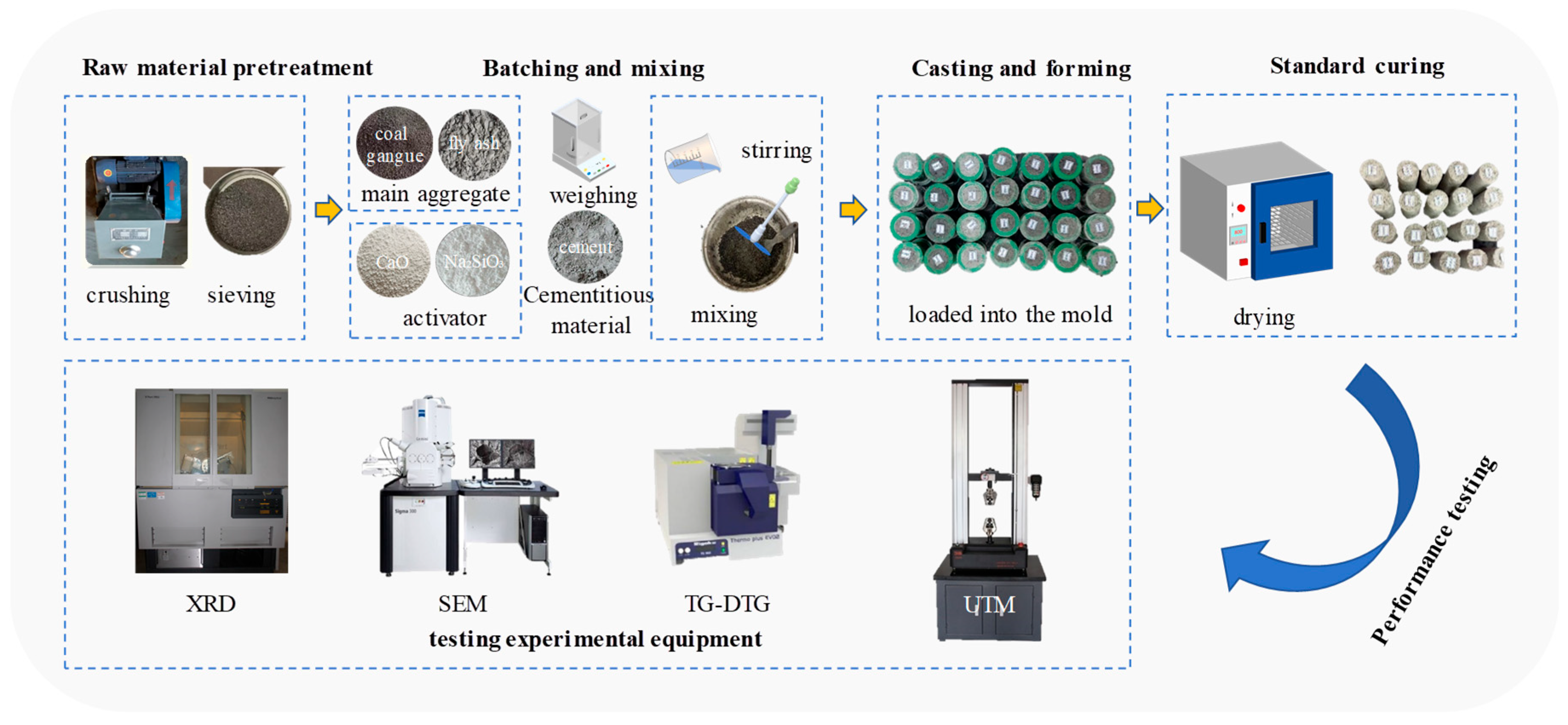
3.1. XRD
XRD is a key technique for analyzing the phase composition of mineral-based materials. Crystalline phases can be identified based on the position (2θ value) and relative intensity of characteristic diffraction peaks. The XRD patterns of coal gangue, fly ash, cement, calcium oxide, and sodium metasilicate are presented in
Figure 2.
The typical mineral composition of coal gangue includes quartz, kaolinite, illite, feldspar, and calcite. In the XRD pattern, characteristic diffraction peaks of quartz are observed at 2θ = 20.8°, 26.6°, 36.5°, and 50.1°, with the most intense peak appearing at 26.6°, corresponding to the (101) crystallographic plane. The peaks at 2θ = 12.4° ((001) plane), 24.9°, and 35.0° are indicative of kaolinite. Illite is identified by its characteristic peaks at 2θ = 8.8°, 17.7°, and 26.6°, with the peak at 26.6° overlapping with that of quartz. Feldspar is characterized by peaks at 2θ = 27.5° and 28.4°, while the peak at 2θ = 29.4° corresponds to CaCO3, indicating the presence of calcite as a carbonate impurity. In summary, the XRD pattern of coal gangue is dominated by quartz peaks, while the intensities of the clay mineral peaks (kaolinite and illite) vary depending on the degree of weathering and the depositional environment of the gangue.
For fly ash, a broad hump within the 2θ range of 20–35°—often referred to as a “hump peak”—is indicative of its amorphous glassy phase and represents a primary characteristic of fly ash. Additionally, a diffraction peak at 2θ = 26.6°, corresponding to the (101) plane of quartz, is commonly observed and is typically attributed to unburned residual minerals. Characteristic peaks of mullite appear at 2θ = 16.5°, 26.2°, and 33.0°, with the peak at 33.0° corresponding to the high-temperature sintering product 3Al2O3·2SiO2. Peaks at 2θ = 30.2°, 35.6°, and 43.3° are associated with magnetite (Fe3O4), where the peak at 43.3° indicates iron oxide phases formed during coal combustion. The pozzolanic activity of fly ash is primarily governed by the content of amorphous glassy phases—with the amorphous phase proportion of the fly ash used in this study being 60%—as reflected by the intensity of the broad hump. In contrast, crystalline minerals such as quartz and mullite are considered inert phases. In high-calcium fly ash (e.g., from lignite), additional peaks corresponding to Ca(OH)2 at 2θ = 18.0° and CaCO3 at 2θ = 29.4° may also be observed.
For cement, the typical mineral composition includes tricalcium silicate (Alite, C3S), dicalcium silicate (Belite, C2S), tricalcium aluminate (Aluminate, C3A), tetracalcium aluminoferrite (Ferrite, C4AF), and gypsum (CaSO4·2H2O). Among these, C3S is the primary contributor to early strength, with characteristic peaks at 2θ = 32.2°, 32.9°, and 52.6°. C2S significantly contributes to the later strength development, with characteristic peaks at 2θ = 32.5°, 33.1°, and 46.4°. C3A hydrates rapidly and requires gypsum control, with characteristic peaks at 2θ = 30.4° and 37.4°. C4AF exhibits characteristic peaks at 2θ = 27.7° and 32.0°, while gypsum (CaSO4·2H2O) regulates the setting time and shows characteristic peaks at 2θ = 11.6° and 23.3°. In general, the XRD pattern of cement clinker is dominated by the peaks of C3S and C2S, while the intensity of the gypsum peaks depends on the gypsum content in the cement. After hydration (hardened cement paste), the characteristic peaks of Ca(OH)2 at 2θ = 18.0° and ettringite (Ca6Al2(SO4)3(OH)6·26H2O) at 2θ = 9.1° are observed.
For Na2SiO3, its typical mineral composition includes anhydrous sodium metasilicate (α-Na2SiO3), pentahydrate sodium metasilicate (Na2SiO3·5H2O), and nonahydrate sodium metasilicate (Na2SiO3·9H2O). The characteristic peaks of α-Na2SiO3 appear at 2θ = 25.9°, 27.8°, and 31.3°, representing the high-temperature stable phase. The characteristic peaks of Na2SiO3·5H2O are found at 2θ = 11.7°, 23.5°, and 35.4°, which correspond to the common industrial crystalline form. For Na2SiO3·9H2O, the characteristic peaks are located at 2θ = 7.6°, 15.3°, and 22.9°, indicating a low-temperature crystallization product. Therefore, the XRD pattern of sodium metasilicate is influenced by the content of crystallization water. The anhydrous phase remains stable at high temperatures (>100 °C), while the industrial form is typically the pentahydrate, characterized by sharp and intense peaks, indicating good crystallinity.
As summarized, a comparison of the crystalline phase structures of coal gangue, fly ash, cement, calcium oxide, and sodium metasilicate is presented in
Table 2.
