1. Introduction
Although global freshwater resources are substantial, nearly half of the world’s population faces water scarcity during certain periods of the year [
1]. This challenge is primarily driven by climate change, rapid population growth, and unregulated urbanization. The continuously increasing demand for water highlights the need for efficient and sustainable water resource management. Such management is essential for ensuring equitable access to water and for preserving it in the long term. In this context, Sun et al. (2022) [
2] conducted a comprehensive bibliometric and geographic analysis, revealing that research on water scarcity is largely focused on affluent and populous countries, with GDP and population size being the main influencing factors. Their study identified drought and climate change as key causes of water scarcity and highlighted the need to extend research efforts to developing countries facing both physical and economic water scarcity. Promoting effective solutions is vital to achieving sustainable water management objectives by 2030. Rainwater emerges as a viable alternative source, capable of partially meeting daily water needs. As a form of atmospheric precipitation, rainwater has been collected and reused since antiquity through various methods. Recently, the harvesting and reuse of rainwater have gained traction even in developed countries, where they are recognized as sustainable strategies to enhance the resilience of water supply systems. This growing interest is reflected in an increasing number of studies, with significant contributions from the United States, China, and India [
3]. Moreover, countries such as Germany, the United Kingdom, and New Zealand actively promote rainwater use for non-potable applications through dedicated policies and regulations [
3]. Building on this, Unver et al. (2024) demonstrated that modular passive rainwater harvesting systems for existing buildings can achieve high efficiency in water collection and reuse, offering a sustainable and cost-effective solution to improve water resilience, especially in regions facing water stress [
4].
Similarly, in Nigeria and other African countries affected by water stress, rainwater is often used for human consumption due to inadequate water supply infrastructure, low living standards, and the minimal costs associated with its collection [
5]. However, in these regions, water quality remains a critical challenge, raising concerns about the safety of direct consumption. Although rainwater harvesting and reuse can substantially reduce potable water demand, regulations governing its integration into water supply systems are frequently restrictive, primarily due to stringent water quality requirements. In Romania, the lack of a clear and coherent legislative framework regarding the use of alternative water sources constitutes a significant barrier to the widespread adoption of such practices. Currently, rainwater is discharged into public sewer systems without reuse, while major investments continue to focus on drainage infrastructure rather than on reuse systems.
Sustainability is broadly defined as the capacity of a system to maintain its functionality and development over time without depleting or causing irreversible damage to natural resources essential for future generations [
6]. In the context of water resource management, sustainability entails ensuring the long-term availability of sufficient water quantities to meet human needs despite increasing environmental pressures and conditions of water stress. This approach emphasizes not only the efficient use of water but also the implementation of adaptive strategies to enhance resilience in the face of climate variability and anthropogenic impacts. The reuse of rainwater in residential buildings offers multiple benefits, including meeting a substantial portion of daily water demand, reducing potable water consumption and associated costs, preventing drought, and mitigating flood risks. This study aims to evaluate the potential for rainwater reuse in small residential buildings for non-potable purposes by treating and analyzing its physicochemical and microbiological properties.
The characteristics of rainwater are influenced by various factors, including the frequency and intensity of precipitation, the type and material of the collection surface, and the degree of atmospheric pollution. According to the study by Mazurkiewicz et al., stored rainwater collected from rooftops and adjacent surfaces generally exhibited physicochemical parameters in line with European Union standards for drinking water. However, the microbiological quality was often unsatisfactory, with high levels of coliform bacteria, psychrophilic, and mesophilic bacteria, highlighting the need for appropriate treatment before reuse [
7]. In this study, a dual-medium pre-filtration system composed of sand and washed fine gravel was used to improve the quality of the collected rainwater. Among the available treatment technologies, filtration based on granular materials such as sand and gravel has proven to be highly effective, economically accessible, and environmentally sustainable. The first sand filters were introduced as early as 1829, when James Simpson implemented sand filtration in England to purify water from the River Thames. As noted by Wegelin, “no other filtration process can improve the physical, chemical, and organoleptic properties of surface waters more effectively than sand filtration.” This method can remove up to 99% of bacteria and up to 93.9% of fecal coliforms [
8]. Physical filtration relies on the capacity of granular media to retain particles and support the development of biological activity that degrades organic contaminants, thereby improving the physicochemical parameters of the water. Specifically, sand filters facilitate mechanical filtration and adsorption processes within the granular layer while simultaneously reducing microbiological risks through the formation of an active biological layer known as the
schmutzdecke at the filter bed surface [
9]. The
schmutzdecke is a complex biofilm, essential for biological purification processes, which develops on the upper surface of slow sand filters. The term originates from German and translates as “dirty layer,” reflecting its intense biological activity and its critical role in enhancing the microbiological quality of the water [
10].
