790. [Google Scholar] [CrossRef]
- Yamchelou, M.T.; Law, D.; Brkljača, R.; Gunasekara, C.; Li, J.; Patnaikuni, I. Geopolymer synthesis using low-grade clays. Constr. Build. Mater. 2021, 268, 121066. [Google Scholar] [CrossRef]
- Hamdi, N.; Ben Messaoud, I.; Srasra, E. Production of geopolymer binders using clay minerals and industrial wastes. Comptes Rendus Chim. 2019, 22, 220–226. [Google Scholar]
- Tole, I.; Habermehl-Cwirzen, K.; Cwirzen, A. Mechanochemical activation of natural clay minerals: An alternative to produce sustainable cementitious binders–review. Miner. Petrol. 2019, 113, 449–462. [Google Scholar] [CrossRef]
- Ferone, C.; Liguori, B.; Capasso, I.; Colangelo, F.; Cioffi, R.; Cappelletto, E.; Di Maggio, R. Thermally treated clay sediments as geopolymer source material. Appl. Clay Sci. 2015, 107, 195–204. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Ogundiran, M.B.; Kumar, S. Synthesis and characterisation of geopolymer from Nigerian Clay. Appl. Clay Sci. 2015, 108, 173–181. [Google Scholar] [CrossRef]
- Yamchelou, M.T.; Law, D.W.; Patnaikuni, I.; Li, J. Alkali activation of mechanically activated low-grade clay. J. Sustain. Cem. Mater. 2021, 10, 272–288. [Google Scholar]
- Lekshmi, S.; Sudhakumar, J. Engineering and durability performances of fly ash based geopolymer mortar containing aluminosilicate rich flood soil waste with and without lime treatment. Silicon 2022, 14, 6141–6156. [Google Scholar] [CrossRef]
- Lekshmi, S.; Sudhakumar, J. An assessment on the durability performance of fly ash-clay based geopolymer mortar containing clay enhanced with lime and GGBS. Clean. Mater. 2022, 5, 100129. [Google Scholar] [CrossRef]
- Lekshmi, S.; Sudhakumar, J.; Thomas, S. Application of clay in geopolymer system: A state-of-the-art review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Dener, M.; Kılıç, H.; Benli, A. Enhancing mechanical performance of one-part sodium sulfate-activated slag mortars using calcium-rich materials. Constr. Build. Mater. 2025, 476, 141285. [Google Scholar]
- Adufu, Y.D.; Sore, S.O.; Nshimiyimana, P.; Messan, A.; Escadeillas, G. Effect of calcium-rich additions on the mechanical and microstructural properties of metakaolin-based geopolymer concrete cured in ambient sub-Saharan climate. Constr. Build. Mater. 2024, 453, 139009. [Google Scholar] [CrossRef]
- Silva, T.H.; Lara, L.F.; Silva, G.J.; Provis, J.L.; Bezerra, A.C. Alkali-activated materials produced using high-calcium, high-carbon biomass ash. Cem. Concr. Compos. 2022, 132, 104646. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Calcium wastes as an additive for a low calcium fly ash geopolymer. Sci. Rep. 2023, 13, 16351. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Dai, E.; Fu, H.; Xu, Z.; Du, E.; Gu, L.; Feng, J. Hydration Kinetics and Performance of MgO-Activated Slag Binder with Available Carbonate and Silicate. J. Mater. Civ. Eng. 2025, 37, 04025126. [Google Scholar] [CrossRef]
- Özen, Ö.C.; Oktay, D.; Aktürk, B. One-part sodium carbonate-activated slag/r-MgO based mixes: Influence of nano-silica incorporation on compressive strength and microstructural development. Constr. Build. Mater. 2024, 422, 135844. [Google Scholar] [CrossRef]
- Qian, P.; Chen, P.; Qian, X.; Wang, X.; Wang, L. Enhancing the performance of alkali-activated slag using plant-derived xylitol as a multi-functional additive. Mag. Concr. Res. 2025, 77, 766–779. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Z.; Chen, J.; Zhang, Y.; Wu, P. Effect of borax-modified activator on mechanical properties and drying shrinkage of alkali-activated slag/metakaolin mortar. Sci. Rep. 2024, 14, 8202. [Google Scholar] [CrossRef]
- Li, P.; Chen, D.; Jia, Z.; Li, Y.; Li, S.; Yu, B. Effects of borax, sucrose, and citric acid on the setting time and mechanical properties of alkali-activated slag. Materials 2023, 16, 3010. [Google Scholar] [CrossRef]
- Oderji, S.Y.; Chen, B.; Mohseni, E. The efficiency of borax as an additive on properties of one-part fly ash/slag-based alkali-activated materials. Eur. J. Environ. Civ. Eng. 2023, 27, 4237–4249. [Google Scholar] [CrossRef]
- ASTM C191; Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C1437; Standard Test Method for Flow of Hydraulic Cement Mortar. ASTM International: West Conshohocken, PA, USA, 2020.