In the alkali-activated coal gangue-based solid waste filling material system, the ratio of CaO to Na
2SiO
3 significantly influences the geopolymerization process and the final mineral phase composition by controlling factors such as alkalinity, silicon and aluminum ion concentration, and reaction pathways. The results are shown in
Figure 3. During the activation of coal gangue-based materials with CaO and Na
2SiO
3, the primary mineral phases formed include C-A-S-H gel (calcium aluminosilicate hydrate), N-A-S-H gel (sodium aluminosilicate hydrate), Ca(OH)
2 (portlandite), calcite (CaCO
3), and potentially ettringite. Among these, the characteristic diffraction peaks of C-A-S-H gel are located at 2θ = 29.5° (corresponding to the Ca-Si-O bond) and 32.0° (corresponding to the Ca-Al-O bond), which are the main contributors to the material’s strength. N-A-S-H gel exhibits a broad amorphous peak in the 2θ range of 20–30° and is readily formed in strongly alkaline environments rich in sodium. Ca(OH)
2 shows sharp characteristic peaks at 2θ = 18.0° and 34.0° and tends to form in excess when a high CaO ratio is used. The characteristic peak of calcite appears at 2θ = 29.4°, typically formed by the carbonation of Ca(OH)
2 when it absorbs CO
2 from the air. If sulfate ions are present in the system (e.g., from the oxidation of pyrite residues in coal gangue), ettringite may form, with a characteristic peak at 2θ = 9.1°.
For the single CaO alkali activator, when the content is 0%, the XRD pattern predominantly exhibits the original mineral phases of coal gangue, with major diffraction peaks corresponding to quartz (2θ = 20.8°, 26.6°), kaolinite (2θ = 12.3°, 24.8°), and illite (2θ = 8.8°, 17.8°). No new phases are formed, indicating that no significant alkali activation reaction has occurred, and the mineral phases remain in their original state. When the alkali activator content increases to 9%, the intensity of the diffraction peaks for the original minerals significantly decreases (with a 30–40% reduction in the quartz peak intensity). At the same time, new diffraction peaks appear at 2θ = 29.4°, 34.1°, and 31.8°, corresponding to Ca(OH)2, a broad peak of C-S-H gel, and ettringite, respectively. This suggests that CaO effectively activates the dissolution of aluminosilicate minerals, generating cementitious products, with a small amount of unreacted Ca(OH)2 remaining. The degree of mineral phase transformation is moderate. As the alkali activator content increases further to 12%, the intensity of the diffraction peaks for the original minerals decreases even more. However, the intensity of the Ca(OH)2 diffraction peak (29.4°) significantly increases (about 60% higher than that for the 9% group). Additionally, a new diffraction peak appears at 2θ = 36.0°, corresponding to the CaCO3 peak (due to excess Ca(OH)2 reacting with CO2). The broadening of the C-S-H gel peak weakens, indicating that excessive CaO leads to the accumulation of free Ca(OH)2 and CaCO3, which inhibits the continuous formation of cementitious products. The mineral phase consists of a mixture of original minerals, excess calcium salts, and a small amount of cementitious phases.
Compared to the coal gangue-based solid waste filling material without the addition of alkali activators, the incorporation of 2% sodium metasilicate results in a slight decrease in the intensity of the diffraction peaks for the original minerals. A broadening diffraction peak appears at 2θ = 31.8°, corresponding to the characteristic peak of C-S-H gel, and the quartz peak intensity decreases by approximately 20%. This indicates that a low dose of sodium metasilicate can partially activate the dissolution of aluminosilicate minerals in coal gangue, generating a small amount of cementitious products. However, the reaction extent is limited, and the material remains dominated by the original minerals. When the sodium metasilicate content increases to 8%, the intensity of the original mineral diffraction peaks significantly weakens (with a reduction of more than 50% in the quartz peak intensity). The intensity of the C-S-H gel broadening peak (31.8°) increases substantially, and a characteristic peak of zeolite appears at 2θ = 29.5°. This suggests that a higher dosage of sodium metasilicate effectively promotes the activation reaction, facilitating the dissolution of aluminosilicate minerals and their reorganization into stable cementitious phases and zeolitic minerals. As a result, the mineral phase is predominantly composed of reaction products, with a significant reduction in the proportion of original minerals, leading to improved cementitious properties of the material.
For the CaO-Na2SiO3 composite alkali activator (with a ratio of 10:2), the characteristic peaks of Ca(OH)2 at 2θ = 18.0° and 34.0° exhibit very high and sharp intensities, indicating a high CaO content in the system. The dissolution of CaO has led to the formation of excess Ca(OH)2. Since CaO dissolves in water to produce Ca2+ and OH− ions, under the low Na2SiO3 ratio, the OH− concentration remains relatively low (pH ≈ 12.5–13.0), which is insufficient to completely consume the Ca2+ generated by CaO dissolution. As a result, Ca(OH)2 precipitates in large quantities as crystals. The characteristic peaks of C-A-S-H gel at 2θ = 29.5° and 32.0° show moderate intensity and relatively sharp peak shapes, suggesting that, under the promotion of higher Ca2+ concentrations, a certain amount of C-A-S-H gel is formed with relatively good crystallinity. The high concentration of Ca2+ ions combines with the silicon and aluminum ions dissolved from the coal gangue, directing the formation of C-A-S-H gel structures. Additionally, the intensity of the N-A-S-H amorphous peak in the range of 2θ = 20–30° is very weak. This is because the Na+ concentration in the system is low, insufficient to provide a sufficiently strong alkaline environment to fully promote the dissolution of aluminosilicate minerals in coal gangue and the polymerization of silicon and aluminum ions, thereby inhibiting the formation of N-A-S-H gel.
3.2. SEM
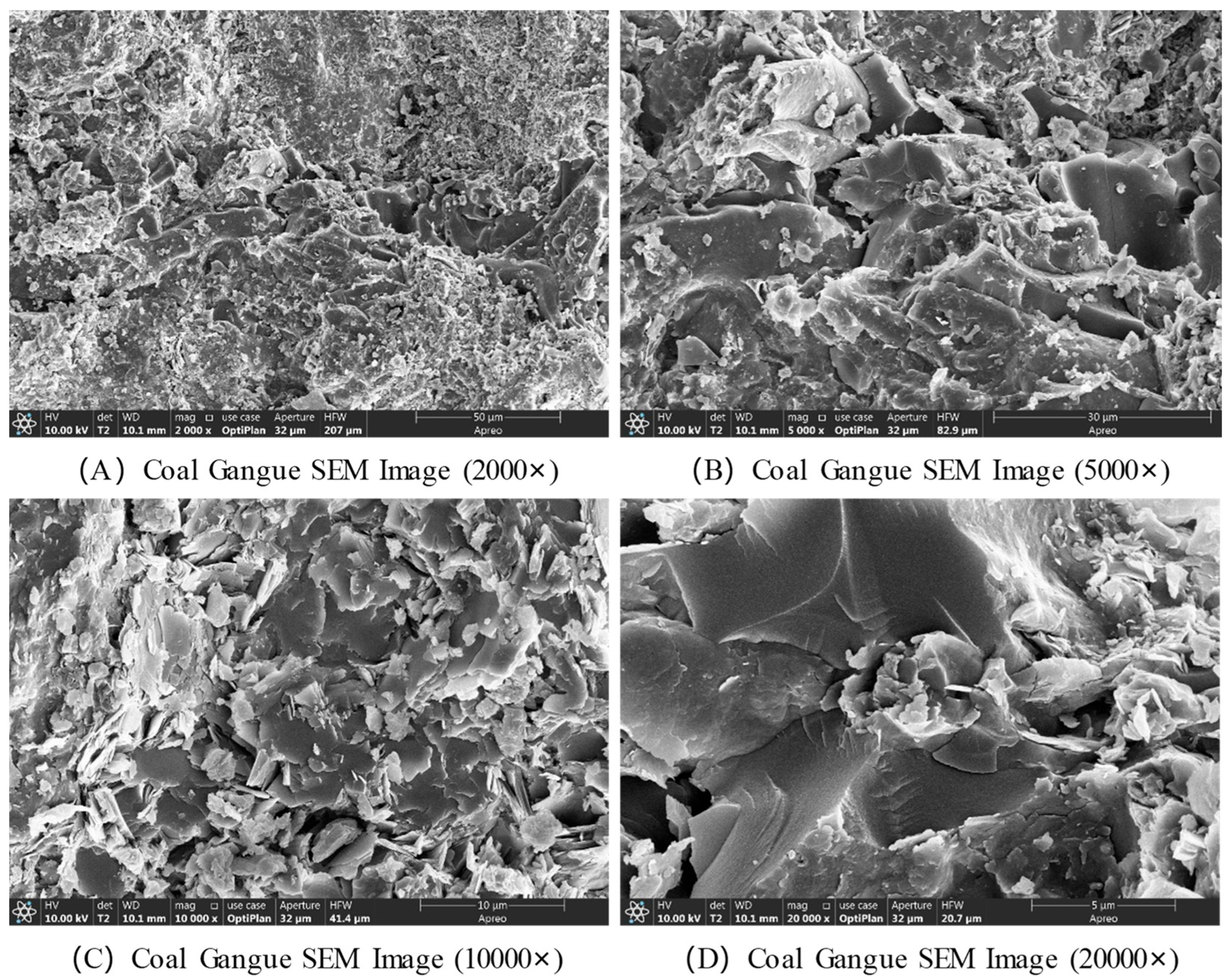
The microstructural characteristics of coal gangue were observed using scanning electron microscopy (SEM), with the results presented in
Figure 4. At magnifications of 20,000× and 10,000×, the SEM images reveal many nanometer-sized particles, which are small crystals of mineral components such as quartz and kaolinite in the coal gangue. These crystals exhibit irregular shapes and varying sizes. Additionally, some nanometer-sized pores and cracks are visible on the surface, which likely result from weathering, erosion, or other processes during the formation or later stages of coal gangue. These pores and cracks provide pathways for gas and liquid adsorption and transport. Moreover, these cracks may influence the mechanical properties of coal gangue. At a magnification of 5000×, the SEM image shows that coal gangue is primarily composed of particles of varying sizes, with some gaps between the particles. At 2000× magnification, the SEM image clearly shows that coal gangue is an aggregate of particles with different diameters, ranging from several micrometers to tens of micrometers.
