Abstract
In this study, gangue-based solid waste was utilized as the primary raw material to prepare filling materials using a composite alkali activator comprising calcium oxide (CaO) and sodium metasilicate (Na2SiO3). By varying the proportions and total content of the alkali activators, with the total content fixed at 12 wt% of coal gangue, the resulting filling materials were systematically investigated. The mineralogical composition, morphology, and hydration degree of the materials were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), and thermogravimetric analysis (TG). In addition, the compressive strength of the materials was measured. The results demonstrated that both the type and dosage of alkali activators significantly influenced the mineral phases and surface morphology of the filling materials. CaO and Na2SiO3 exhibited distinct effects on the degree of hydration, and the curing age was also found to be a critical influencing factor. For single-component activators, the compressive strength of the filling materials initially increased and then decreased with increasing activator content, with optimal values observed at CaO contents between 6% and 9% and Na2SiO3 content around 2%. In the case of the composite CaO–Na2SiO3 system, the uniaxial compressive strength exhibited a similar trend, increasing first and then decreasing with the CaO-to-Na2SiO3 ratio, with the optimal ratio determined to be 3:1. Furthermore, a positive correlation between curing age and compressive strength was observed. This study elucidates the synergistic mechanism of CaO and Na2SiO3, identifies optimal mix proportions, and quantifies empirical relationships between raw material properties, reaction conditions (activator ratio/content, curing age), and compressive strength. These relationships serve as core data for subsequent construction of a “raw material–reaction condition–strength” correlation model, providing support for formulation optimization of gangue-based filling materials.
1. Introduction
Coal has long served as the primary energy source in China, playing an irreplaceable role in ensuring national energy security and driving industrialization. However, the large quantities of solid waste generated during coal mining—such as coal gangue, fly ash, and slag—have emerged as critical bottlenecks hindering the sustainable development of the mining industry [1,2]. It is estimated that coal gangue, a major byproduct of coal extraction, accounts for approximately 15–20% of the total coal production. To date, more than 5 billion tons of gangue have been accumulated across the country. These massive stockpiles not only occupy vast land resources but also lead to serious ecological problems, such as soil contamination and groundwater deterioration, due to leaching and wind erosion [3]. Simultaneously, mining-induced voids have resulted in frequent geological hazards, including surface subsidence and roadway collapse, which demand substantial financial resources for remediation. Against this backdrop, transforming mining solid waste into underground backfilling materials has gained significant attention as a promising strategy to achieve coordinated goals of waste reduction, goaf remediation, and resource recycling [4,5]. The integration of efficient coal resource recovery with solid waste utilization addresses both the environmental issues caused by gangue accumulation and the economic challenges associated with backfill mining. Moreover, it helps alleviate the pressure of deep mining and mitigates the operational strain related to continuous excavation [6,7].
Traditional backfill materials are predominantly based on cementitious systems. However, cement production is associated with significant carbon emissions—approximately 0.8 tons of CO2 per ton of cement produced—and is heavily dependent on high-grade limestone resources. These characteristics are in stark contradiction with the development of green mining practices [8]. As a low-carbon alternative, alkali-activated technology employs alkaline activators (e.g., NaOH, sodium silicate) to chemically react with aluminosilicate-based solid wastes, forming hydrated products with cementitious properties [9]. This approach generates only one-fifth to one-third of the carbon emissions of ordinary Portland cement (OPC) and enables efficient utilization of industrial solid waste, making it a promising substitute for cement in sustainable construction [10,11]. In addition to the gangue-based alkali-activated materials focused on in this study, fly ash geopolymers are also important alternatives to traditional cement. From the perspective of carbon emissions, the production process of fly ash geopolymers generates almost no additional carbon emissions, reducing the carbon footprint by more than 90% compared with cement. In terms of raw materials, they mainly use fly ash, an industrial solid waste, without consuming natural resources such as limestone. Moreover, they can absorb large amounts of fly ash, alleviating the problem of solid waste accumulation. In terms of mechanical properties, fly ash geopolymers exhibit fast early strength development; their 28-day compressive strength can reach 40–80 MPa, which is comparable to that of medium-to-high strength grade cement, and their long-term strength stability is better [12]. However, they also have certain limitations: for example, they are relatively sensitive to curing temperature, with slow strength development in low-temperature environments, and the uneven geographical distribution of fly ash (the raw material) may restrict their application scope. Through the comparison with traditional cement and fly ash geopolymers, it can be seen that different alternative materials have their own characteristics. The gangue-based alkali-activated filling material in this study has unique advantages in utilizing gangue, reducing transportation costs, and adapting to the specific environment of mines, thus providing more diversified material options for green mining.
Zhu et al. [13] prepared alkali-activated coal gangue-slag concrete (ACSC) by using coal gangue-slag and a Na2SiO3 and NaOH complex activator. In recent years, alkali-activated backfill materials based on fly ash and blast furnace slag have been applied in certain mining operations. However, these materials rely heavily on highly reactive solid waste resources, which are scarce in many coal mining regions of China. In contrast, low-reactivity wastes such as coal gangue are abundant, resulting in elevated material costs and limiting the scalability of this technology [14]. Coal gangue, the most prevalent solid waste generated in coal mining, primarily consists of aluminosilicate minerals such as quartz, kaolinite, and illite. In theory, these minerals can be converted into cementitious binders through alkali activation. Coal gangue has poor reactivity due to its stable crystal structure, so CaO and Na2SiO3 were selected as composite activators because CaO can provide high-concentration Ca2+, reaction heat, and alkalinity to destroy coal gangue’s crystal structure, while Na2SiO3 can supplement silicon sources and maintain system alkalinity to promote cementitious product formation. Compared with NaOH (high cost and strong corrosiveness) and Ca(OH)2 (weak alkalinity and low activation efficiency), this composite system has better cost-effectiveness, environmental safety, and activation efficiency, which is more suitable for preparing gangue-based solid waste filling materials [15]. However, limited attention has been given to the underlying microscopic mechanisms governing the geopolymerization–mineralization process. This process—termed “polymerization-induced mineralization”—refers to the formation of rock-like solidified bodies through chemical reactions during alkali activation. It involves multi-scale physicochemical processes, including mineral dissolution, ion reorganization, gel crystallization, and structural evolution. A comprehensive understanding of these mechanisms is crucial for the precise regulation of material performance.