Kuslu et al. demonstrated the effectiveness of sand and gravel medium filters in removing suspended solids from irrigation water. Their findings indicated that combined pumice and sand–gravel filters achieved high solid removal efficiency alongside sustained flow rates, underscoring the suitability of sand and gravel filtration for enhancing water quality in small-scale applications [
11]. Similarly, Pipil et al. conducted a study in Delhi, India, demonstrating that sand and gravel filtration effectively removes suspended solids and reduces water hardness caused by calcium and magnesium ions in stormwater runoff. The study noted that runoff from residential areas, with higher levels of ammonium, nitrate, phosphate, and organic carbon, requires additional treatment stages such as constructed wetlands and disinfection to meet potable water standards. This highlights the important role of sand and gravel filtration as part of a sustainable stormwater treatment system [
12]. Their primary advantages include ease of installation, minimal maintenance requirements, and adaptability to local conditions, which make them especially appropriate for household-scale systems aimed at the collection and reuse of rainwater. However, the effectiveness of these filters in removing microorganisms can be limited, which necessitates the inclusion of an additional disinfection stage within the treatment process. In the conducted study, calcium hypochlorite disinfection was used as a complementary method for water treatment.
Calcium hypochlorite disinfection is an effective and widely used method for ensuring the microbiological safety of water. As a strong oxidizing compound, it is capable of inactivating a broad spectrum of pathogens, including bacteria, viruses, and protozoa, thereby significantly reducing the risk of waterborne diseases [
13,
14]. The solid and stable form of calcium hypochlorite, combined with its low cost, ease of storage and transportation, and suitability for use in areas with limited infrastructure, provides a significant advantage for its application in decentralized water treatment systems, including the household-scale reuse of rainwater [
15,
16]. Mohammed (2019) [
17] demonstrated enhanced disinfection efficiency of calcium hypochlorite through a Ca(OCl)
2/AgNPs composite, which achieved 100% biocidal activity against pathogenic bacteria at 1.5 mg/L within 180 min. When applied to filter paper, the composite also led to complete inactivation of coliform bacteria, indicating the potential of nanomaterials to improve conventional disinfectants in microbiological water treatment. Compared to other disinfection methods, such as ultraviolet radiation or ozonation, which require continuous energy consumption and specialized equipment, calcium hypochlorite stands out as a cost-effective and more easily implementable alternative, particularly in rural areas or communities with limited resources [
18,
19]. Mezzanotte et al. (2007) [
18] conducted a study on the efficiency of various wastewater disinfection methods and found that chlorination provides superior reduction in total coliforms compared to ozonation and UV radiation. They also emphasized that the choice of microbiological indicator influences the evaluation of disinfection performance, with
Escherichia coli being the most representative indicator of the hygienic safety of treated water. For chlorination, the Hom model was identified as the most suitable for describing the kinetics of the disinfection process.
High levels of organic matter can reduce the disinfectant’s efficacy and promote the formation of harmful by-products such as trihalomethanes [
20,
21]. Under these circumstances, the incorporation of a preliminary filtration step, using granular materials such as sand and gravel, becomes essential to reduce turbidity and organic matter content, thereby significantly enhancing the efficiency of the chlorination process and mitigating risks to public health. The combined approach of granular medium filtration followed by disinfection with calcium hypochlorite represents a practical, adaptable, and sustainable solution for improving the quality and safety of rainwater intended for non-potable purposes. However, research evaluating the effectiveness of this treatment method for rooftop-harvested rainwater remains limited, particularly in small-scale or household settings.
Scope and Highlights
This study presents a systematic and applied evaluation of the feasibility of rainwater reuse in small-scale residential buildings. Rainwater samples were collected and subjected to comprehensive physicochemical and microbiological analyses both prior to and following treatment. The treatment process, comprising filtration followed by disinfection, was designed to improve water quality and assess the potential of treated rainwater as a sustainable alternative source for non-potable applications.