The SEM images of fly ash are presented in
Figure 5. At a magnification of 2000×, the fly ash appears as an aggregate composed of particles with varying sizes, exhibiting a significant difference in particle diameters. The morphology is predominantly spherical, accompanied by a small number of irregular block-like particles. There are observable voids between particles, and localized agglomeration leads to the formation of a porous structure, resulting in an overall loose and heterogeneous texture. At a magnification of 10,000×, the surfaces of the spherical particles display microstructural defects such as small pits and cracks. Some particle surfaces are covered with nanoscale deposits, which are likely microcrystals formed via crystallization. The irregular particles exhibit rough surfaces with clearly visible pores, which may serve as active sites for chemical reactions. The interfaces between particles are more distinct at this scale, allowing the observation of inter-particle bonding modes and the degree of interconnectivity. At a magnification of 20,000×, the nanoscale features of fly ash particles become evident. The smooth spherical particles display predominantly amorphous characteristics, though localized crystallization is observed, including the presence of needle-like or plate-like microcrystalline structures.
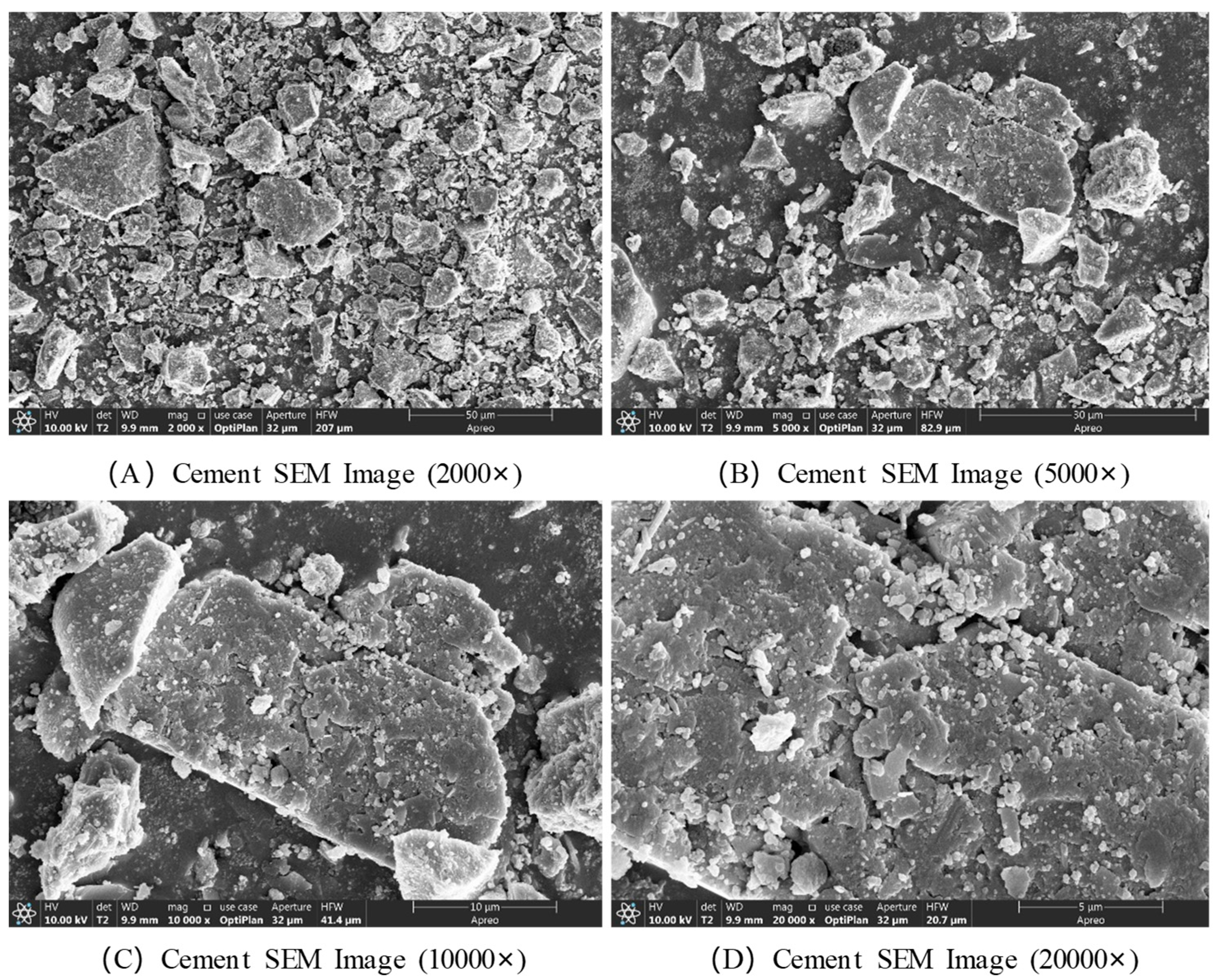
The microstructural morphology of cement was characterized using SEM, and the results are presented in
Figure 6. The SEM images reveal that cement is a heterogeneous aggregate composed of various mineral phases. Its morphology is primarily characterized by numerous blunt-edged, ellipsoidal particles of varying sizes, exhibiting irregular shapes overall. At a magnification of 2000×, all visible particles appear to be smaller than 50 μm. At 5000× magnification, the surfaces of larger particles are observed to be smooth and flat, with a substantial number of smaller particles irregularly attached to them. At higher magnifications of 10,000× and 20,000×, many needle-like, rod-shaped, columnar, and ellipsoidal granular structures are clearly seen to be distributed and embedded on the surfaces of larger particles, approximately 20 μm in size.
The SEM images of CaO are shown in
Figure 7. At a magnification of 2000×, the SEM images reveal that the size of CaO particles is uneven, with irregular blocky or granular shapes. Significant gaps between the particles are observed, and in some areas, particle aggregation is evident, forming a porous structure. At a magnification of 5000×, the SEM images show that the particle surfaces are relatively rough, with some areas exhibiting uneven topography. The particles are composed of smaller aggregating particles, with tiny gaps between them, forming numerous small pores, which is consistent with the porous nature of calcium oxide. To quantify the specific surface area corresponding to this porous structure, the Brunauer–Emmett–Teller (BET) method was used to determine the specific surface area of the CaO powder employed in this study, and the result showed that its specific surface area was 23 m
2/g. At 10,000× magnification, many micropores are visible, with varying sizes and shapes. Some particles also show cracks on their surfaces, likely due to stress changes during the preparation process or calcination conditions. Additionally, small nanometer-sized particles are observed to be attached to the surface of larger particles, which may be microcrystals formed during the crystallization process of calcium oxide. At 20,000× magnification, the SEM images clearly show the crystalline structure of the CaO particles, with observable edges and crystal faces.