Existing studies have demonstrated that the strength development of alkali-activated materials is closely related to the type, morphology, and spatial distribution of the hydration products formed during the activation process [16,17]. In slag-based alkali-activated systems, the primary reaction product is typically an amorphous calcium (alumino) silicate hydrate (C-(A)-S-H) gel [18], whereas fly ash-based systems often generate crystalline zeolite phases alongside amorphous gels. However, due to the complex composition of coal gangue—which contains inert minerals such as quartz and mica [19]—the reaction products of alkali-activated gangue-based materials often exhibit multiphase coexistence, characterized by the presence of amorphous gels, microcrystalline phases, and unreacted particles. Traditional cement hydration theories are insufficient to fully explain the geopolymerization-induced mineralization behavior of such systems. Moreover, factors such as the particle size distribution of gangue, surface defects, and interfacial interactions with alkali activators significantly affect ion dissolution kinetics and the growth direction of hydration products, thereby influencing the macroscopic performance of the resulting materials [20]. Wang et al. [21] used the calcined coal gangue (CG) as aluminosilicate precursors, and studied the effects of alkali activators (i.e., Na2SiO3/NaOH, NaOH concentration, and liquid–solid) on the mechanical characteristics and microstructure of CG-based geopolymers. Despite growing interest in gangue-based alkali-activated materials, a systematic understanding of the dynamic transformation process—spanning solid waste dissolution, ion migration, gel nucleation, and structural consolidation—remains lacking. In particular, the mechanisms by which factors such as activator composition and curing conditions regulate the geopolymerization–mineralization pathways have not been clearly elucidated [22,23]. Therefore, developing methods to improve the performance of filling materials—especially their mechanical strength—holds significant theoretical value for advancing sustainable and green mining practices [24,25]. Optimizing the mechanical performance of gangue-based backfill materials using low-cost alkali activators not only reduces reliance on expensive binders but also maximizes the potential reactivity of low-activity solid wastes, such as coal gangue. This approach offers a low-carbon, environmentally friendly solution for green backfill mining. However, limited attention has been paid to the role of coal gangue’s latent reactivity in enhancing the mechanical behavior of alkali-activated backfill materials, and fundamental studies on the geopolymerization-mineralization mechanisms in such systems are scarce. This knowledge gap has significantly constrained the broader application of alkali activation technologies in mine backfilling. In response to the urgent needs of coal mine solid waste utilization and sustainable backfilling, this study employs coal gangue as the principal raw material, activated by a composite alkali system comprising calcium oxide (CaO) and Na2SiO3, with supplementary activators including fly ash and cement. A combination of microscopic characterization techniques—including scanning electron microscopy (SEM), X-ray diffraction (XRD), and thermogravimetric analysis (TGA)—and mechanical testing systems is utilized to investigate the effects of activator content, activator ratio, and curing age on the microstructure of gangue-based cemented backfill materials. From a microstructural perspective, this study aims to elucidate the mechanisms of mechanical performance regulation and quantify empirical relationships between raw material properties, reaction conditions, and macroscopic behavior, laying the groundwork for subsequent construction of a “raw material–reaction condition–strength” correlation model. The innovation of this study in the field of alkali-activated coal gangue-based solid waste backfill materials lies in two main aspects. First, a low-cost composite activation system was developed to overcome the limitations of conventional approaches that rely heavily on highly reactive slag or single activators. Using low-reactivity coal gangue as the primary raw material, a novel combination of CaO and Na2SiO3 was employed as the composite activator. This synergistic system effectively stimulates the latent reactivity of coal gangue, significantly reduces material costs, and mitigates the adverse effects of high-sodium systems on mechanical strength. Second, the ion migration behavior and reaction product evolution under composite activation were systematically elucidated through multi-scale characterizations, including XRD, SEM, and TG analyses. The results reveal that CaO acts as a calcium source to promote the crystallization of calcium–aluminum–silicate–hydrate (C-A-S-H) gels, while Na2SiO3 modulates the alkalinity to enhance the dissolution of silicon and aluminum phases. The findings are expected to provide theoretical support for the formulation optimization and performance control of gangue-based backfill materials, thereby promoting large-scale utilization of coal mine solid waste and the construction of environmentally sustainable mining operations.
2. Materials and Methods
2.1. Materials
In the present study, the aggregates employed were coal gangue and fly ash. The initial moisture content of the coal gangue was 1.3%, and its primary mineral phases included quartz, kaolinite, and illite. The alkali activators employed were calcium oxide (CaO, purity ≥ 90%) and Na2SiO3 with a modulus ranging from 2.0 to 2.5. The total dosage of alkali activators was fixed at 12 wt% of the coal gangue. A series of CaO-to-Na2SiO3 mass ratios were designed as follows: 0:0 (Control Group), 3:0, 0:2, 10:2, 9:3, and 8:4. These ratios were selected to comprehensively cover the key interaction range between Ca2+ and SiO32− ions, enabling a systematic investigation of their synergistic effects in the alkali activation process.
2.2. Preparation
First, the coal gangue was pretreated by crushing and sieving through an 80-mesh sieve, with the specific surface area controlled within the range of 350–400 m2/kg. Then, according to the designed mix proportions, coal gangue, CaO, and Na2SiO3 were weighed and thoroughly mixed with water at a water-to-solid ratio of 0.38. The resulting slurry was poured into 50 × 100 mm cylindrical molds to prepare the test specimens. The specimens were then cured under standard conditions (20 °C, 95% relative humidity) for designated curing periods of 1 d, 3 d, 7 d, and 28 d, with the experimental scheme for sample preparation presented in Table 1.

Table 1.
Experimental scheme for sample preparation.
2.3. Characterization and Performance Testing
The microstructural morphology of the specimens at different curing ages—including gel phase distribution, crystalline phase characteristics, and pore structure—was observed using scanning electron microscopy (SEM, SIGMA 300, ZEISS, Oberkochen, Germany). The mineralogical composition of the geopolymeric phases was analyzed by X-ray diffraction (XRD, X’Pert PRO MPD, Panalytical, Almelo, The Netherlands) within a 2θ range of 5–60°. The relative content of specific phases was semi-quantitatively estimated based on characteristic diffraction peaks, such as C-A-S-H at 29.5°, N-A-S-H at 22°, and Ca(OH)2 at 18°. Thermogravimetric and differential thermogravimetric analysis (TG-DTG, TG-DTA8122, Rigaku Corporation, Tokyo, Japan) was performed from 30 °C to 1000 °C at a heating rate of 10 °C/min under a nitrogen atmosphere to evaluate the thermal decomposition behavior, gel phase stability, and crystallinity. Compressive strength tests were conducted using a universal testing machine (UTM, CMT6103, SASTest, Sansi Eternal Technology (Zhejiang) Co., Ltd., Ningbo, China) for all samples cured for 1 d, 3 d, 7 d, and 28 d. Each test group included three parallel specimens, and the average value was reported. The material preparation experimental process is shown in Figure 1.
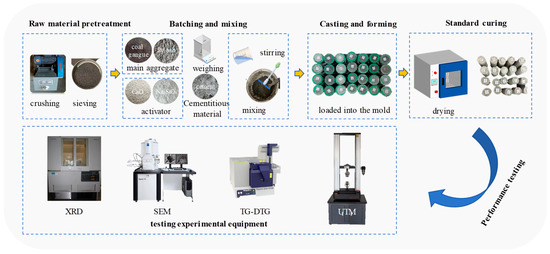
Figure 1.
Schematic diagram of the material preparation experimental process.
3. Results and Discussion
3.1. XRD
XRD is a key technique for analyzing the phase composition of mineral-based materials. Crystalline phases can be identified based on the position (2θ value) and relative intensity of characteristic diffraction peaks. The XRD patterns of coal gangue, fly ash, cement, calcium oxide, and sodium metasilicate are presented in Figure 2.