To address existing research gaps, this study investigates the integrated system’s capacity to enhance key water quality parameters, including pH, turbidity, electrical conductivity, total hardness (as the combined concentration of calcium and magnesium), color, odor, E. coli, Enterococci, Coliform Bacteria, and Clostridium perfringens. These parameters were selected based on their relevance to public health and their effectiveness in evaluating the efficiency of the filtration and disinfection processes. The findings provide empirical evidence supporting the viability of treated rainwater for safe and sustainable non-potable household use, thereby contributing to the advancement of sustainable water resource management strategies.
The highlights of the present case study can be summarized as follows:
- (i)
Demonstrating the potential of rainwater as a valuable resource for sustainable household water management.
- (ii)
Promoting the conservation of natural water resources by reducing potable water consumption and associated costs.
- (iii)
Encouraging the adoption of efficient systems for rainwater collection, treatment, and reuse.
- (iv)
Addressing the current limited or virtually nonexistent rainwater reuse practices within the national context.
- (v)
Assessing the effectiveness of an integrated treatment system to optimize technological processes for rainwater purification.
- (vi)
Establishing a comprehensive experimental database to serve as a foundational reference for future research on rainwater treatment and reuse, both nationally and internationally.
- (vii)
Facilitating the implementation of sustainable water management strategies that foster responsible utilization of rainwater as a renewable resource.
- (viii)
Contributing to environmental protection through enhanced water resource management.
2. Materials and Methods
2.1. General Description of the Study
This study was conducted in a residential building located in Cluj County, Romania, over a period of three months (May–July 2025), with the primary objective of investigating the functional performance of a treatment system designed to improve rainwater quality. The proposed system integrated two treatment stages: stratified filtration using successive layers of sand and gravel, and chemical disinfection with a calcium hypochlorite solution. The efficiency of the treatment system was evaluated by analyzing the physicochemical and microbiological parameters of the treated water, in order to assess its potential for non-potable reuse.
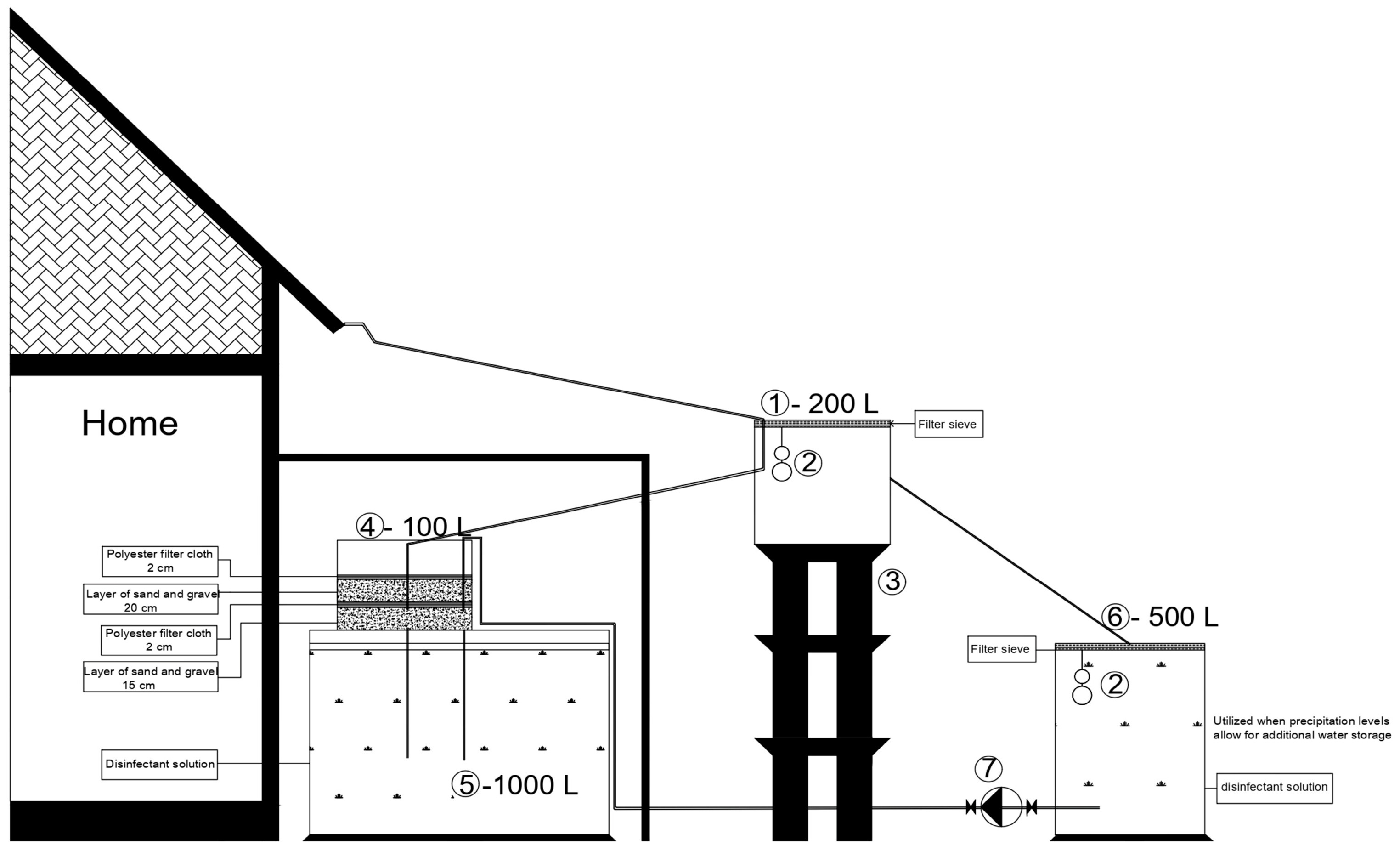
To quantitatively assess the available resource, the total volume of rainwater collected during the study period was monitored. The water was collected from a 50 m2 section of the building’s roof, made of ceramic tiles with a 45° slope, and directed via a system of gutters and downspouts into an intermediate polyethylene tank with a capacity of 200 L. This tank was equipped with a mesh screen designed to retain coarse particles such as leaves, sand, and plant debris.