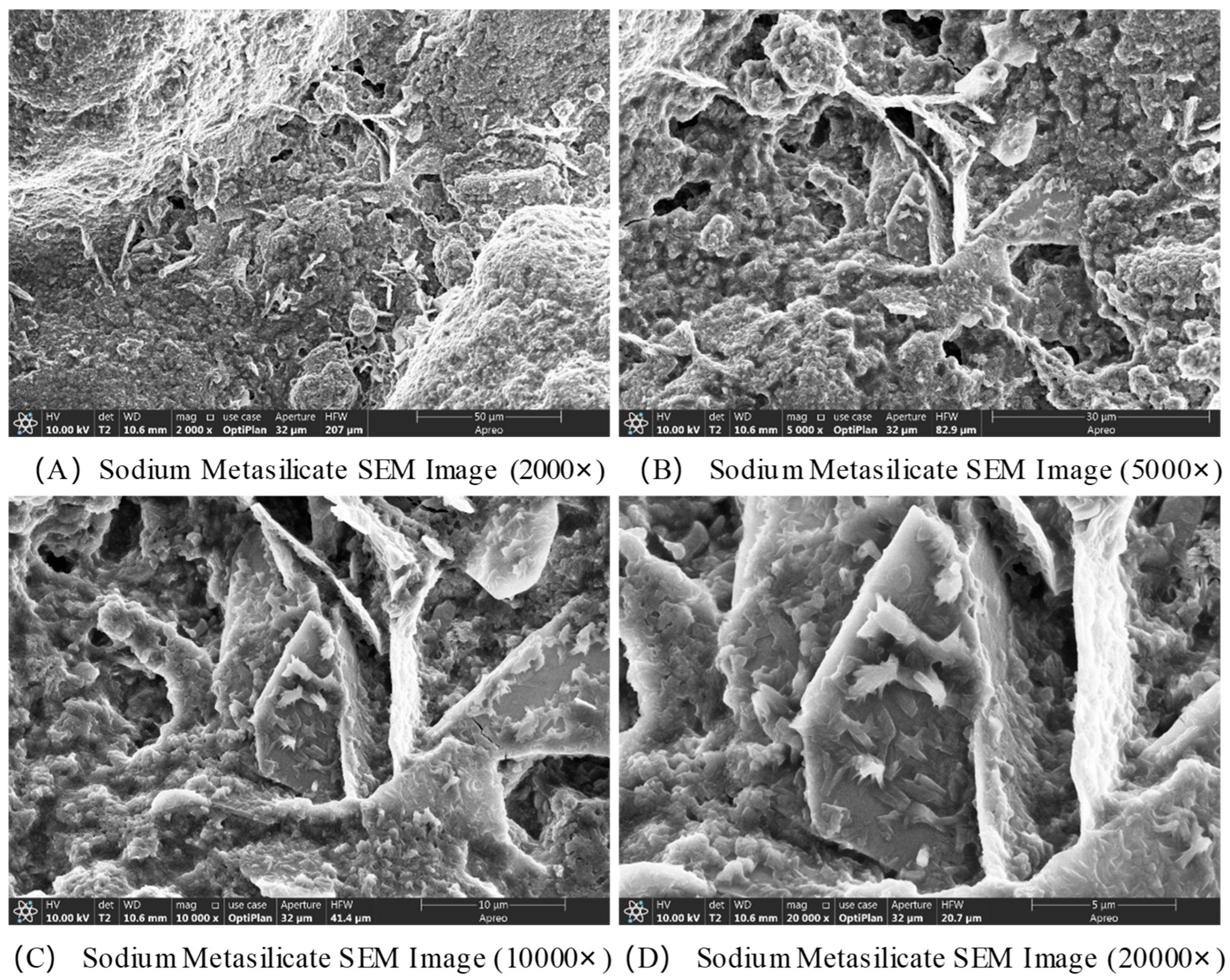
The SEM images of Na
2SiO
3 are shown in
Figure 8. Sodium metasilicate overall exhibits an irregular blocky or granular agglomerate structure, with noticeable variations in particle size. Significant gaps between particles are observed, accompanied by slight aggregation, resulting in a relatively loose overall structure. At a magnification of 5000×, the surface roughness of the particles is clearly visible, with some regions displaying layered or flaky structures. At magnifications of 10,000× and 20,000×, the finer surface morphology of the particles is accentuated, revealing edges and step-like structures formed by crystallization, or pits and cracks resulting from defects. In some areas, nano-sized microcrystal agglomerates may be present, forming a porous surface texture. These structures provide reactive sites for interactions with other substances, such as coal gangue and calcium oxide.
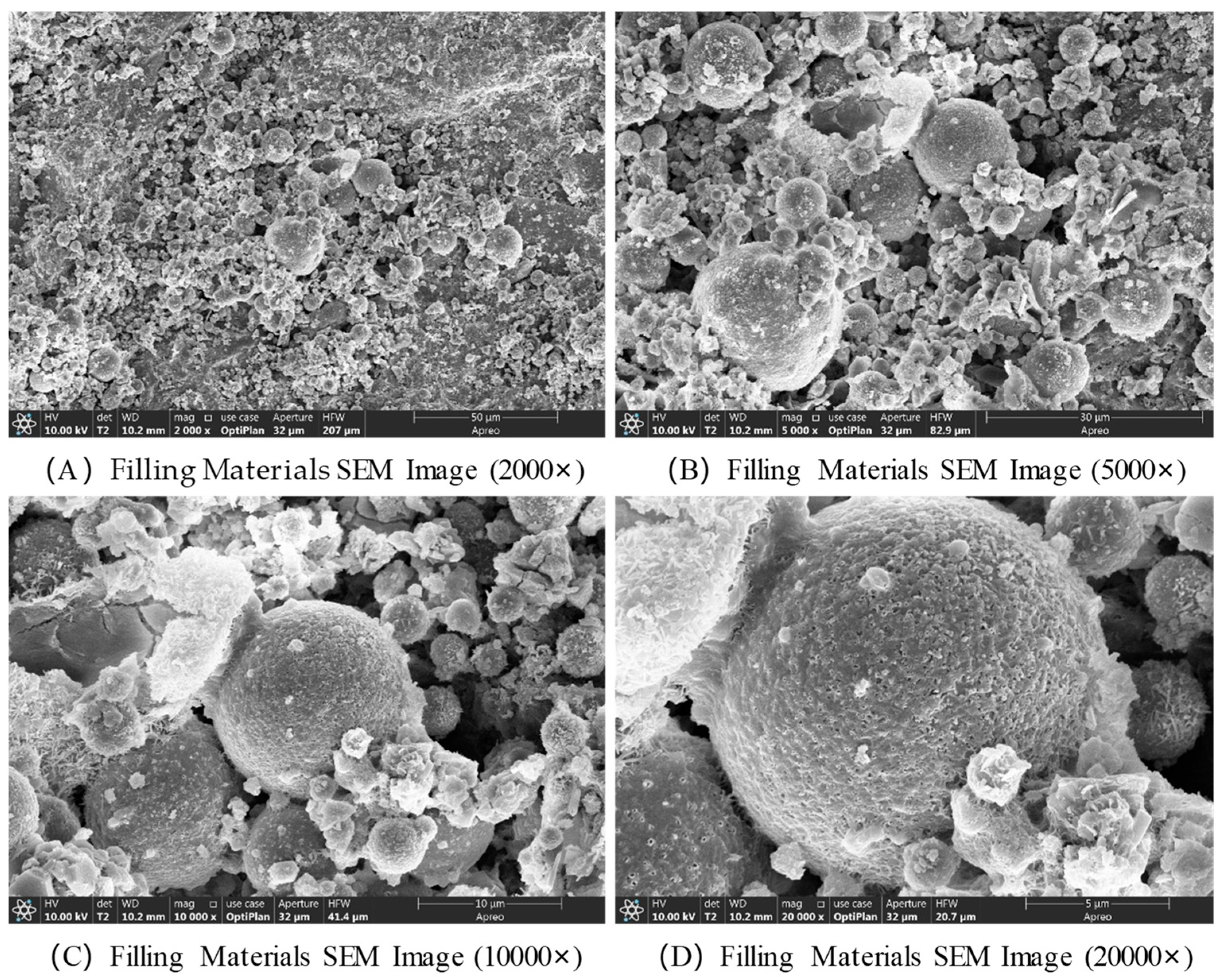
The SEM images of the filling material at a curing age of 28 d are shown in
Figure 9. The geopolymerization process of the alkali-activated coal gangue-based solid waste filling material (i.e., the hardening of the gel system and the formation of products) exhibits characteristic changes from the macroscopic structure to the microscopic interface in SEM images at different magnifications. The core manifestations include the formation of reaction products, particle bonding, and pore evolution. At a magnification of 2000×, the original coal gangue particles (mostly spherical, angular, or blocky) are encapsulated or connected by the cementitious products, forming a continuous matrix framework. Unreacted coal gangue particles are dispersed within, with fibrous and gel-like alkali activation products (such as C-S-H and N-A-S-H gels) filling the spaces between the particles. The overall pore distribution is broad, including larger inter-particle gaps and micropores within the gel body, and the structural density increases as the reaction progresses. At 5000× magnification, the original particles are covered by one or more layers of gel products, with some areas showing gel formation growing outward from the particle surface, creating “bridging” structures that fill the inter-particle voids. At 10,000× magnification, the gel body exhibits an amorphous or semi-crystalline dense structure with a rough surface and subtle wrinkles. At 20,000× magnification, nano-sized microcrystals can be observed within the gel body, such as the fibrous nanocrystals of C-S-H gel. These nanoscale structures enhance the material’s strength through accumulation and crosslinking. At the particle–gel interface, a transition zone is observed, with a gradient change due to elemental diffusion. In some areas, nano-sized pores or defects are present, and their quantity and distribution directly influence the material’s density and durability.