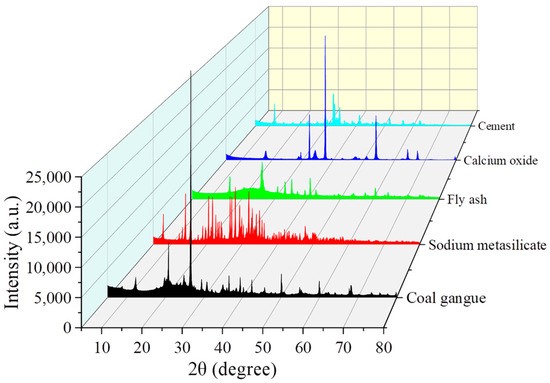
Figure 2.
XRD patterns of solid waste raw materials.
The typical mineral composition of coal gangue includes quartz, kaolinite, illite, feldspar, and calcite. In the XRD pattern, characteristic diffraction peaks of quartz are observed at 2θ = 20.8°, 26.6°, 36.5°, and 50.1°, with the most intense peak appearing at 26.6°, corresponding to the (101) crystallographic plane. The peaks at 2θ = 12.4° ((001) plane), 24.9°, and 35.0° are indicative of kaolinite. Illite is identified by its characteristic peaks at 2θ = 8.8°, 17.7°, and 26.6°, with the peak at 26.6° overlapping with that of quartz. Feldspar is characterized by peaks at 2θ = 27.5° and 28.4°, while the peak at 2θ = 29.4° corresponds to CaCO3, indicating the presence of calcite as a carbonate impurity. In summary, the XRD pattern of coal gangue is dominated by quartz peaks, while the intensities of the clay mineral peaks (kaolinite and illite) vary depending on the degree of weathering and the depositional environment of the gangue.
For fly ash, a broad hump within the 2θ range of 20–35°—often referred to as a “hump peak”—is indicative of its amorphous glassy phase and represents a primary characteristic of fly ash. Additionally, a diffraction peak at 2θ = 26.6°, corresponding to the (101) plane of quartz, is commonly observed and is typically attributed to unburned residual minerals. Characteristic peaks of mullite appear at 2θ = 16.5°, 26.2°, and 33.0°, with the peak at 33.0° corresponding to the high-temperature sintering product 3Al2O3·2SiO2. Peaks at 2θ = 30.2°, 35.6°, and 43.3° are associated with magnetite (Fe3O4), where the peak at 43.3° indicates iron oxide phases formed during coal combustion. The pozzolanic activity of fly ash is primarily governed by the content of amorphous glassy phases—with the amorphous phase proportion of the fly ash used in this study being 60%—as reflected by the intensity of the broad hump. In contrast, crystalline minerals such as quartz and mullite are considered inert phases. In high-calcium fly ash (e.g., from lignite), additional peaks corresponding to Ca(OH)2 at 2θ = 18.0° and CaCO3 at 2θ = 29.4° may also be observed.
For cement, the typical mineral composition includes tricalcium silicate (Alite, C3S), dicalcium silicate (Belite, C2S), tricalcium aluminate (Aluminate, C3A), tetracalcium aluminoferrite (Ferrite, C4AF), and gypsum (CaSO4·2H2O). Among these, C3S is the primary contributor to early strength, with characteristic peaks at 2θ = 32.2°, 32.9°, and 52.6°. C2S significantly contributes to the later strength development, with characteristic peaks at 2θ = 32.5°, 33.1°, and 46.4°. C3A hydrates rapidly and requires gypsum control, with characteristic peaks at 2θ = 30.4° and 37.4°. C4AF exhibits characteristic peaks at 2θ = 27.7° and 32.0°, while gypsum (CaSO4·2H2O) regulates the setting time and shows characteristic peaks at 2θ = 11.6° and 23.3°. In general, the XRD pattern of cement clinker is dominated by the peaks of C3S and C2S, while the intensity of the gypsum peaks depends on the gypsum content in the cement. After hydration (hardened cement paste), the characteristic peaks of Ca(OH)2 at 2θ = 18.0° and ettringite (Ca6Al2(SO4)3(OH)6·26H2O) at 2θ = 9.1° are observed.
For Na2SiO3, its typical mineral composition includes anhydrous sodium metasilicate (α-Na2SiO3), pentahydrate sodium metasilicate (Na2SiO3·5H2O), and nonahydrate sodium metasilicate (Na2SiO3·9H2O). The characteristic peaks of α-Na2SiO3 appear at 2θ = 25.9°, 27.8°, and 31.3°, representing the high-temperature stable phase. The characteristic peaks of Na2SiO3·5H2O are found at 2θ = 11.7°, 23.5°, and 35.4°, which correspond to the common industrial crystalline form. For Na2SiO3·9H2O, the characteristic peaks are located at 2θ = 7.6°, 15.3°, and 22.9°, indicating a low-temperature crystallization product. Therefore, the XRD pattern of sodium metasilicate is influenced by the content of crystallization water. The anhydrous phase remains stable at high temperatures (>100 °C), while the industrial form is typically the pentahydrate, characterized by sharp and intense peaks, indicating good crystallinity.
As summarized, a comparison of the crystalline phase structures of coal gangue, fly ash, cement, calcium oxide, and sodium metasilicate is presented in Table 2.

Table 2.
Summary and comparison of phase analysis.
In the alkali-activated coal gangue-based solid waste filling material system, the ratio of CaO to Na2SiO3 significantly influences the geopolymerization process and the final mineral phase composition by controlling factors such as alkalinity, silicon and aluminum ion concentration, and reaction pathways. The results are shown in Figure 3. During the activation of coal gangue-based materials with CaO and Na2SiO3, the primary mineral phases formed include C-A-S-H gel (calcium aluminosilicate hydrate), N-A-S-H gel (sodium aluminosilicate hydrate), Ca(OH)2 (portlandite), calcite (CaCO3), and potentially ettringite. Among these, the characteristic diffraction peaks of C-A-S-H gel are located at 2θ = 29.5° (corresponding to the Ca-Si-O bond) and 32.0° (corresponding to the Ca-Al-O bond), which are the main contributors to the material’s strength. N-A-S-H gel exhibits a broad amorphous peak in the 2θ range of 20–30° and is readily formed in strongly alkaline environments rich in sodium. Ca(OH)2 shows sharp characteristic peaks at 2θ = 18.0° and 34.0° and tends to form in excess when a high CaO ratio is used. The characteristic peak of calcite appears at 2θ = 29.4°, typically formed by the carbonation of Ca(OH)2 when it absorbs CO2 from the air. If sulfate ions are present in the system (e.g., from the oxidation of pyrite residues in coal gangue), ettringite may form, with a characteristic peak at 2θ = 9.1°.
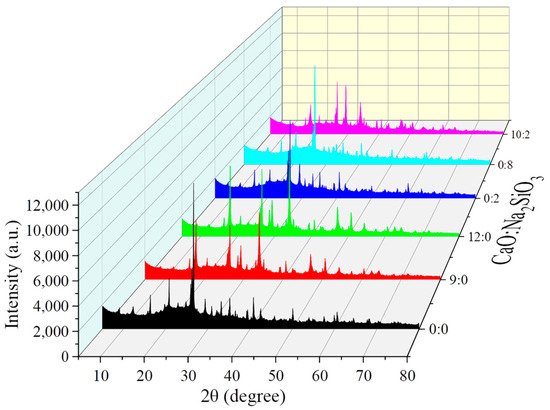
Figure 3.