Subsequently, the water was conveyed by gravity through a DN75 mm polypropylene pipe into a second tank with a capacity of 100 L, where the filtration system was installed. This system consisted of successive layers of sand and washed fine gravel, supplemented with two additional mesh screens. The filtered water was then stored in a 1000 L tank, where it underwent disinfection using a calcium hypochlorite solution.
To optimize the management of rainwater resources and ensure additional storage during periods of heavy rainfall, the system incorporates an auxiliary collection tank with a capacity of 500 L, constructed from the same material as the main storage tank. During intense precipitation events, a portion of the rainwater collected by the main system is directed to the filtration unit and then flows into the primary tank intended for storage and disinfection, while the excess is temporarily diverted to the auxiliary tank.
In the context of non-potable rainwater reuse, when the water level in the main tank falls below the optimal threshold of approximately 100 L per day, it is replenished by transferring water from the auxiliary tank via a small-scale pumping station. The water then undergoes filtration and disinfection processes as outlined in the proposed treatment system.
For non-potable domestic applications, such as toilet flushing or garden irrigation, the treated rainwater is distributed from the main storage tank via a pumping system and a dedicated pipe network, installed either inside or outside the building depending on functional requirements. The reuse of rainwater for household non-potable purposes is permitted only when the physicochemical and microbiological parameters meet the applicable regulatory standards.
Figure 1 illustrates the functional diagram of the collection, treatment, and storage system, along with the main components used in the experiment.
2.2. Sand and Fine Gravel Filtration
In the 100 L tank labeled as (4) in
Figure 1, a rainwater treatment system was installed, consisting of successive layers of sand and washed fine gravel, supplemented by two additional polyester filter meshes. The first layer, composed of sand (with particle sizes of approximately 0.4–0.8 mm) and gravel (with particle sizes between 2–6 mm), with a thickness of 15 cm, was placed at the bottom of the tank after the materials were thoroughly washed and cleaned three times to eliminate impurities and prevent any negative impact on the quality of the treated water (step 1 in
Figure 2). Above this layer, a 2 cm thick polyester filter mesh was added (step 2 in
Figure 2), followed by a second layer of washed fine sand and gravel, 20 cm thick, with the same particle sizes as the first layer (step 3 in
Figure 2). Next, a second polyester filter mesh, also 2 cm thick, was installed to provide an additional barrier for particle retention (step 4 in
Figure 2). Rainwater is transported by gravity to this filtration tank, where it passes sequentially through the first polyester mesh, the 20 cm sand and gravel layer, the second polyester mesh, and the final 15 cm sand and gravel layer, for further purification. The treated water is then stored in the 1000 L tank labeled as (5) in
Figure 1, where it undergoes disinfection. The functional schematic of the filter medium used in the treatment process is presented in
Figure 2.