- Omur, T.; Miyan, N.; Kabay, N.; Birol, B.; Oktay, D. Characterization of ferrochrome ash and blast furnace slag based alkali-activated paste and mortar. Constr. Build. Mater. 2023, 363, 129805. [Google Scholar] [CrossRef]
- ASTM C109/C109M; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 50 mm [2 in.] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM C642; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM C596; Standard Test Method for Drying Shrinkage of Mortar Containing Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2023.
- Zhou, S.; Zhou, S.; Zhang, J.; Tan, X.; Chen, D. Relationship between moisture transportation, efflorescence and structure degradation in fly ash/slag geopolymer. Materials 2020, 13, 5550. [Google Scholar] [CrossRef]
- Allahverdi, A.; Najafi Kani, E.; Hossain, K.M.A.; Lachemi, M. Methods to control efflorescence in alkali-activated cement-based materials. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 463–483. [Google Scholar]
- NT Build 492; Concrete, Mortar and Cement-Based Repair Materials: Chloride Migration Coefficient from Non-Steady-State Migration Experiments. Nordtest: Espoo, Finland, 1999.
- Wang, L.; Fu, H.; Gao, Q.; Luo, J.; Tang, J.; Song, J.; Li, Y.; Yu, G. Engineering Performance and Mechanism of Alkali-Activated Ground Granulated Blast Furnace Slag–Zeolite Powder Grouting Materials. Appl. Sci. 2025, 15, 3345. [Google Scholar] [CrossRef]
- Shoaei, P.; Momenzadeh, A.; Hosseini, H.; Rajaei, S.; Ameri, F.; Pilehvar, S. One-part slag/zeolite geopolymer mortars under ambient and heat curing conditions. Case Stud. Constr. Mater. 2024, 20, e02677. [Google Scholar] [CrossRef]
- Hu, M.; Dong, T.; Cui, Z.; Li, Z. Mechanical behavior and microstructure evaluation of quicklime-activated cement kiln dust-slag binder pastes. Materials 2024, 17, 1253. [Google Scholar] [CrossRef]