XRD patterns of filling materials under different types and dosages of alkali activators.
For the single CaO alkali activator, when the content is 0%, the XRD pattern predominantly exhibits the original mineral phases of coal gangue, with major diffraction peaks corresponding to quartz (2θ = 20.8°, 26.6°), kaolinite (2θ = 12.3°, 24.8°), and illite (2θ = 8.8°, 17.8°). No new phases are formed, indicating that no significant alkali activation reaction has occurred, and the mineral phases remain in their original state. When the alkali activator content increases to 9%, the intensity of the diffraction peaks for the original minerals significantly decreases (with a 30–40% reduction in the quartz peak intensity). At the same time, new diffraction peaks appear at 2θ = 29.4°, 34.1°, and 31.8°, corresponding to Ca(OH)2, a broad peak of C-S-H gel, and ettringite, respectively. This suggests that CaO effectively activates the dissolution of aluminosilicate minerals, generating cementitious products, with a small amount of unreacted Ca(OH)2 remaining. The degree of mineral phase transformation is moderate. As the alkali activator content increases further to 12%, the intensity of the diffraction peaks for the original minerals decreases even more. However, the intensity of the Ca(OH)2 diffraction peak (29.4°) significantly increases (about 60% higher than that for the 9% group). Additionally, a new diffraction peak appears at 2θ = 36.0°, corresponding to the CaCO3 peak (due to excess Ca(OH)2 reacting with CO2). The broadening of the C-S-H gel peak weakens, indicating that excessive CaO leads to the accumulation of free Ca(OH)2 and CaCO3, which inhibits the continuous formation of cementitious products. The mineral phase consists of a mixture of original minerals, excess calcium salts, and a small amount of cementitious phases.
Compared to the coal gangue-based solid waste filling material without the addition of alkali activators, the incorporation of 2% sodium metasilicate results in a slight decrease in the intensity of the diffraction peaks for the original minerals. A broadening diffraction peak appears at 2θ = 31.8°, corresponding to the characteristic peak of C-S-H gel, and the quartz peak intensity decreases by approximately 20%. This indicates that a low dose of sodium metasilicate can partially activate the dissolution of aluminosilicate minerals in coal gangue, generating a small amount of cementitious products. However, the reaction extent is limited, and the material remains dominated by the original minerals. When the sodium metasilicate content increases to 8%, the intensity of the original mineral diffraction peaks significantly weakens (with a reduction of more than 50% in the quartz peak intensity). The intensity of the C-S-H gel broadening peak (31.8°) increases substantially, and a characteristic peak of zeolite appears at 2θ = 29.5°. This suggests that a higher dosage of sodium metasilicate effectively promotes the activation reaction, facilitating the dissolution of aluminosilicate minerals and their reorganization into stable cementitious phases and zeolitic minerals. As a result, the mineral phase is predominantly composed of reaction products, with a significant reduction in the proportion of original minerals, leading to improved cementitious properties of the material.
For the CaO-Na2SiO3 composite alkali activator (with a ratio of 10:2), the characteristic peaks of Ca(OH)2 at 2θ = 18.0° and 34.0° exhibit very high and sharp intensities, indicating a high CaO content in the system. The dissolution of CaO has led to the formation of excess Ca(OH)2. Since CaO dissolves in water to produce Ca2+ and OH− ions, under the low Na2SiO3 ratio, the OH− concentration remains relatively low (pH ≈ 12.5–13.0), which is insufficient to completely consume the Ca2+ generated by CaO dissolution. As a result, Ca(OH)2 precipitates in large quantities as crystals. The characteristic peaks of C-A-S-H gel at 2θ = 29.5° and 32.0° show moderate intensity and relatively sharp peak shapes, suggesting that, under the promotion of higher Ca2+ concentrations, a certain amount of C-A-S-H gel is formed with relatively good crystallinity. The high concentration of Ca2+ ions combines with the silicon and aluminum ions dissolved from the coal gangue, directing the formation of C-A-S-H gel structures. Additionally, the intensity of the N-A-S-H amorphous peak in the range of 2θ = 20–30° is very weak. This is because the Na+ concentration in the system is low, insufficient to provide a sufficiently strong alkaline environment to fully promote the dissolution of aluminosilicate minerals in coal gangue and the polymerization of silicon and aluminum ions, thereby inhibiting the formation of N-A-S-H gel.
3.2. SEM
The microstructural characteristics of coal gangue were observed using scanning electron microscopy (SEM), with the results presented in Figure 4. At magnifications of 20,000× and 10,000×, the SEM images reveal many nanometer-sized particles, which are small crystals of mineral components such as quartz and kaolinite in the coal gangue. These crystals exhibit irregular shapes and varying sizes. Additionally, some nanometer-sized pores and cracks are visible on the surface, which likely result from weathering, erosion, or other processes during the formation or later stages of coal gangue. These pores and cracks provide pathways for gas and liquid adsorption and transport. Moreover, these cracks may influence the mechanical properties of coal gangue. At a magnification of 5000×, the SEM image shows that coal gangue is primarily composed of particles of varying sizes, with some gaps between the particles. At 2000× magnification, the SEM image clearly shows that coal gangue is an aggregate of particles with different diameters, ranging from several micrometers to tens of micrometers.
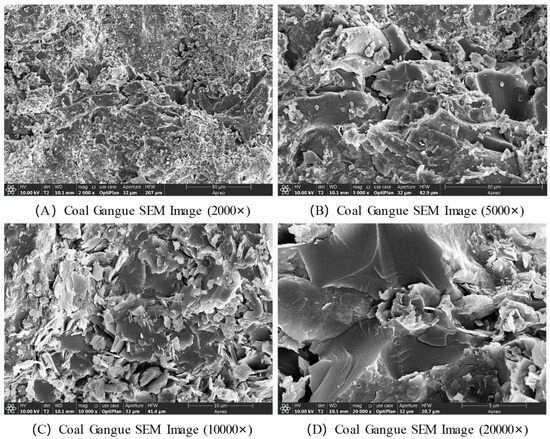
Figure 4.
SEM images of coal gangue.
The SEM images of fly ash are presented in Figure 5. At a magnification of 2000×, the fly ash appears as an aggregate composed of particles with varying sizes, exhibiting a significant difference in particle diameters. The morphology is predominantly spherical, accompanied by a small number of irregular block-like particles. There are observable voids between particles, and localized agglomeration leads to the formation of a porous structure, resulting in an overall loose and heterogeneous texture. At a magnification of 10,000×, the surfaces of the spherical particles display microstructural defects such as small pits and cracks. Some particle surfaces are covered with nanoscale deposits, which are likely microcrystals formed via crystallization. The irregular particles exhibit rough surfaces with clearly visible pores, which may serve as active sites for chemical reactions. The interfaces between particles are more distinct at this scale, allowing the observation of inter-particle bonding modes and the degree of interconnectivity. At a magnification of 20,000×, the nanoscale features of fly ash particles become evident. The smooth spherical particles display predominantly amorphous characteristics, though localized crystallization is observed, including the presence of needle-like or plate-like microcrystalline structures.