2.3. Chemical Disinfection Using Calcium Hypochlorite
After the filtration stage, the water was transferred into the 1000 L tank labeled as (5) in
Figure 1, which served a dual function of storage and disinfection. Chemical disinfection was carried out by applying a calcium hypochlorite solution, prepared by dissolving commercially available bleaching powder (commonly referred to as “chloride of lime”), a technical mixture predominantly composed of Ca(ClO)
2, with additional components such as CaCl
2 and Ca(OH)
2. Upon contact with water, calcium hypochlorite releases hypochlorite ions (ClO
−), which exhibit biocidal properties and contribute to the microbiological inactivation of the water. The working solution was prepared according to the recommended dosage for surface water treatment (8–12 g of calcium hypochlorite per liter of water). Accordingly, 12 g of calcium hypochlorite were dissolved in 1 L of water. This solution was gradually dosed into the disinfection tank every three days throughout the experimental period, in order to maintain a relatively constant concentration of active oxidizing agent in the treated water volume.
2.4. Physicochemical and Microbiological Parameters Analyzed in Laboratory Samples
Throughout the entire monitoring period, water samples were collected both from untreated rainwater and from rainwater that had undergone the filtration and disinfection processes. These samples were analyzed to determine various physicochemical parameters (pH, turbidity, total hardness, electrical conductivity, free residual chlorine, color, and odor) and microbiological parameters (Total colony count, Coliform bacteria,
E. coli, Enterococci, and
C. perfringens) in order to evaluate the efficiency of the applied treatment. The water samples were collected in 500 mL laboratory-grade sampling bottles specifically designed for physicochemical and microbiological analysis (
Figure 3).
Coliform bacteria are Gram-negative, rod-shaped, aerobic or facultative anaerobic organisms commonly used as indicators for assessing the sanitary quality of food and water [
22].
E. coli, the primary species within the fecal coliform group, is considered the most reliable indicator of potential pathogenic contamination. This bacterium does not grow or multiply in the external environment [
23]. Enterococci are Gram-positive bacteria that develop in the intestinal tracts of humans and animals. Although not generally harmful to human health, their presence in the environment or water may indicate possible contamination with other pathogens or viruses [
24].
C. perfringens is a Gram-positive, rod-shaped, anaerobic bacterium associated with acute gastrointestinal infections. This pathogen produces a range of toxins that contribute to disease development and has the ability to form spores that are resistant to harsh environmental conditions [
25].
The first set of samples was collected and analyzed in May, following the initial rainfall event of 20 mm (equivalent to 20 L/m
2), which resulted in the accumulation of 1000 L of rainwater in the storage tank. The selection of the storage tank capacity was based on the catchment area of 50 m
2 and the average precipitation from a single rainfall event, which ranges between 20–25 mm. Thus, for a 50 m
2 catchment area and a rainfall of 20 mm, a storage tank with a capacity of 1000 L is necessary. According to precipitation statistics for May in the study location, rainfall amounts typically vary between 2 and 20 mm on average [
26]. Therefore, for the given catchment area, the chosen storage tank is adequate to hold the rainwater collected from a single rainfall event. To monitor the water levels, two float switches were installed: one in the rainwater collection tank with a capacity of 200 L, labeled as (1)
Figure 1, and the other in the storage and disinfection tank with a capacity of 1000 L, labeled as (5) in
Figure 1. Although May is generally one of the rainiest months of the year, in 2025 the total precipitation recorded was lower than that of the previous year. While the long-term average number of rainy days in May is approximately 17, only 13 rainy days were recorded in the current year [
26,
27]. The amount of precipitation during this month allowed exclusive use of the two tanks mentioned above.
June 2025 registered the lowest precipitation levels in recent decades (1961–2025), with a national average of only 18.9 mm [
28]. This characterized the month as exceptionally dry. Under these conditions, after a brief rain episode, the collection tank accumulated only 30 L of rainwater, an insufficient volume for conducting relevant and conclusive laboratory analyses.
The second set of samples was collected in July, a month characterized by abundant rainfall, with 20 rainy days and intense precipitation reaching approximately 100 mm [
28]. Following the first heavy rain event, the tanks reached their maximum capacity, necessitating the use of an auxiliary tank provided for storing the additional collected water.