3.3. Hydration Degree of Filling Materials
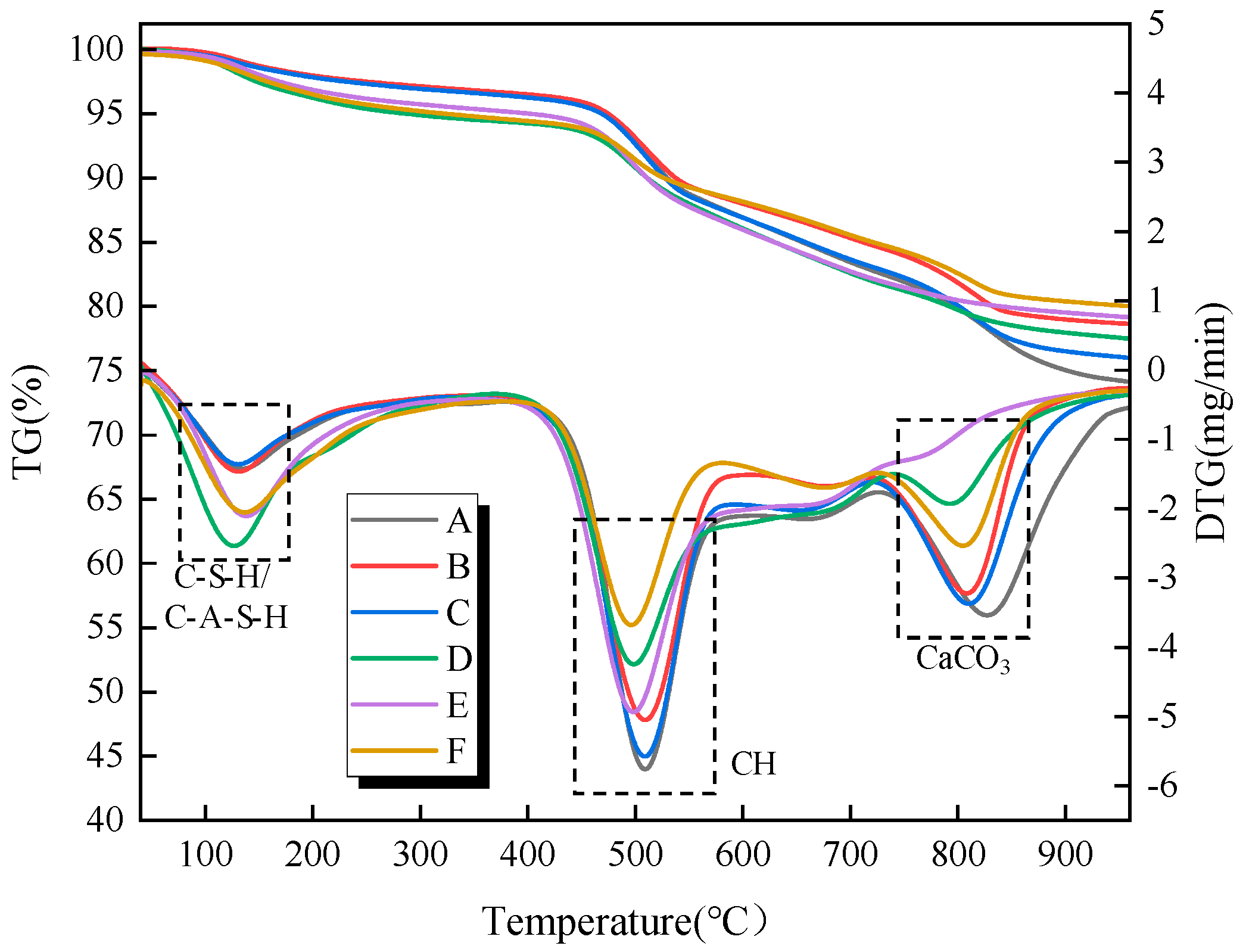
To further investigate the hydration reaction process within the filling material samples under different influencing factors,
Figure 10 and
Figure 11 present the thermogravimetric (TG) and differential thermogravimetric (DTG) curves of the filling material samples at curing ages of 7 and 28 days, respectively, under different types and dosages of activators. The hydration products of the samples under varying activator types and dosages are generally consistent, mainly including C-S-H/C-A-S-H gels, AFt, CH, and calcium carbonate. According to the DTG curves, three distinct weight loss peaks are observed during the heating process from 50 °C to 1000 °C. The first weight loss peak occurs in the temperature range of 70 °C to 200 °C and is primarily attributed to the loss of free water molecules from hydration products such as C-S-H/C-A-S-H gels and AFt. The second weight loss peak is observed in the temperature range of 400 °C to 600 °C and is primarily related to the loss of chemically bound water from CH. The third weight loss peak occurs in the temperature range of 670 °C to 830 °C, and it is primarily attributed to the decomposition of calcium carbonate, which releases carbon dioxide upon heating [
26,
27].
Under the curing age of 7 d, for the single CaO alkali activator, as the CaO content increases, the weight loss corresponding to C-S-H/C-A-S-H gel and AFt first increases and then decreases. This suggests that an appropriate activator content can promote the formation of hydration products such as C-S-H/C-A-S-H gel and AFt, contributing to higher strength in the filling material samples. Additionally, as the activator content increases, the weight loss corresponding to CH also decreases to some extent, indicating that the reactive components (SiO
2 and Al
2O
3) in the gangue powder undergo pozzolanic reactions, continuously consuming CH and reducing its content in the reaction system. Therefore, by calculating the CH content in the reaction system, the influence of the activator on the degree of hydration within the sample can be quantitatively analyzed. According to reports by Zhai et al. [
23] and He et al. [
28], the thermal decomposition of CH produces calcium oxide and water, with molecular weights of 74 and 18, respectively. By calculating the mass loss resulting from the thermal decomposition of CH, the CH content can be further determined. The corresponding calculation formula is as follows:
Here, WCH represents the CH content in the hydration reaction system, and WH represents the content of chemically bound water produced from the thermal decomposition of CH in the hydration reaction system. WCH indirectly reflects the degree of hydration in the filling material; a lower WCH indicates that more CH has participated in the pozzolanic reaction, which in turn suggests a higher degree of hydration in the filling material.
Based on the above equation, the CH content and chemically bound water content of the samples under different activator types and dosages are presented in
Table 3. It can be observed that as the activator (single CaO) content increases, the CH content in the samples initially decreases and then increases. The sample with 3% activator content shows the lowest CH content, indicating that an optimal activator content can promote more CH to participate in the pozzolanic reaction, thereby enhancing the hydration degree of the system and generating a greater amount of hydration products.
Under the curing age of 7 d, for the single Na
2SiO
3 alkali activator, as the Na
2SiO
3 content increases, the weight loss corresponding to C-S-H/C-A-S-H gel and AFt increases (
Figure 9A,D), indicating that the Na
2SiO
3 alkali activator promotes the formation of hydration products such as C-S-H/C-A-S-H gel and AFt. Additionally, as the Na
2SiO
3 content increases, the weight loss corresponding to CH decreases. The CH content and chemically bound water content of the samples under different activator (single Na
2SiO
3) dosages are calculated and presented in
Table 3. It can be observed that as the activator content increases, the CH content within the samples decreases.