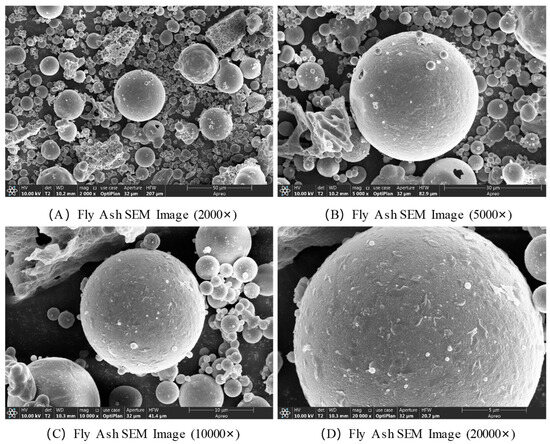
Figure 5.
SEM images of fly ash.
The microstructural morphology of cement was characterized using SEM, and the results are presented in Figure 6. The SEM images reveal that cement is a heterogeneous aggregate composed of various mineral phases. Its morphology is primarily characterized by numerous blunt-edged, ellipsoidal particles of varying sizes, exhibiting irregular shapes overall. At a magnification of 2000×, all visible particles appear to be smaller than 50 μm. At 5000× magnification, the surfaces of larger particles are observed to be smooth and flat, with a substantial number of smaller particles irregularly attached to them. At higher magnifications of 10,000× and 20,000×, many needle-like, rod-shaped, columnar, and ellipsoidal granular structures are clearly seen to be distributed and embedded on the surfaces of larger particles, approximately 20 μm in size.
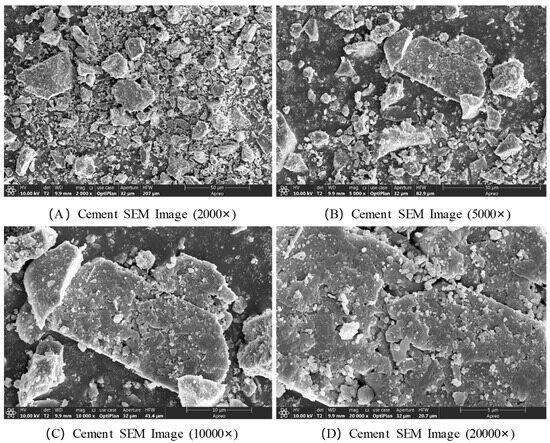
Figure 6.
SEM images of cement.
The SEM images of CaO are shown in Figure 7. At a magnification of 2000×, the SEM images reveal that the size of CaO particles is uneven, with irregular blocky or granular shapes. Significant gaps between the particles are observed, and in some areas, particle aggregation is evident, forming a porous structure. At a magnification of 5000×, the SEM images show that the particle surfaces are relatively rough, with some areas exhibiting uneven topography. The particles are composed of smaller aggregating particles, with tiny gaps between them, forming numerous small pores, which is consistent with the porous nature of calcium oxide. To quantify the specific surface area corresponding to this porous structure, the Brunauer–Emmett–Teller (BET) method was used to determine the specific surface area of the CaO powder employed in this study, and the result showed that its specific surface area was 23 m2/g. At 10,000× magnification, many micropores are visible, with varying sizes and shapes. Some particles also show cracks on their surfaces, likely due to stress changes during the preparation process or calcination conditions. Additionally, small nanometer-sized particles are observed to be attached to the surface of larger particles, which may be microcrystals formed during the crystallization process of calcium oxide. At 20,000× magnification, the SEM images clearly show the crystalline structure of the CaO particles, with observable edges and crystal faces.

Figure 7.
SEM images of calcium oxide.
The SEM images of Na2SiO3 are shown in Figure 8. Sodium metasilicate overall exhibits an irregular blocky or granular agglomerate structure, with noticeable variations in particle size. Significant gaps between particles are observed, accompanied by slight aggregation, resulting in a relatively loose overall structure. At a magnification of 5000×, the surface roughness of the particles is clearly visible, with some regions displaying layered or flaky structures. At magnifications of 10,000× and 20,000×, the finer surface morphology of the particles is accentuated, revealing edges and step-like structures formed by crystallization, or pits and cracks resulting from defects. In some areas, nano-sized microcrystal agglomerates may be present, forming a porous surface texture. These structures provide reactive sites for interactions with other substances, such as coal gangue and calcium oxide.
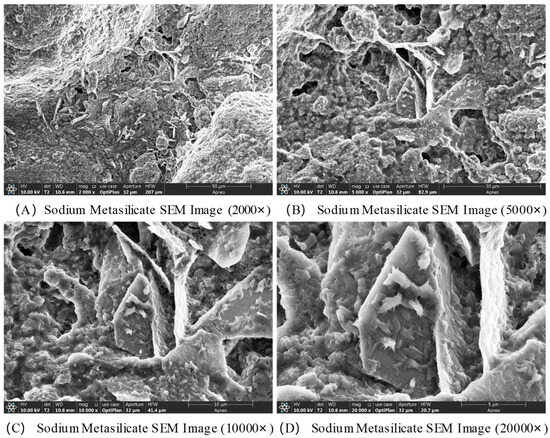
Figure 8.
SEM images of sodium metasilicate.
The SEM images of the filling material at a curing age of 28 d are shown in Figure 9. The geopolymerization process of the alkali-activated coal gangue-based solid waste filling material (i.e., the hardening of the gel system and the formation of products) exhibits characteristic changes from the macroscopic structure to the microscopic interface in SEM images at different magnifications. The core manifestations include the formation of reaction products, particle bonding, and pore evolution. At a magnification of 2000×, the original coal gangue particles (mostly spherical, angular, or blocky) are encapsulated or connected by the cementitious products, forming a continuous matrix framework. Unreacted coal gangue particles are dispersed within, with fibrous and gel-like alkali activation products (such as C-S-H and N-A-S-H gels) filling the spaces between the particles. The overall pore distribution is broad, including larger inter-particle gaps and micropores within the gel body, and the structural density increases as the reaction progresses. At 5000× magnification, the original particles are covered by one or more layers of gel products, with some areas showing gel formation growing outward from the particle surface, creating “bridging” structures that fill the inter-particle voids. At 10,000× magnification, the gel body exhibits an amorphous or semi-crystalline dense structure with a rough surface and subtle wrinkles. At 20,000× magnification, nano-sized microcrystals can be observed within the gel body, such as the fibrous nanocrystals of C-S-H gel. These nanoscale structures enhance the material’s strength through accumulation and crosslinking. At the particle–gel interface, a transition zone is observed, with a gradient change due to elemental diffusion. In some areas, nano-sized pores or defects are present, and their quantity and distribution directly influence the material’s density and durability.