2.5. Sequence of Laboratory Testing
The first set of laboratory samples, collected in May, was analyzed following a well-defined chronological sequence. The first sample, raw rainwater, was collected ten hours after accumulation in the collection tank to determine the physicochemical and microbiological characteristics of the water in its initial state, prior to the treatment process. The second sample was taken one day after passing through the filtration layer and after the disinfectant solution was applied in the 1000 L tank, labeled as (5) in
Figure 1. The third sample was collected ten days later, after the water had passed through the filtration layer and the disinfectant solution had been applied at 72-h intervals. This monitoring period was chosen to evaluate changes in water composition under real storage conditions, influenced by the filtration process and disinfectant treatment, aiming to determine the feasibility of reuse.
The second set of samples, collected in July, started with the collection of a raw rainwater sample ten hours after the first rainfall event, aiming to analyze the impact of high temperatures and heavy precipitation on the physicochemical and microbiological properties of untreated water. The second and third samples were collected according to the same methodology as the previous set, at one day and ten days after filtration and disinfection applied every three days. Additionally, a fourth sample was collected from an auxiliary storage tank three days after the initial collection. To maintain water quality during storage, the same type of disinfectant was applied, but at a reduced concentration of 3 g of calcium hypochlorite dissolved in water.
For each set of analyses, the filtration layer composed of sand and gravel was replaced with a new one of identical composition to ensure consistency and efficiency of the filtration process. Throughout the study, the rainwater was not reused for non-potable activities but was exclusively subjected to quantitative and qualitative evaluation to determine its reuse potential. All water samples were analyzed in accordance with the national legislation in force, using standardized laboratory methods recognized both nationally and internationally.
Table 1 presents the parameters analyzed during the laboratory tests and the applied determination methods for each.
3. Results
For both the first and second sets of samples, the initial stage involved determining the physicochemical properties (pH, turbidity, color, odor) and microbiological parameters (Colony count,
E. coli, Enterococci, Coliform bacteria,
C. perfringens) of the raw rainwater, aiming to assess its quality based on precipitation intensity and meteorological conditions.
Table 2 presents the characteristics of the raw rainwater determined during the two sampling campaigns.
After collection, the rainwater underwent a filtration process and was then stored in the 1000 L tank, identified as (5) in
Figure 1, according to the previously presented scheme. The second sample was taken one day after passing through the filtration layer and the application of the disinfectant solution. Unlike the first sample, in addition to the usual physicochemical parameters, additional indicators were analyzed in the second sample, such as residual chlorine concentration, total hardness (determined as the sum of calcium and magnesium concentrations), and electrical conductivity, considering the presence of calcium hypochlorite solution in the tank. The results are presented in
Table 3.
The third sample was collected ten days after water storage and the application of the disinfectant treatment. The obtained results are presented in
Table 4.
The fourth sample was collected from the auxiliary storage tank, designated as (6) in
Figure 1, three days after the rainfall event. To maintain water quality, the same disinfectant solution was applied, but at a reduced concentration. This auxiliary tank was designed to enable the storage of a larger volume of raw rainwater during high-intensity precipitation events (
Table 5).
4. Discussion
4.1. Physicochemical Indicators Obtained
According to the results obtained from laboratory analyses, the pH values of the collected rainwater fall within the limits recommended by the World Health Organization (WHO), as outlined in the Guidelines for Drinking Water Quality [
39]. After passing through the filtration layer and applying the disinfectant solution, a slight increase in pH was observed; however, the values remained within the acceptable range. This increase can be attributed to the presence of calcium ions released either through leaching from the filter medium or through the dissolution of calcium hypochlorite in water—a phenomenon that may also explain the increase in turbidity [
40].
The sand and gravel used in the filter layer were washed exclusively with water, which did not ensure complete removal of fine particles and thus contributed to increased turbidity. The electrical conductivity of the filtered water showed elevated values, influenced by the ionic concentration of the solution and the contact time with the filter medium [
41,
42]. Residual chlorine concentrations measured after the disinfectant was applied ranged between 2.169–2.517 mg/L, due to the substantial amount of disinfectant solution used. These values exceed the limits set by national regulations, namely O.G.N No. 7/18.01.2023 and H.G. 971/2023 [
43,
44].
The higher disinfectant concentration is justified by the need to eliminate bacterial contamination in the raw rainwater, as confirmed by the microbiological analysis presented in
Table 2. Total hardness, defined as the sum of calcium and magnesium ion concentrations, remained within acceptable values and was dependent on the concentration of these ions.