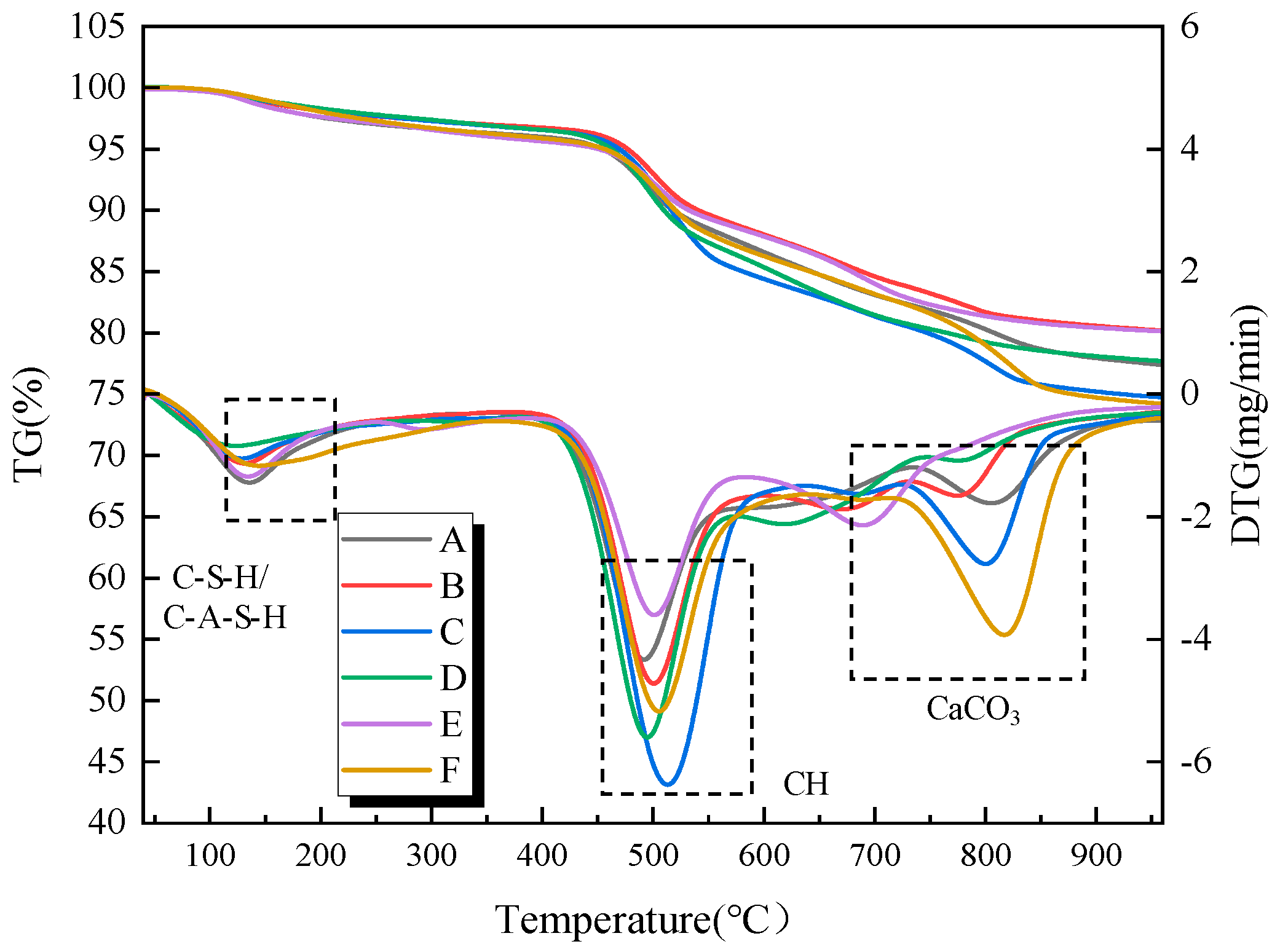
Figure 11 presents the thermogravimetric analysis curves of the filling material samples at a curing age of 28 d. Compared to the 7-day curing age, the weight loss corresponding to C-S-H/C-A-S-H gel and AFt increases, indicating that with the extension of the curing age, the hydration products gradually increase. Notably, the sample without alkali activator at 28 days shows a weight loss peak around 700 °C, and the DTG curve exhibits some fluctuations. This may be due to the instability of the C-S-H gel structure system within the sample as the curing age increases, which is prone to structural decomposition under high-temperature conditions.
Furthermore, during the early curing stage (7 d), the CH content within the samples exhibits a gradual increase, while in the later curing stage (28 d), the CH content shows a gradual decrease. This can be attributed to the fact that in the early curing stage, the rate of CH formation from cement hydration exceeds the rate at which CH is consumed by the pozzolanic reaction. However, as the curing age progresses, the pozzolanic effect of the gangue powder gradually dominates the hydration system, leading to a reduction in the CH content within the system. The chemically bound water and CH content of samples with different alkali activator types and dosages at 28 d curing age are presented in
Table 4. Furthermore, from the perspective of practical mine application scenarios, the underground backfill body is in a relatively closed environment where the CO
2 concentration in the air is much lower than that in the conventional laboratory environment. Therefore, the impact of carbonation on CH content in actual engineering will be further weakened, and it will not pose a significant threat to the bearing performance of the backfill body. Meanwhile, combined with the XRD analysis results, no obvious enhancement of the characteristic diffraction peak of CaCO
3 (2θ = 29.4°) was observed in the samples cured for 28 days.
3.4. Mechanical Performance Analysis
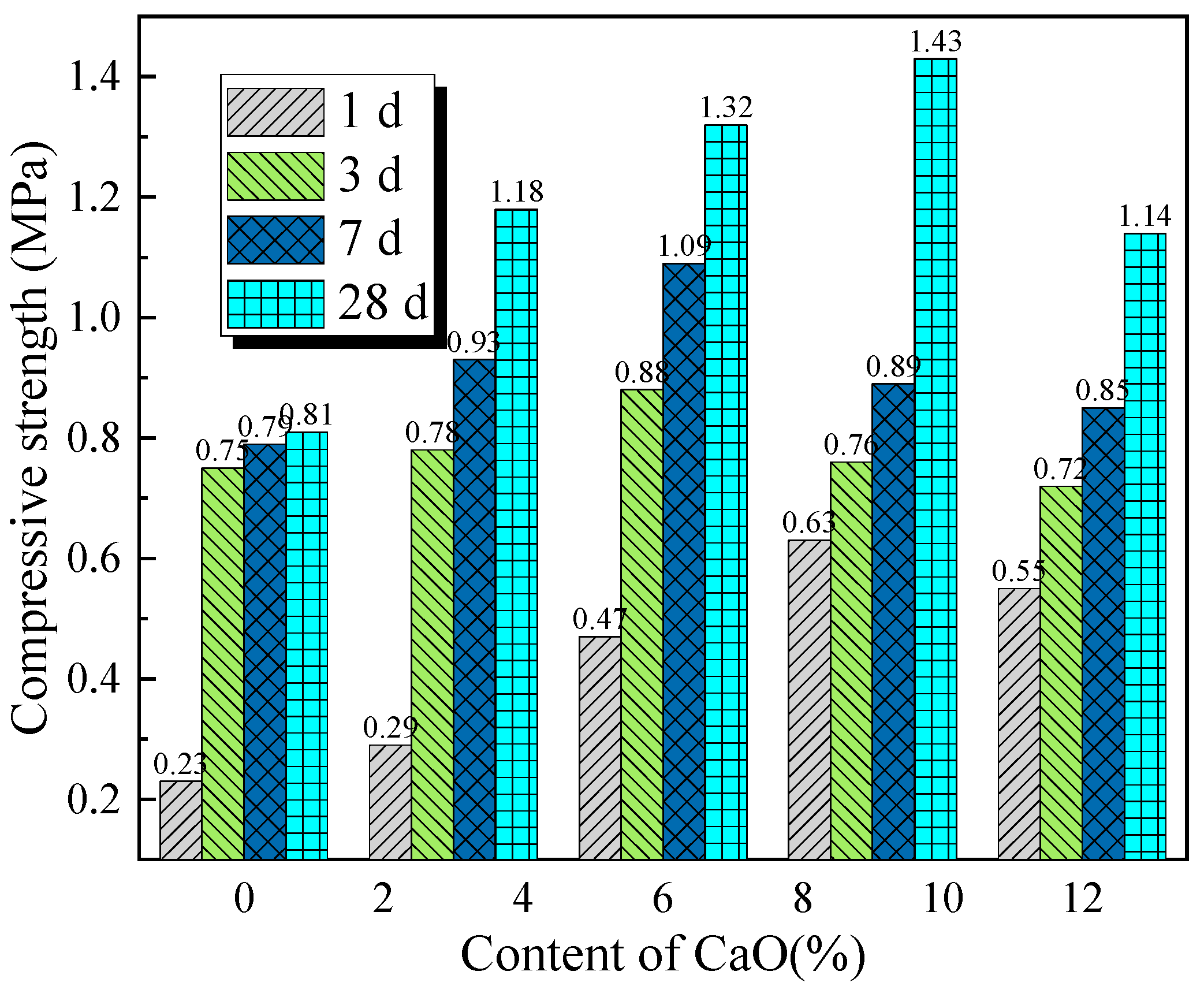
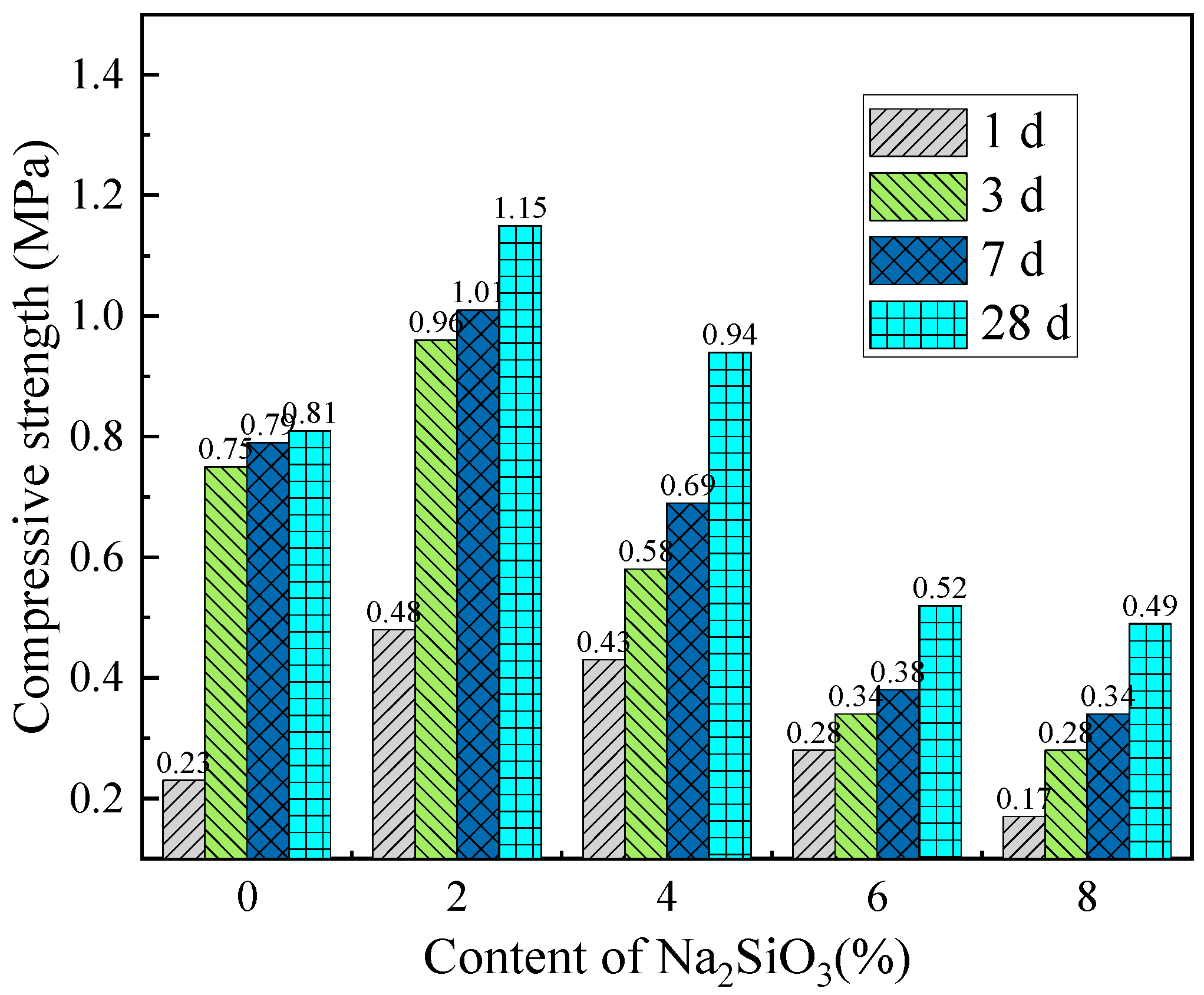
Compressive strength reflects the load-bearing capacity of cemented backfill materials under overburden pressure, making it a key parameter for evaluating the performance of backfill materials and an important indicator for assessing the safety and stability of mining operations. To investigate the effect of activator content on the compressive strength of backfill materials,
Figure 12 and
Figure 13 show the variation in compressive strength with the content of single activators at different curing ages. As shown in
Figure 12, under the same curing age, the compressive strength of the backfill material samples increases initially with the activator content and then decreases. The CaO activator content that yields the maximum compressive strength of the backfill material is between 6% and 9%, while the optimal Na