- Liu, H.; Sanjayan, J.G.; Bu, Y. The application of sodium hydroxide and anhydrous borax as composite activator of class F fly ash for extending setting time. Fuel 2017, 206, 534–540. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A. Influence of calcium aluminate cement on geopolymerization of natural pozzolan. Constr. Build. Mater. 2016, 114, 290–296. [Google Scholar] [CrossRef]
- Omur, T.; Miyan, N.; Kabay, N. Utilization of eggshell powder in one-part alkali-activated metakaolin based binder. Constr. Build. Mater. 2024, 445, 137981. [Google Scholar] [CrossRef]
- Rabie, M.; Irshidat, M.R.; Al-Nuaimi, N. Ambient and heat-cured geopolymer composites: Mix design optimization and life cycle assessment. Sustainability 2022, 14, 4942. [Google Scholar] [CrossRef]
- Alves, L.; Leklou, N.; de Simone e Souza, F.; de Barros, S. Effects of Thermal Activation on Mechanical Performance and Sustainability of Slag-Based Geopolymers. Materials 2025, 18, 4419. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tang, D.; Yang, K.; Zhang, Z.; Li, Q.; Pan, Q.; Yang, C. Effect of Ca(OH)2 on shrinkage characteristics and microstructures of alkali-activated slag concrete. Constr. Build. Mater. 2018, 175, 467–482. [Google Scholar] [CrossRef]
- He, J.; Bai, W.; Zheng, W.; He, J.; Sang, G. Influence of hydrated lime on mechanical and shrinkage properties of alkali-activated slag cement. Constr. Build. Mater. 2021, 289, 123201. [Google Scholar] [CrossRef]
- Válek, J.; van Halem, E.; Viani, A.; Pérez-Estébanez, M.; Ševčík, R.; Šašek, P. Determination of optimal burning temperature ranges for production of natural hydraulic limes. Constr. Build. Mater. 2014, 66, 771–780. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Zhou, Z.; Du, P.; Xu, D.; Xie, N.; Cheng, X.; Liu, Y. Effect of zeolite on waste based alkali-activated inorganic binder efflorescence. Constr. Build. Mater. 2018, 158, 683–690. [Google Scholar] [CrossRef]