Organoleptic parameters such as color and odor remained within acceptable limits, with no significant deviations observed before or after the treatment process. Chemical indicators such as nitrates, nitrites, and aluminum were not included in the analysis, as the purpose of this study was to assess the potential for rainwater reuse in non-potable domestic applications, where such indicators are not essential for evaluating water quality.
4.2. Microbiological Indicators Obtained
From a microbiological perspective, rainwater collected from the building’s roof exhibited a total colony count exceeding 300 CFU/mL at both 22 °C and 36 °C. Enterococci were detected at concentrations ranging between 103 and 119 CFU/100 mL, Coliform bacteria exceeded 80 CFU/100 mL, and C. perfringens ranged between 64 and 72 CFU/100 mL. Notably, E. coli was absent from all analyzed samples. Consequently, the rainwater presented a high bacterial load, attributable to climatic conditions as well as microorganisms present in the air, on the roof surface, and within the collection tank walls. Although this water is intended for non-potable reuse, treatment and disinfection are essential to ensure hygienic conditions, especially if reused for toilet flushing.
After passage through the filtration layer and application of the disinfectant solution, analyzed 24 h later, a significant reduction in microbial load was observed. Total colony counts decreased to 70–80 CFU/1 mL at 22 °C incubation and 16–25 CFU/1 mL at 36 °C. Coliform bacteria numbers dropped to 29–40 CFU/100 mL, and Enterococci as well as C. perfringens were no longer detected in the samples. Ten days post-storage and periodic disinfectant application, microbiological water quality further improved due to prolonged contact time with the disinfectant. Colony counts reduced to 2–4 CFU/1 Ml at 22 °C and 3 CFU/1 mL at 36 °C, with Coliform bacteria completely eliminated.
Application of calcium hypochlorite solution resulted in a significant microbial load reduction within 24 h. At 22 °C, Colony counts dropped from >300 CFU/1 mL to 70–80 CFU/1 mL, and at 36 °C, from 300 to 16–25 CFU/1 mL. After ten days, with three successive applications of the disinfectant solution, values further decreased to 4–2 CFU/1 mL at 22 °C and 3 CFU/1 mL at 36 °C. These results highlight the high efficacy of calcium hypochlorite in reducing bacterial load in treated rainwater. The bacteriological values obtained fall within acceptable limits even for potable water use, according to national and international regulations. Regarding the additionally collected rainwater, favorable conditions were maintained three days after collection due to the presence of the disinfectant.
In comparison with other studies focusing on rainwater treatment using similar filtration materials such as sand and gravel layers, the results of this study demonstrate superior efficiency in reducing Total Coliforms [
45]. This enhanced performance is primarily attributed to the subsequent application of calcium hypochlorite, a broad-spectrum disinfectant highly effective against pathogenic microorganisms. Regarding combined treatment approaches, whether sand and gravel filtration followed by calcium hypochlorite disinfection or ultraviolet (UV) radiation, a significant decrease in bacterial colony counts was observed in both cases [
9,
46]. While both methods yield effective microbiological outcomes, the use of UV disinfection entails higher installation and maintenance costs, potentially limiting its feasibility in decentralized systems or individual households.
These findings demonstrate the efficiency of sand and gravel filtration layers, as well as the effectiveness of calcium hypochlorite as a disinfectant agent in eliminating microorganisms. This disinfectant is primarily used for water treatment, and its dosage must comply with public health and environmental protection standards [
47]. Samples were analyzed in an accredited laboratory according to current standards.
4.3. Assessment of Rainwater Volume Throughout the Study
In May, the total recorded precipitation in the area was 78.38 mm. Based on a collection surface area of 50 m2, an estimated volume of approximately 3919 L (3.919 m3) of rainwater could have been harvested. If this water had been reused for non-potable domestic purposes, such as toilet flushing, it would have resulted in a significant reduction in the consumption of potable water from the public supply. For example, a standard toilet tank with a volume of 6 L, used on average 5 times per day, generates a daily water consumption of approximately 30 L. Over the 31 days of May, the total water demand for this purpose would be around 930 L. This amount could have been fully supplied by the collected rainwater volume, without affecting potable water reserves. The remaining volume of approximately 2989 L could have been used for other non-potable activities, such as irrigation, or stored for later use during months with lower precipitation.