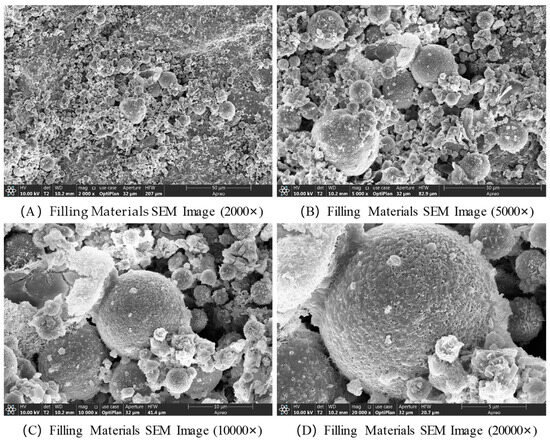
Figure 9.
SEM images of filling materials at 28 d curing age.
3.3. Hydration Degree of Filling Materials
To further investigate the hydration reaction process within the filling material samples under different influencing factors, Figure 10 and Figure 11 present the thermogravimetric (TG) and differential thermogravimetric (DTG) curves of the filling material samples at curing ages of 7 and 28 days, respectively, under different types and dosages of activators. The hydration products of the samples under varying activator types and dosages are generally consistent, mainly including C-S-H/C-A-S-H gels, AFt, CH, and calcium carbonate. According to the DTG curves, three distinct weight loss peaks are observed during the heating process from 50 °C to 1000 °C. The first weight loss peak occurs in the temperature range of 70 °C to 200 °C and is primarily attributed to the loss of free water molecules from hydration products such as C-S-H/C-A-S-H gels and AFt. The second weight loss peak is observed in the temperature range of 400 °C to 600 °C and is primarily related to the loss of chemically bound water from CH. The third weight loss peak occurs in the temperature range of 670 °C to 830 °C, and it is primarily attributed to the decomposition of calcium carbonate, which releases carbon dioxide upon heating [26,27].
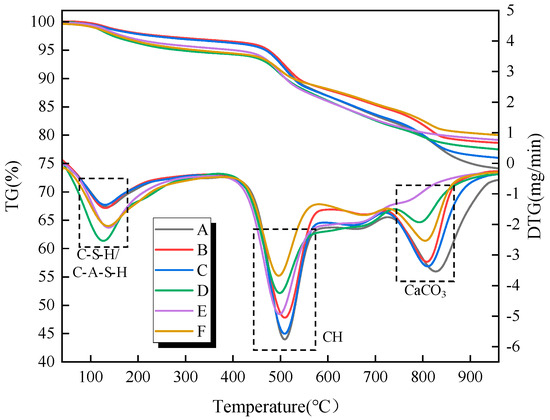
Figure 10.
TG and DTG of the samples under the action of different alkaline activators at the 7-day curing age. A is no alkali activator, B is 3% CaO, C is 12% CaO, D is 2% Na2SiO3, E is 8% Na2SiO3, and F is 8% CaO + 4% Na2SiO3.
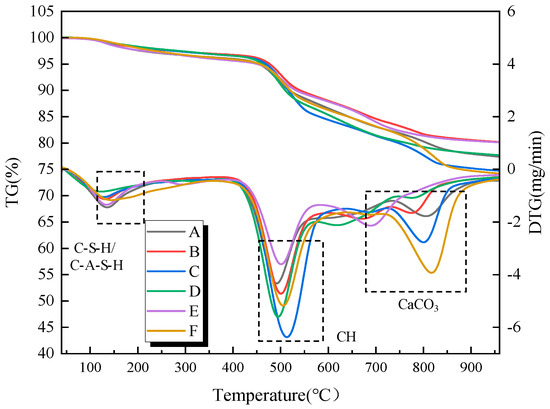
Figure 11.
TG and DTG of the samples under the action of different alkaline activators at the 28-day curing age. A is no alkali activator, B is 3% CaO, C is 12% CaO, D is 2% Na2SiO3, E is 8% Na2SiO3, and F is 8% CaO + 4% Na2SiO3.
Under the curing age of 7 d, for the single CaO alkali activator, as the CaO content increases, the weight loss corresponding to C-S-H/C-A-S-H gel and AFt first increases and then decreases. This suggests that an appropriate activator content can promote the formation of hydration products such as C-S-H/C-A-S-H gel and AFt, contributing to higher strength in the filling material samples. Additionally, as the activator content increases, the weight loss corresponding to CH also decreases to some extent, indicating that the reactive components (SiO2 and Al2O3) in the gangue powder undergo pozzolanic reactions, continuously consuming CH and reducing its content in the reaction system. Therefore, by calculating the CH content in the reaction system, the influence of the activator on the degree of hydration within the sample can be quantitatively analyzed. According to reports by Zhai et al. [23] and He et al. [28], the thermal decomposition of CH produces calcium oxide and water, with molecular weights of 74 and 18, respectively. By calculating the mass loss resulting from the thermal decomposition of CH, the CH content can be further determined. The corresponding calculation formula is as follows:
Here, WCH represents the CH content in the hydration reaction system, and WH represents the content of chemically bound water produced from the thermal decomposition of CH in the hydration reaction system. WCH indirectly reflects the degree of hydration in the filling material; a lower WCH indicates that more CH has participated in the pozzolanic reaction, which in turn suggests a higher degree of hydration in the filling material.
Based on the above equation, the CH content and chemically bound water content of the samples under different activator types and dosages are presented in Table 3. It can be observed that as the activator (single CaO) content increases, the CH content in the samples initially decreases and then increases. The sample with 3% activator content shows the lowest CH content, indicating that an optimal activator content can promote more CH to participate in the pozzolanic reaction, thereby enhancing the hydration degree of the system and generating a greater amount of hydration products.

Table 3.
Chemically bound water and CH content of samples with different alkali activator types and dosages at 7 d curing age.
Under the curing age of 7 d, for the single Na2SiO3 alkali activator, as the Na2SiO3 content increases, the weight loss corresponding to C-S-H/C-A-S-H gel and AFt increases (Figure 9A,D), indicating that the Na2SiO3 alkali activator promotes the formation of hydration products such as C-S-H/C-A-S-H gel and AFt. Additionally, as the Na2SiO3 content increases, the weight loss corresponding to CH decreases. The CH content and chemically bound water content of the samples under different activator (single Na2SiO3) dosages are calculated and presented in Table 3. It can be observed that as the activator content increases, the CH content within the samples decreases.
Figure 11 presents the thermogravimetric analysis curves of the filling material samples at a curing age of 28 d. Compared to the 7-day curing age, the weight loss corresponding to C-S-H/C-A-S-H gel and AFt increases, indicating that with the extension of the curing age, the hydration products gradually increase. Notably, the sample without alkali activator at 28 days shows a weight loss peak around 700 °C, and the DTG curve exhibits some fluctuations. This may be due to the instability of the C-S-H gel structure system within the sample as the curing age increases, which is prone to structural decomposition under high-temperature conditions.