- Dung, N.; Hooper, T.; Unluer, C. Improving the carbonation resistance of Na2CO3-activated slag mixes via the use of reactive MgO and nucleation seeding. Cem. Concr. Compos. 2021, 115, 103832. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. Comparison of alkali and silica sources in one-part alkali-activated blast furnace slag mortar. J. Clean. Prod. 2018, 187, 171–179. [Google Scholar] [CrossRef]
- Omur, T.; Miyan, N.; Özkan, H.; Kabay, N. Characterization of rapid-hardening alkali-activated binders incorporating basic oxygen furnace slag and blast furnace slag. J. Build. Eng. 2025, 111, 113616. [Google Scholar] [CrossRef]
- Yuan, Z.; Jia, Y.; Xie, X.; Xu, J. Study on the Macroscopic Properties and Microstructure of High Fly Ash Content Alkali-Activated Fly Ash Slag Concrete Cured at Room Temperature. Materials 2025, 18, 547. [Google Scholar] [CrossRef]
- Kunther, W.; Ferreiro, S.; Skibsted, J. Influence of the Ca/Si ratio on the compressive strength of cementitious calcium–silicate–hydrate binders. J. Mater. Chem. A 2017, 5, 17401–17412. [Google Scholar] [CrossRef]
- Revathi, T.; Jeyalakshmi, R. Fly ash–GGBS geopolymer in boron environment: A study on rheology and microstructure by ATR FT-IR and MAS NMR. Constr. Build. Mater. 2021, 267, 120965. [Google Scholar] [CrossRef]
- Ben Haha, M.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- He, J.; Zheng, W.; Bai, W.; Hu, T.; He, J.; Song, X. Effect of reactive MgO on hydration and properties of alkali-activated slag pastes with different activators. Constr. Build. Mater. 2021, 271, 121608. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.; Ouyang, X.; Fu, J.; Li, Z. Effect of CaO on the shrinkage and microstructure of alkali-activated slag/fly ash microsphere. Constr. Build. Mater. 2024, 421, 135672. [Google Scholar] [CrossRef]