In July, the recorded precipitation in the study area ranged between 101 mm and 125 mm, corresponding to a potential collectible water volume between 5050 and 6250 L for the same 50 m2 collection area. This volume could provide up to 6.25 m3 of water usable for non-potable purposes, covering most of the daily household demand for such activities. Moreover, any surplus could be stored and reused in August, following the treatment procedures described in this study.
The average annual precipitation in the study area is estimated to be between 500 and 600 mm. Based on a collection surface area of 50 m2, similar to that analyzed in the study, an estimated annual volume of 25–30 m3 of rainwater can be harvested and reused. For non-potable domestic uses such as toilet flushing, the average daily water consumption per person is approximately 30 L, which corresponds to an annual consumption of about 10.95 m3. This amount can be fully met by the collected rainwater, even in years when precipitation is at the lower end of the average climatic range. The remaining available volume, estimated between 14 and 19 m3 per year, can be allocated for other non-potable uses such as garden irrigation, outdoor cleaning, and washing of patios or walkways, or stored for later use, especially during dry periods, depending on the monthly distribution of rainfall. The integration of an efficient filtration, storage, and distribution system enables optimal utilization of this renewable resource throughout the year.
By reducing the consumption of potable water supplied by the public network, implementing such a system contributes to the optimization of water resource use and the reduction in associated monthly costs. Although the system’s efficiency is influenced by seasonal variability in precipitation, investing in a rainwater harvesting and reuse solution remains economically justifiable in the long term and environmentally responsible, especially in the current context of increasing pressure on freshwater resources and intensifying climate change.
4.4. Economic Assessment of Rainwater Treatment and Reuse
To evaluate the economic impact of rainwater reuse in residential buildings for non-potable purposes, a specific scenario was analyzed: the water consumption of a household with two occupants. The analysis compares the total monthly water consumption with the volume of rainwater that could potentially be collected and reused, using May and July as reference months. Based on this comparison (see
Table 6), potential financial savings and the extent to which water demand could be met are assessed, thereby contributing to an estimation of the real benefits of implementing a rainwater harvesting and reuse system (
Table 6).
May—Rainwater Scenario:
Estimated collected rainwater: 3.919 m3
Monthly consumption: 8.4 m3
Percentage of consumption covered by rainwater:
By collecting rainwater in May, nearly half of the monthly water consumption (46.7%) could be covered, resulting in a savings of approximately EUR 6.70. Since the rainwater is used for non-potable purposes, it can be stored and reused following the treatment methods previously described. In July, due to higher precipitation levels, over 74% of the monthly water demand could be supplied from alternative sources, generating a saving of EUR 10.69. Similarly, the use of rainwater for non-potable purposes allows for its storage and reuse through the mentioned treatment methods. The implementation of a rainwater harvesting system can significantly reduce the monthly water bill, especially during months with abundant rainfall. Over just two months, total savings can reach ~EUR 17.39. Annually, such savings may exceed ~EUR 100 for an average household located in areas with frequent rainfall.
The implementation costs of the system studied were low due to the use of affordable filter media and durable materials for the equipment used in rainwater harvesting and treatment. Four polypropylene tanks of different capacities were purchased for the system. Polypropylene is a durable material with low costs, does not negatively affect water quality, and has an expected lifespan of 10 to 25 years. The prefiltration system costs are minimal or even free if materials are sourced externally. In this study, the filter medium was replaced monthly at a low acquisition cost.
Another important cost factor is the disinfectant solution, which was purchased in a quantity of 250 g for this study. The water level measuring equipment used has a low cost and an estimated lifespan of about 3 years. The pumping station included in the system has low energy consumption, helping to keep operational costs down. The expected lifespan of the pump is approximately 5 to 7 years, depending on usage and maintenance. Additionally, the pipes used in the system are made of polypropylene, which are affordable and durable, contributing to the overall low acquisition and maintenance costs. The total investment required to implement the system was approximately EUR 593, which includes all equipment. Maintenance costs are low and include cleaning, filter medium replacement (including purchased sand), and disinfectant purchase, estimated at around EUR 2.3 per month, depending on disinfectant demand and environmental conditions.
Regarding space and storage requirements, in this study, the filtration, storage, and disinfection tanks, along with the disinfectant solution, were stored in an annex to the building with an area of approximately 2 m2. The rainwater collection tanks were installed outdoors, requiring dedicated space for placement. A key element of this research is the rainwater itself, which has no acquisition cost, representing a major benefit in terms of resource utilization.