2SiO
3 activator content is 2%. This indicates that an appropriate activator content can significantly optimize the strength properties of the backfill material. Furthermore, the compressive strength of the samples with Na
2SiO
3 as the sole activator is higher than that of the samples with CaO alone. However, increasing the Na
2SiO
3 content inevitably raises the cost of backfill mining.
It is noteworthy that the compressive strength of the samples using composite activators is higher than that of the samples with only CaO or Na
2SiO
3, as shown in
Figure 14. This indicates that the addition of CaO and Na
2SiO
3 can work synergistically to activate the pozzolanic effect of coal gangue powder, leading to the formation of more hydration products, such as calcium silicate hydrate (C-S-H) and calcium aluminate hydrate (C-A-S-H), thereby enhancing the compressive strength of the cemented backfill material samples. Therefore, an appropriate activator content can significantly optimize the strength properties of the backfill material. Furthermore, the use of composite activators ensures that the backfill material has sufficient load-bearing capacity while reducing the cost of backfill mining.
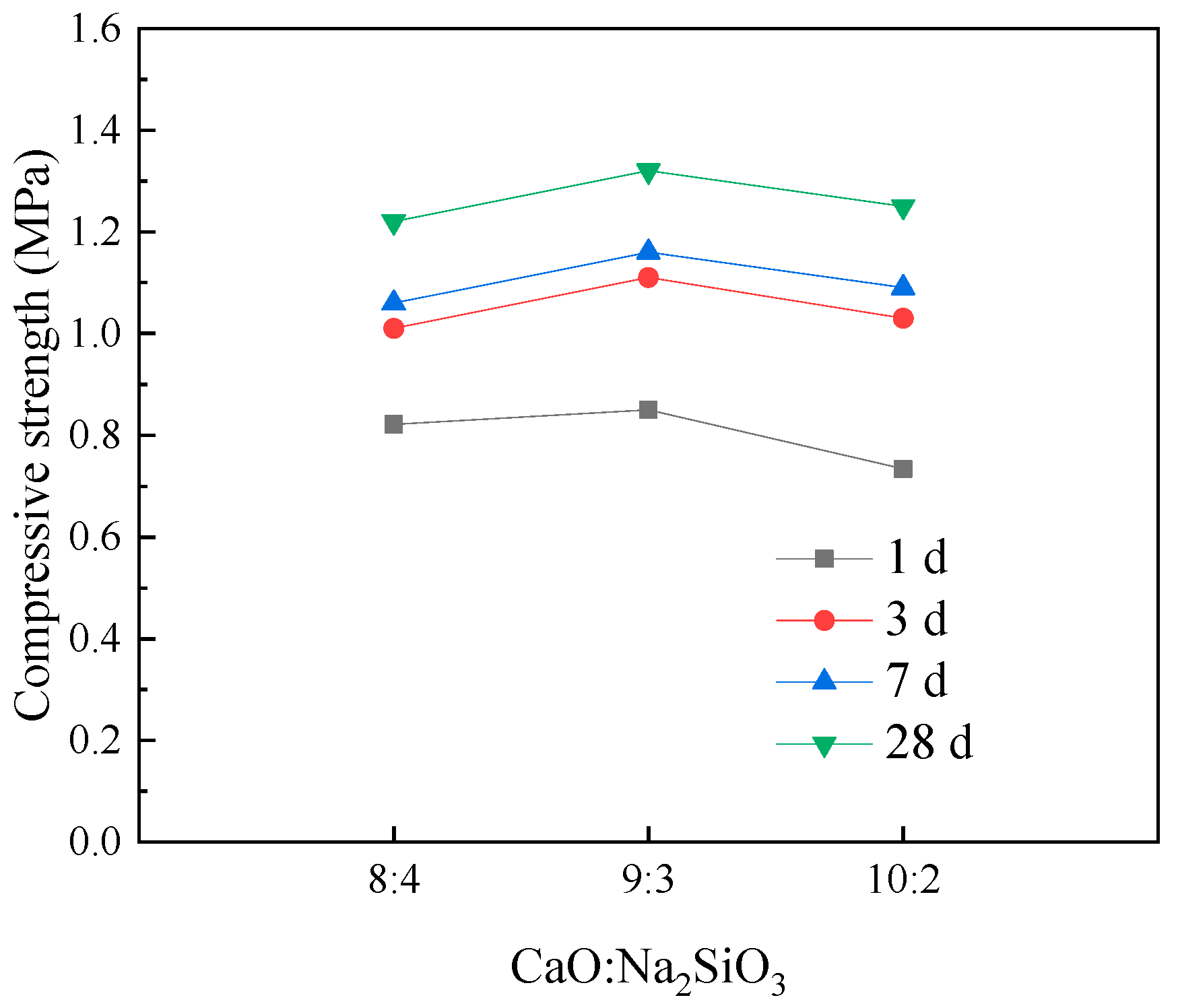
To investigate the effect of activator ratio on the compressive strength of the filling material samples,
Figure 14 presents the variation in compressive strength with the activator ratio under different curing ages. As shown in the figure, under the same alkali activator content (12%) and curing age, as the ratio of CaO to Na
2SiO
3 increases, the compressive strength of the filling material samples first increases and then decreases. Therefore, by adjusting the activator ratio, the strength properties of the filling material samples can be optimized. The activator ratio that corresponds to the maximum compressive strength of the samples is 3:1, indicating that properly controlling the activator ratio can enhance the load-bearing capacity of the cemented backfill material and effectively mitigate the movement and deformation of the overburden in the goaf area. The reason for this behavior is that, although CaO and Na
2SiO
3 can synergistically activate the latent reactivity of coal gangue powder, leading to the formation of more hydration products, when the proportion of Na
2SiO
3 is too high, the free sodium ions react first with the reactive substances in the coal gangue powder, inhibiting the hydration of tricalcium aluminate, dicalcium silicate, and tricalcium silicate, thus reducing the formation of hydration products. On the other hand, when the proportion of CaO is too high, excess CaO reacts with carbon dioxide in the air to form carbonates, which deteriorates the strength properties of the backfill material. Additionally, the low solubility of CaO means that excess CaO, upon precipitation, also lowers the compressive strength of the filling material.