- Zheng, D.; Ji, T.; Wang, G. Effect of CaO on the Autogenous Shrinkage of Alkali-Activated Slag Mortar. Adv. Mater. Sci. Eng. 2021, 2021, 9918834. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, G.; Han, Z.; Zhang, F.; Liu, M.; Hao, J. Evolution of Mechanical Properties, Mineral Crystallization, and Micro-Gel Formation in Alkali-Activated Carbide Slag Cementitious Materials. Crystals 2025, 15, 731. [Google Scholar] [CrossRef]
- Rashad, A.M. Calcium hydroxide in geopolymers—A critical overview. Eur. J. Environ. Civ. Eng. 2025, 29, 351–379. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Zhao, J. Mitigating the drying shrinkage and autogenous shrinkage of alkali-activated slag by NaAlO2. Materials 2020, 13, 3499. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, D.; Cheng, Z.-Q. Effect of zeolite powder on the hydration and microstructure evolution of hardened cement paste at low water-binder ratio. Rev. Romana Mater. 2020, 50, 331–336. [Google Scholar]
- Liu, C.; Li, Z.; Ye, G. Mechanisms of efflorescence of alkali-activated slag. Cem. Concr. Compos. 2024, 155, 105811. [Google Scholar] [CrossRef]
- Maslyk, M.; Gäb, T.; Matveeva, G.; Opitz, P.; Mondeshki, M.; Krysiak, Y.; Kolb, U.; Tremel, W. Multistep Crystallization Pathways in the Ambient-Temperature Synthesis of a New Alkali-Activated Binder. Adv. Funct. Mater. 2022, 32, 2108126. [Google Scholar] [CrossRef]
- Seilkhanova, G.A.; Rakhym, A.B.; Kan, A.V.; Kenessova, A.K.; Mastai, Y. The use of natural zeolite and chamotte clay-based sorbents for the extraction of sodium and potassium ions from saline water: A preliminary study. Chem. Bull. Kazakh Natl. Univ. 2022, 105, 44–53. [Google Scholar] [CrossRef]
- Criado, M.; Provis, J.L. Alkali activated slag mortars provide high resistance to chloride-induced corrosion of steel. Front. Mater. 2018, 5, 34. [Google Scholar] [CrossRef]
- Ke, X.; Bernal, S.A.; Hussein, O.H.; Provis, J.L. Chloride binding and mobility in sodium carbonate-activated slag pastes and mortars. Mater. Struct. 2017, 50, 252. [Google Scholar] [CrossRef]