Furthermore, during the early curing stage (7 d), the CH content within the samples exhibits a gradual increase, while in the later curing stage (28 d), the CH content shows a gradual decrease. This can be attributed to the fact that in the early curing stage, the rate of CH formation from cement hydration exceeds the rate at which CH is consumed by the pozzolanic reaction. However, as the curing age progresses, the pozzolanic effect of the gangue powder gradually dominates the hydration system, leading to a reduction in the CH content within the system. The chemically bound water and CH content of samples with different alkali activator types and dosages at 28 d curing age are presented in Table 4. Furthermore, from the perspective of practical mine application scenarios, the underground backfill body is in a relatively closed environment where the CO2 concentration in the air is much lower than that in the conventional laboratory environment. Therefore, the impact of carbonation on CH content in actual engineering will be further weakened, and it will not pose a significant threat to the bearing performance of the backfill body. Meanwhile, combined with the XRD analysis results, no obvious enhancement of the characteristic diffraction peak of CaCO3 (2θ = 29.4°) was observed in the samples cured for 28 days.

Table 4.
Chemically bound water and CH content of samples with different alkali activator types and dosages at 28 d curing age.
3.4. Mechanical Performance Analysis
Compressive strength reflects the load-bearing capacity of cemented backfill materials under overburden pressure, making it a key parameter for evaluating the performance of backfill materials and an important indicator for assessing the safety and stability of mining operations. To investigate the effect of activator content on the compressive strength of backfill materials, Figure 12 and Figure 13 show the variation in compressive strength with the content of single activators at different curing ages. As shown in Figure 12, under the same curing age, the compressive strength of the backfill material samples increases initially with the activator content and then decreases. The CaO activator content that yields the maximum compressive strength of the backfill material is between 6% and 9%, while the optimal Na2SiO3 activator content is 2%. This indicates that an appropriate activator content can significantly optimize the strength properties of the backfill material. Furthermore, the compressive strength of the samples with Na2SiO3 as the sole activator is higher than that of the samples with CaO alone. However, increasing the Na2SiO3 content inevitably raises the cost of backfill mining.
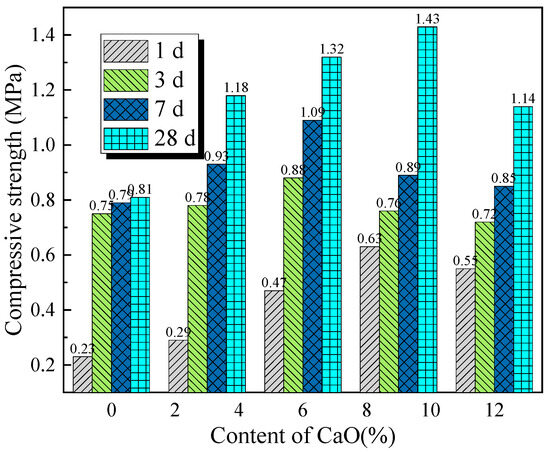
Figure 12.
Effect of single-dose CaO activator content on compressive strength of backfill materials at different curing times.
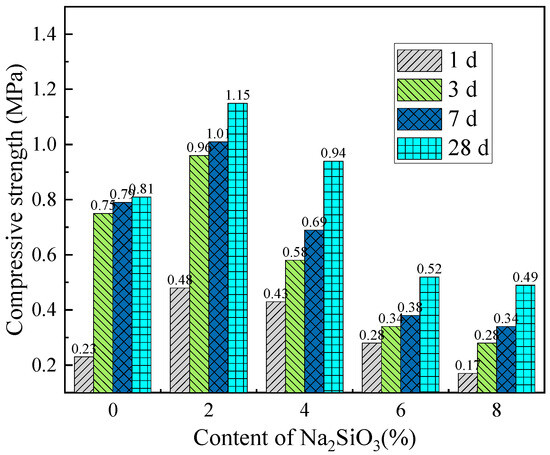
Figure 13.
Effect of single-dose Na2SiO3 activator content on compressive strength of backfill materials at different curing times.
It is noteworthy that the compressive strength of the samples using composite activators is higher than that of the samples with only CaO or Na2SiO3, as shown in Figure 14. This indicates that the addition of CaO and Na2SiO3 can work synergistically to activate the pozzolanic effect of coal gangue powder, leading to the formation of more hydration products, such as calcium silicate hydrate (C-S-H) and calcium aluminate hydrate (C-A-S-H), thereby enhancing the compressive strength of the cemented backfill material samples. Therefore, an appropriate activator content can significantly optimize the strength properties of the backfill material. Furthermore, the use of composite activators ensures that the backfill material has sufficient load-bearing capacity while reducing the cost of backfill mining.
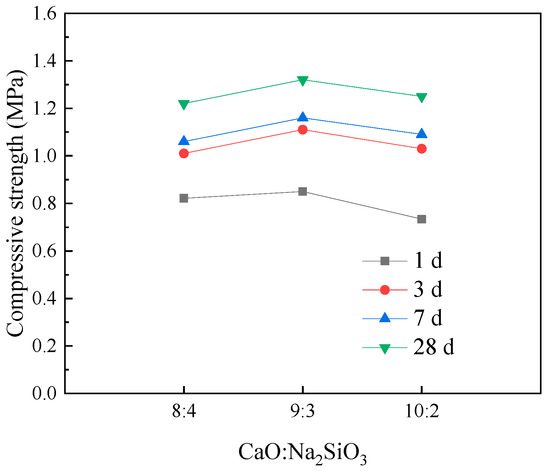
Figure 14.
Effect of the ratio of CaO to Na2SiO3 in the CaO-Na2SiO3 composite activator on compressive strength of backfill materials at different curing times.
To investigate the effect of activator ratio on the compressive strength of the filling material samples, Figure 14 presents the variation in compressive strength with the activator ratio under different curing ages. As shown in the figure, under the same alkali activator content (12%) and curing age, as the ratio of CaO to Na2SiO3 increases, the compressive strength of the filling material samples first increases and then decreases. Therefore, by adjusting the activator ratio, the strength properties of the filling material samples can be optimized. The activator ratio that corresponds to the maximum compressive strength of the samples is 3:1, indicating that properly controlling the activator ratio can enhance the load-bearing capacity of the cemented backfill material and effectively mitigate the movement and deformation of the overburden in the goaf area. The reason for this behavior is that, although CaO and Na2SiO3 can synergistically activate the latent reactivity of coal gangue powder, leading to the formation of more hydration products, when the proportion of Na2SiO3 is too high, the free sodium ions react first with the reactive substances in the coal gangue powder, inhibiting the hydration of tricalcium aluminate, dicalcium silicate, and tricalcium silicate, thus reducing the formation of hydration products. On the other hand, when the proportion of CaO is too high, excess CaO reacts with carbon dioxide in the air to form carbonates, which deteriorates the strength properties of the backfill material. Additionally, the low solubility of CaO means that excess CaO, upon precipitation, also lowers the compressive strength of the filling material.
Combining Figure 12, Figure 13 and Figure 14, it is evident that the compressive strength of the filling material samples is positively correlated with the curing age. Therefore, appropriately extending the curing period can improve the strength properties of the filling material. Furthermore, rational control of the activator content and ratio contributes to the stable increase in the compressive strength of the filling material.