Combining
Figure 12,
Figure 13 and
Figure 14, it is evident that the compressive strength of the filling material samples is positively correlated with the curing age. Therefore, appropriately extending the curing period can improve the strength properties of the filling material. Furthermore, rational control of the activator content and ratio contributes to the stable increase in the compressive strength of the filling material.
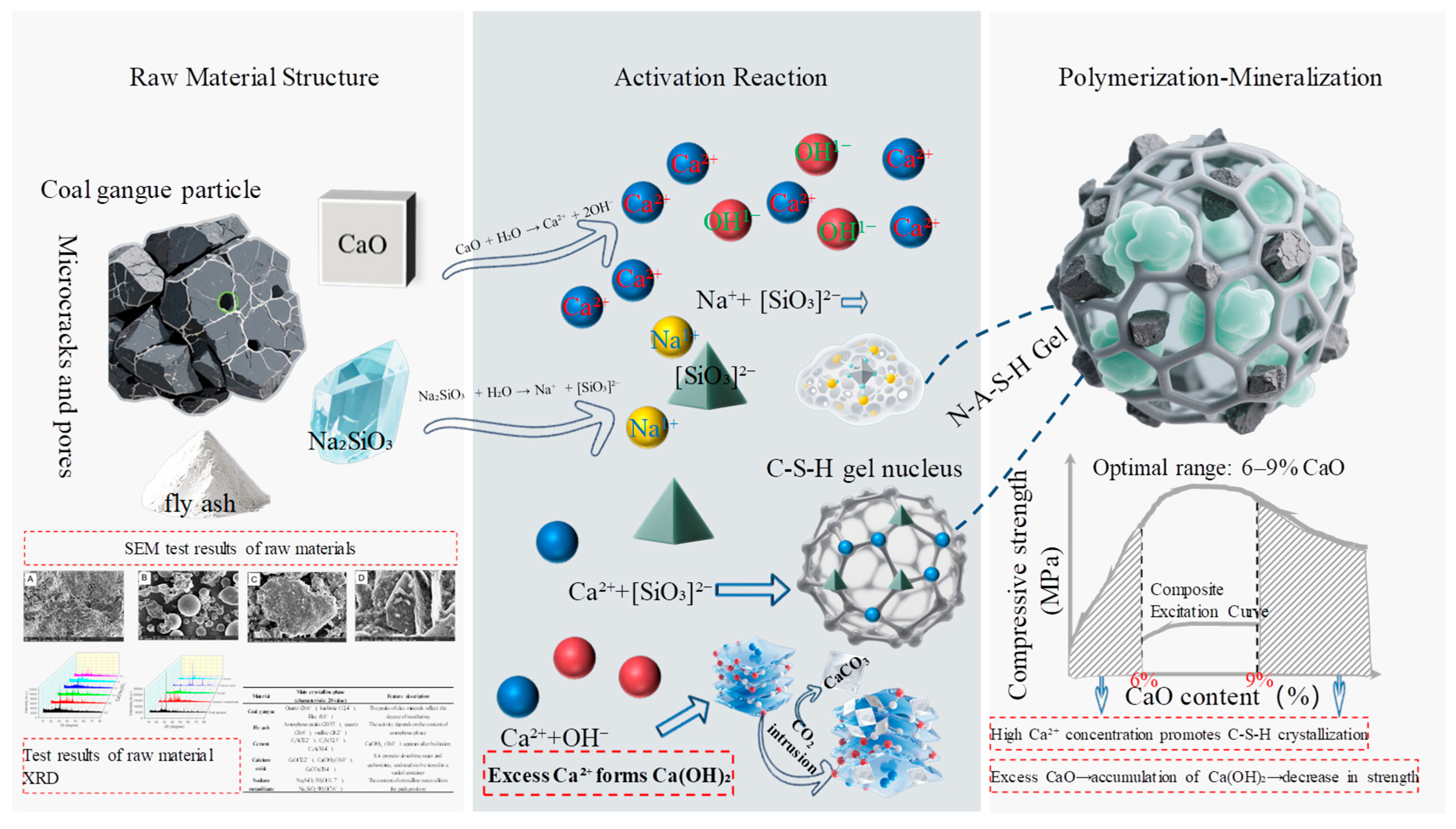
3.5. Laws of Polymeric Mineralization
The physicochemical properties of raw materials such as coal gangue, fly ash, and cement, along with the action of alkali activators (CaO and Na
2SiO
3), collectively provide the material foundation for the geopolymerization and mineralization of gangue-based solid waste filling materials. SEM images of coal gangue (
Figure 4) reveal angular block-like particles with smooth surfaces. XRD patterns (
Figure 2) indicate the presence of strong diffraction peaks corresponding to quartz, mullite, and kaolinite, suggesting that the Si-Al components exist in stable crystalline phases with inherently low reactivity. In contrast, SEM images of fly ash (
Figure 5) show spherical glassy particles accompanied by minor quartz content. The corresponding XRD patterns display a broad diffuse hump attributed to the amorphous glassy phase, superimposed with peaks of quartz and mullite. This amorphous nature enhances the reactivity of fly ash compared to coal gangue. Cement, serving as an auxiliary binder, exhibits characteristic peaks of C
3S and C
2S in the XRD pattern (
Figure 2), which can rapidly release Ca
2+ and silicate ions upon early hydration, thereby providing initial strength support. As a major component of the alkali activator system, SEM images show that CaO consists of irregular particles (
Figure 7), which react with water to form Ca(OH)
2, elevating the system pH to 12–13 and promoting the dissolution of aluminosilicate phases. Simultaneously, the released Ca
2+ serves as a precursor for the formation of calcium-based gels. Na
2SiO
3 appears as flocculent agglomerates under SEM (
Figure 8); upon dissolution, it releases silicate (SiO
32−) and sodium ions (Na
+), enhancing the alkalinity and providing additional reactive silica, thus accelerating the polycondensation of Si and Al species.
The geopolymerization and mineralization of the gangue-based filling material essentially involve a transformation process from ion dissolution to crystalline product formation. At the 7-day curing stage, the system enters the initial gelation and early product formation phase. TGA curves (
Figure 10) show the first weight-loss peak within 100–200 °C (DTG maximum at 120 °C), corresponding to gel dehydration (mass loss of 3–5%), indicating the predominance of cement hydration and the onset of alkali activation. When the CaO:Na
2SiO
3 ratio is 10:2, the broad peak reaches its maximum intensity, reflecting the highest yield of C-S-H/C-A-S-H gels. However, excessive CaO content (12%) leads to the appearance of Ca(OH)
2 peaks in the XRD spectra and a secondary weight-loss peak at 400–500 °C in the TGA curve (mass loss ~2%) due to the decomposition of unreacted Ca(OH)
2, resulting in a less compact structure. At the 28-day curing age, the filling material exhibits complete mineralization and structural densification. A second weight-loss peak emerges between 600–800 °C (DTG peak at 790 °C), attributed to the decomposition of calcite (mass loss 2–4%). Additionally, mass loss at 100–200 °C increases to 6–8%, confirming the synergistic accumulation of gels and crystalline phases. Under this curing regime, the composite activator (CaO:Na
2SiO
3 = 10:2) shows no Ca(OH)
2 peak in XRD and exhibits moderate calcite peak intensity (
Figure 3) while achieving the highest overall mass loss (8.5%) in TGA (
Figure 11), suggesting effective reaction among Ca
2+, Si
4+, and Al
3+ and the formation of an interpenetrating “gel–crystal” network.
The compressive strength of the gangue-based filling material increases progressively with curing time during the geopolymerization and mineralization process. From 1 to 3 d, strength development is primarily driven by cement hydration and the formation of C-S-H gels, while the contribution from alkali activation becomes evident (accounting for ~30% of the reaction products at 3 d). When curing is extended to 7 d, strength improvement is predominantly governed by alkali-activated gel network formation (gel content ~60%). Between 7 and 28 d, further strength enhancement results from crystal growth (e.g., calcite, zeolite), leading to a denser matrix through gel–crystal synergism (crystalline phase content 20–30%). Furthermore, the content of alkali activators (CaO and Na
2SiO
3) must be optimized to ensure the balanced reaction of Ca
2+, Si
4+, and Al
3+ at all curing stages. This avoids the formation of residual Ca(OH)
2 or excessive Si
4+ species, ensuring maximal gel and crystalline phase generation and structural compactness (
Figure 12 and
Figure 13). Excess CaO results in discontinuous crystal domains due to Ca(OH)
2 precipitation, while excessive Na
2SiO
3 leads to discrete gel morphology—both negatively affecting compressive strength, with the 28-day samples showing the most pronounced strength deterioration under imbalanced activator ratios.