- Bernal, S.A. Microstructural changes induced by CO2 exposure in alkali-activated slag/metakaolin pastes. Front. Mater. 2016, 3, 43. [Google Scholar] [CrossRef]
- Okoronkwo, M.U.; Mondal, S.K.; Wang, B.; Ma, H.; Kumar, A. Formation and stability of gismondine-type zeolite in cementitious systems. J. Am. Ceram. Soc. 2021, 104, 1513–1525. [Google Scholar] [CrossRef]
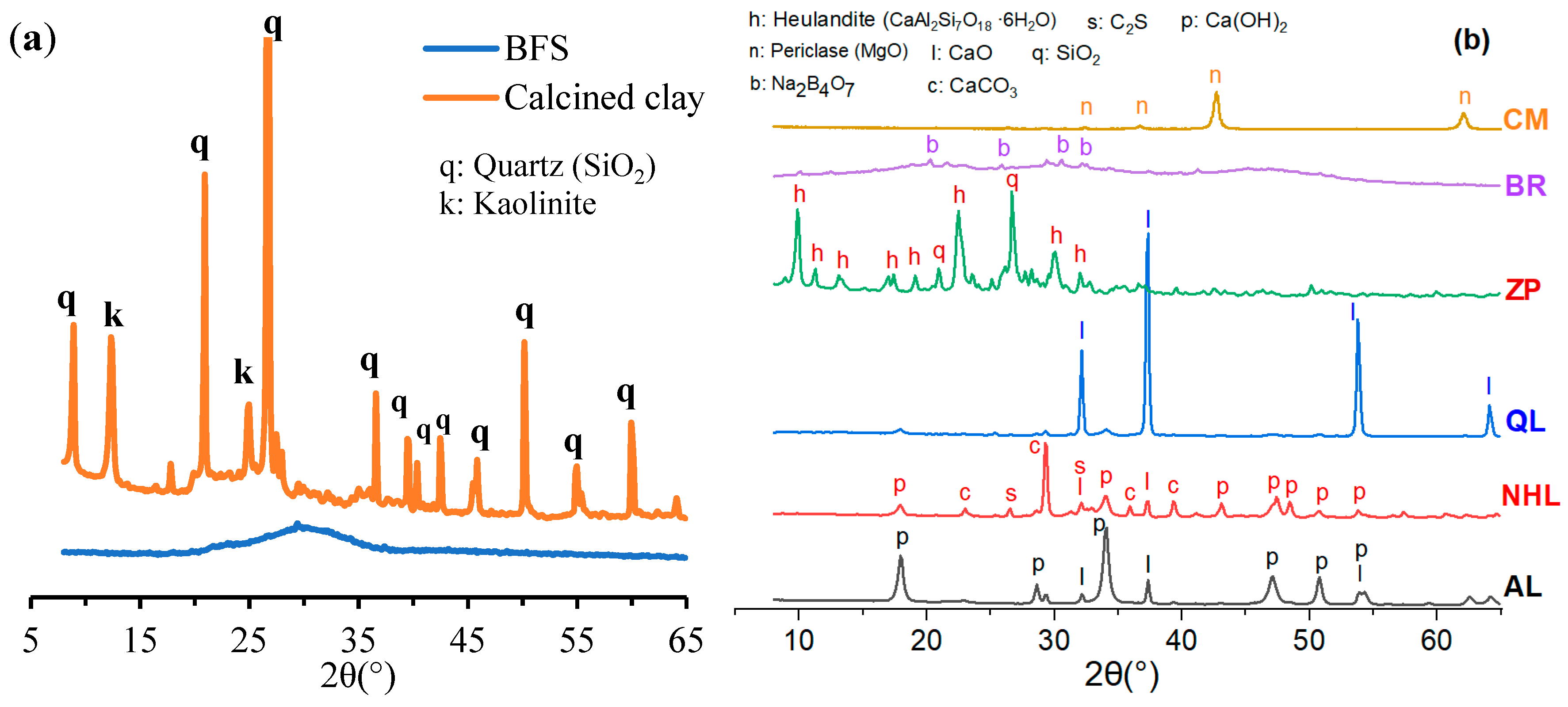
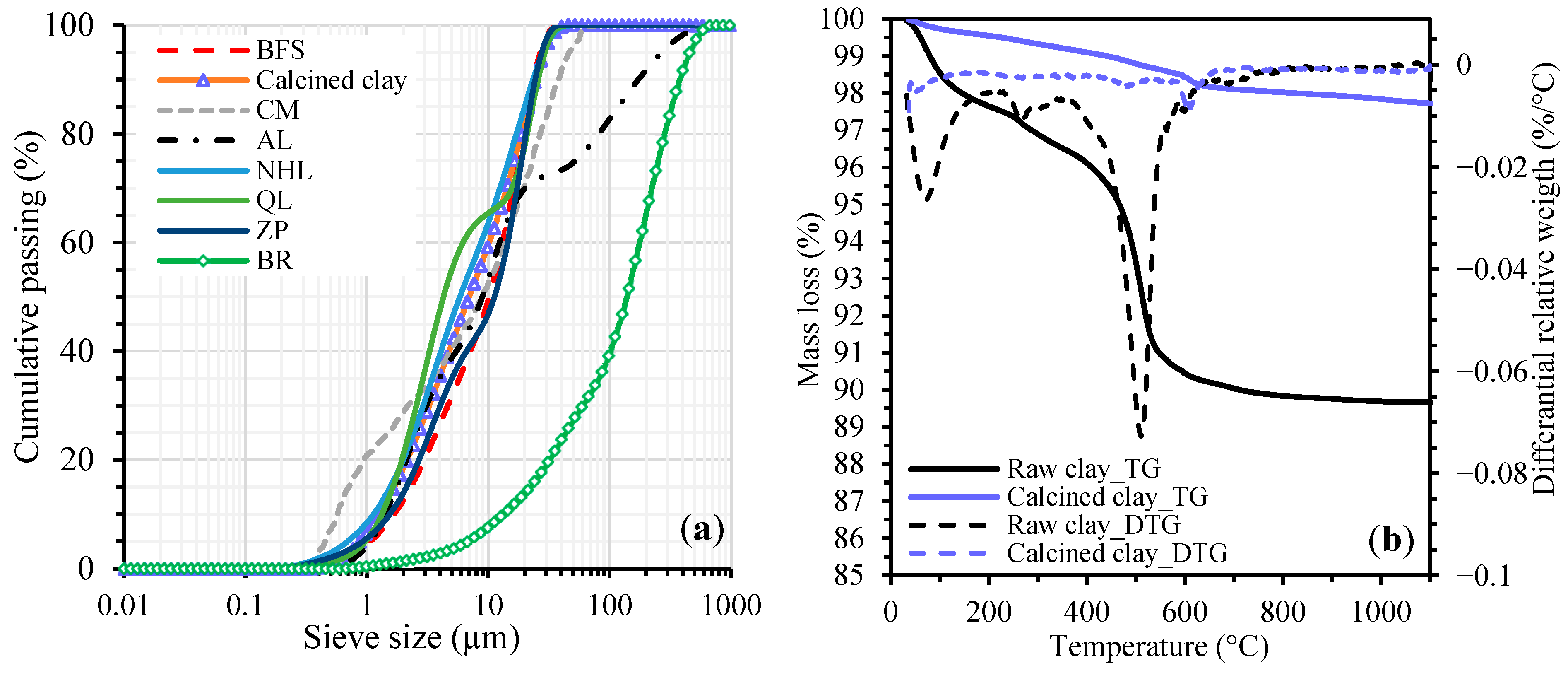
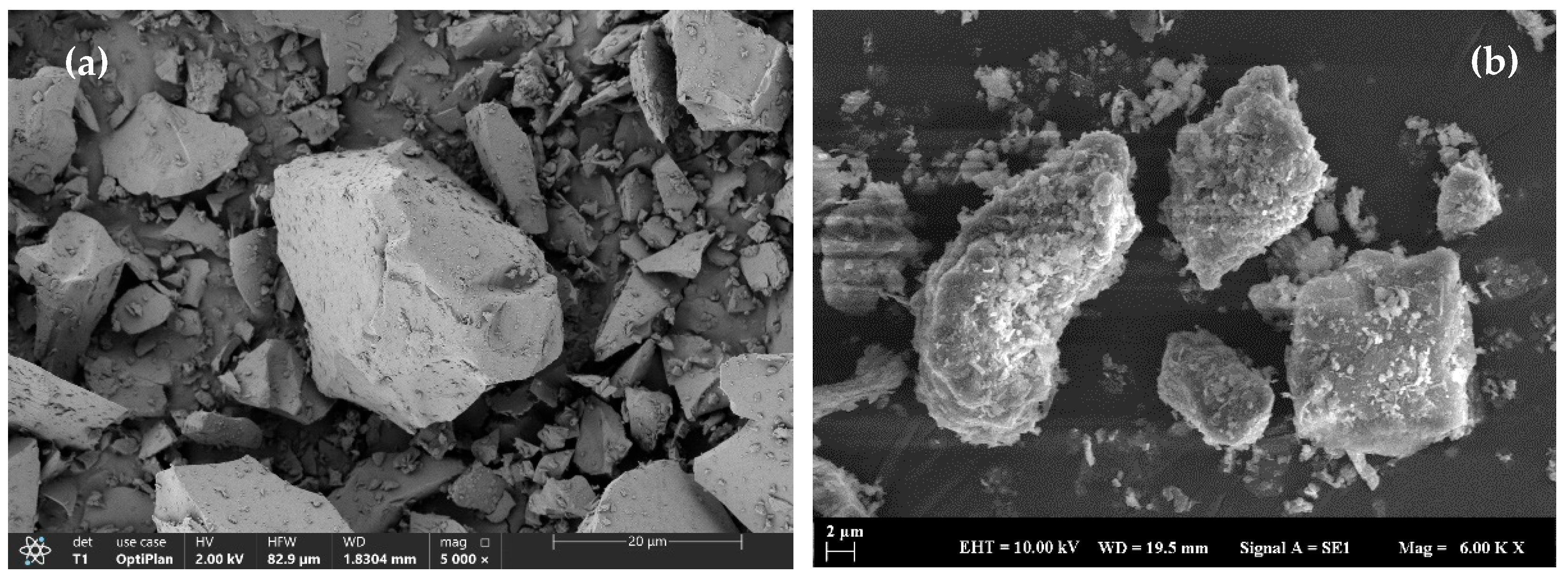






| Oxide Composition (%) | BFS | Calcined Clay | CM | AL | NHL | QL | ZP | BR |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 37.65 | 68.23 | 4.40 | 1.3 | 11.75 | 0.64 | 65.39 | - |
| Al2O3 | 13.61 | 20.11 | 0.10 | 0.6 | 3.59 | 0.17 | 11.31 | - |
| Fe2O3 | 0.71 | 2.74 | 0.60 | 0.3 | 1.55 | 0.09 | 1.45 | - |
| CaO | 27.45 | 1.79 | 5.00 | 70.1 | 52.42 | 85.36 | 3.47 | - |
| MgO | 11.24 | 0.37 | 89.90 | 1.3 | 2.91 | 0.84 | 1.15 | - |
| SO3 | 0.51 | 0.15 | - | 0.6 | 1.43 | 1.01 | 0.03 | - |
| K2O | 1.01 | 1.68 | - | 0.2 | 0.90 | 0.04 | 3.46 | - |
| Na2O | 0.59 | 0.74 | - | 0.0 | 0.16 | 0.09 | 0.30 | 30.41 |
| TiO2 | - | 1.96 | - | 0.5 | 0.33 | 0.35 | 1.26 | - |
| P2O5 | - | 0.05 | - | 0.1 | 0.06 | 0.02 | 0.03 | - |
| Cr2O3 | - | - | - | - | - | - | - | - |
| Mn2O3 | 4.25 | 0.03 | - | - | 0.04 | - | 0.05 | - |
| ZnO | - | 0.01 | - | - | - | - | - | - |
| SrO | 0.08 | 0.01 | - | - | 0.07 | 0.05 | 0.33 | - |
| B2O3 | - | - | - | - | - | - | - | 68.42 |
| Cl | - | 0.12 | - | 0.1 | 0.09 | - | 0.06 | - |
| LOI * | 2.55 | 1.10 | 0.80 | 25.4 | 24.55 | 11.70 | 12.63 | 0.23 |
| Mixture Code | BFS | Calcined Clay | SMS | Water | Sand | Superplasticizer | Minor Additive |
|---|---|---|---|---|---|---|---|
| REF | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | - |
| CM5 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 32.6 |
| CM10 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 65.1 |
| AL5 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 32.6 |
| AL15 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 97.7 |
| NHL5 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 32.6 |
| NHL15 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 97.7 |
| QL2 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 13.0 |
| QL5 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 32.6 |
| QL10 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 65.1 |
| QL15 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 97.7 |
| ZP5 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 32.6 |
| ZP15 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 97.7 |
| Br2 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 13.0 |
| Br5 | 325.7 | 325.7 | 162.8 | 260.5 | 1300 | 9.8 | 32.6 |
| Mixture Code | 1 h | 3 h | 6 h | 1 Day | 3 Days | 5 Days | 7 Days | Deposit Mass (g) |
|---|---|---|---|---|---|---|---|---|
| REF | × | × | × | ✓ | ✓ | ✓ | ✓ | 2.626 |
| CM5 | × | × | × | ✓ | ✓ | ✓ | ✓ | 2.779 |
| CM10 | × | × | × | ✓ | ✓ | ✓ | ✓ | 2.977 |
| AL5 | × | × | × | ✓ | ✓ | ✓ | ✓ | 3.028 |
| AL15 | × | × | × | ✓ | ✓ | ✓ | ✓ | 3.069 |
| NHL5 | × | × | × | ✓ | ✓ | ✓ | ✓ | 1.957 |
| NHL15 | × | × | × | ✓ | ✓ | ✓ | ✓ | 3.913 |
| QL2 | × | × | × | ✓ | ✓ | ✓ | ✓ | 2.568 |
| QL5 | × | × | × | ✓ | ✓ | ✓ | ✓ | 4.143 |
| QL10 | × | × | × | ✓ | ✓ | ✓ | ✓ | 2.993 |
| QL15 | × | × | × | ✓ | ✓ | ✓ | ✓ | 2.256 |
| ZP5 | × | × | × | × | ✓ | ✓ | ✓ | 1.548 |
| ZP15 | × | × | × | × | ✓ | ✓ | ✓ | 1.087 |
| Br2 | × | × | × | × | × | ✓ | ✓ | 0.478 |
| Br5 | × | × | × | × | × | × | ✓ | 0.005 |
| Mixture Code | EDS Spot | O | Na | Mg | Al | Si | Ca | Ca/Si * | Si/Al * |
|---|---|---|---|---|---|---|---|---|---|
| REF | 1 | 48.72 | 9.61 | 0.98 | 6.93 | 22.53 | 11 | 0.52 ± 0.03 | 2.99 ± 0.18 |
| 2 | 47.06 | 10.64 | 2.46 | 8.08 | 19.35 | 10.13 | |||
| CM10 | 1 | 47.52 | 9.88 | 1.22 | 7.22 | 21.45 | 11.22 | 0.51 ± 0.02 | 2.63± 0.26 |
| 2 | 55.18 | 2.65 | 35.33 | 1.23 | 4.29 | 1.31 | |||
| AL15 | 1 | 58.9 | 8.07 | 1.4 | 5.22 | 14.01 | 11.93 | 0.93 ± 0.11 | 2.59 ± 0.13 |
| 2 | 53.08 | 0.94 | 6.43 | 6.46 | 16.22 | 16.41 | |||
| NHL15 | 1 | 61.57 | 8.21 | 4.6 | 5.36 | 11.68 | 7.64 | 0.71 ± 0.02 | 2.53 ± 0.25 |
| 2 | 63.42 | 2.45 | 0.76 | 1.42 | 3.92 | 28.02 | |||
| QL10 | 1 | 57.71 | 3.15 | 0.74 | 7.34 | 15.24 | 12.71 | 0.83 ± 0.01 | 2.35 ± 0.12 |
| 2 | 53.55 | 4.98 | 0.77 | 7.12 | 17.21 | 14.25 | |||
| ZP15 | 1 | 40.71 | 0.13 | - | - | 59.16 | - | 0.53 ± 0.01 | 3.20 ± 0.02 |
| 2 | 47.16 | 5.33 | 0.01 | 6.2 | 25.19 | 13.54 | |||
| Br5 | 1 | 59.89 | 10.64 | - | 6.28 | 14.57 | 7.08 | 0.45 ± 0.08 | 2.61 ± 0.32 |
| 2 | 57.24 | 8.57 | 5.88 | 5.24 | 16.31 | 6.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çalbıyık, S.; Omur, T.; Ozkan, H.; Kabay, N. Influence of Minor Additives on the Performance of Calcined Clay and Blast Furnace Slag Based One Part Alkali-Activated Mortars. Buildings 2025, 15, 3776. https://doi.org/10.3390/buildings15203776
Çalbıyık S, Omur T, Ozkan H, Kabay N. Influence of Minor Additives on the Performance of Calcined Clay and Blast Furnace Slag Based One Part Alkali-Activated Mortars. Buildings. 2025; 15(20):3776. https://doi.org/10.3390/buildings15203776
Chicago/Turabian StyleÇalbıyık, Suat, Tarik Omur, Hakan Ozkan, and Nihat Kabay. 2025. "Influence of Minor Additives on the Performance of Calcined Clay and Blast Furnace Slag Based One Part Alkali-Activated Mortars" Buildings 15, no. 20: 3776. https://doi.org/10.3390/buildings15203776
APA StyleÇalbıyık, S., Omur, T., Ozkan, H., & Kabay, N. (2025). Influence of Minor Additives on the Performance of Calcined Clay and Blast Furnace Slag Based One Part Alkali-Activated Mortars. Buildings, 15(20), 3776. https://doi.org/10.3390/buildings15203776