3.5. Laws of Polymeric Mineralization
The physicochemical properties of raw materials such as coal gangue, fly ash, and cement, along with the action of alkali activators (CaO and Na2SiO3), collectively provide the material foundation for the geopolymerization and mineralization of gangue-based solid waste filling materials. SEM images of coal gangue (Figure 4) reveal angular block-like particles with smooth surfaces. XRD patterns (Figure 2) indicate the presence of strong diffraction peaks corresponding to quartz, mullite, and kaolinite, suggesting that the Si-Al components exist in stable crystalline phases with inherently low reactivity. In contrast, SEM images of fly ash (Figure 5) show spherical glassy particles accompanied by minor quartz content. The corresponding XRD patterns display a broad diffuse hump attributed to the amorphous glassy phase, superimposed with peaks of quartz and mullite. This amorphous nature enhances the reactivity of fly ash compared to coal gangue. Cement, serving as an auxiliary binder, exhibits characteristic peaks of C3S and C2S in the XRD pattern (Figure 2), which can rapidly release Ca2+ and silicate ions upon early hydration, thereby providing initial strength support. As a major component of the alkali activator system, SEM images show that CaO consists of irregular particles (Figure 7), which react with water to form Ca(OH)2, elevating the system pH to 12–13 and promoting the dissolution of aluminosilicate phases. Simultaneously, the released Ca2+ serves as a precursor for the formation of calcium-based gels. Na2SiO3 appears as flocculent agglomerates under SEM (Figure 8); upon dissolution, it releases silicate (SiO32−) and sodium ions (Na+), enhancing the alkalinity and providing additional reactive silica, thus accelerating the polycondensation of Si and Al species.
The geopolymerization and mineralization of the gangue-based filling material essentially involve a transformation process from ion dissolution to crystalline product formation. At the 7-day curing stage, the system enters the initial gelation and early product formation phase. TGA curves (Figure 10) show the first weight-loss peak within 100–200 °C (DTG maximum at 120 °C), corresponding to gel dehydration (mass loss of 3–5%), indicating the predominance of cement hydration and the onset of alkali activation. When the CaO:Na2SiO3 ratio is 10:2, the broad peak reaches its maximum intensity, reflecting the highest yield of C-S-H/C-A-S-H gels. However, excessive CaO content (12%) leads to the appearance of Ca(OH)2 peaks in the XRD spectra and a secondary weight-loss peak at 400–500 °C in the TGA curve (mass loss ~2%) due to the decomposition of unreacted Ca(OH)2, resulting in a less compact structure. At the 28-day curing age, the filling material exhibits complete mineralization and structural densification. A second weight-loss peak emerges between 600–800 °C (DTG peak at 790 °C), attributed to the decomposition of calcite (mass loss 2–4%). Additionally, mass loss at 100–200 °C increases to 6–8%, confirming the synergistic accumulation of gels and crystalline phases. Under this curing regime, the composite activator (CaO:Na2SiO3 = 10:2) shows no Ca(OH)2 peak in XRD and exhibits moderate calcite peak intensity (Figure 3) while achieving the highest overall mass loss (8.5%) in TGA (Figure 11), suggesting effective reaction among Ca2+, Si4+, and Al3+ and the formation of an interpenetrating “gel–crystal” network.
The compressive strength of the gangue-based filling material increases progressively with curing time during the geopolymerization and mineralization process. From 1 to 3 d, strength development is primarily driven by cement hydration and the formation of C-S-H gels, while the contribution from alkali activation becomes evident (accounting for ~30% of the reaction products at 3 d). When curing is extended to 7 d, strength improvement is predominantly governed by alkali-activated gel network formation (gel content ~60%). Between 7 and 28 d, further strength enhancement results from crystal growth (e.g., calcite, zeolite), leading to a denser matrix through gel–crystal synergism (crystalline phase content 20–30%). Furthermore, the content of alkali activators (CaO and Na2SiO3) must be optimized to ensure the balanced reaction of Ca2+, Si4+, and Al3+ at all curing stages. This avoids the formation of residual Ca(OH)2 or excessive Si4+ species, ensuring maximal gel and crystalline phase generation and structural compactness (Figure 12 and Figure 13). Excess CaO results in discontinuous crystal domains due to Ca(OH)2 precipitation, while excessive Na2SiO3 leads to discrete gel morphology—both negatively affecting compressive strength, with the 28-day samples showing the most pronounced strength deterioration under imbalanced activator ratios.
3.6. Mechanisms of Polymeric Mineralization
The reaction mechanism at the microscopic level reveals the essence of the synergistic effect between CaO and Na2SiO3 (Figure 15). The dissolution of CaO provides Ca2+ ions and elevates the system pH (~12–13), effectively disrupting the crystal structure of stable aluminosilicate minerals such as quartz and kaolinite in coal gangue, thereby promoting the dissolution and release of Si4+ and Al3+ ions. Simultaneously, the dissolution of Na2SiO3 not only provides additional OH− to maintain the highly alkaline environment but also introduces reactive SiO32− ions that directly participate in constructing the reaction network. At the optimal ratio (CaO:Na2SiO3 = 3:1), the high concentration of Ca2+ tends to undergo polycondensation reactions with the dissolved [SiO4]4− and [AlO4]5− tetrahedral units, predominantly forming a calcium-based hydrated aluminosilicate gel (C-A-S-H) with relatively high crystallinity and excellent mechanical properties. Its characteristic XRD peaks (29.5°, 32.0°) and dense structure observed under SEM are clearly discernible. Meanwhile, an appropriate amount of Na+ helps stabilize the dissolved aluminosilicate precursors, facilitating the early formation of the gel network. Excessive CaO leads to the accumulation of unreacted Ca(OH)2 (evidenced by prominent XRD peaks at 18.0°, 34.0°) and even carbonation to CaCO3 (peak at 29.4°), occupying space and weakening the matrix continuity. Excessive Na2SiO3 may, due to the high Na+ concentration, interfere with the effective coordination of Ca2+, inhibiting the preferential growth of C-A-S-H and instead promoting the formation of sodium-rich gel (N-A-S-H) with relatively weaker mechanical properties, or causing localized silicate enrichment. This competition and synergy at the ionic level ultimately determine the gel type, crystallinity, micromorphology, and consequently, the macroscopic mechanical performance.
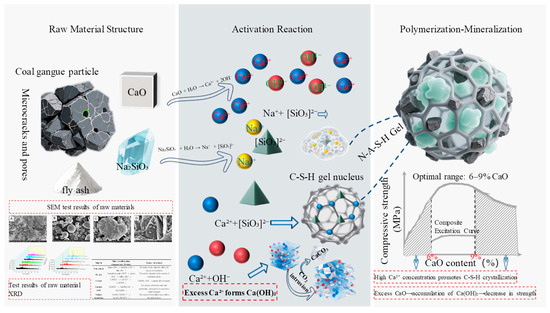
Figure 15.
Polymerization–mineralization mechanisms of alkali-activated gangue-based solid waste backfill materials.
4. Conclusions
In this work, the effects of controlling the content of single CaO and Na2SiO3 alkali activators, as well as the ratio of composite alkali activators, on the surface morphology, mineral phase composition, and degree of hydration of gangue-based solid waste filling materials were investigated. Additionally, the compressive strength of the materials was measured. The type, content, and ratio of CaO to Na2SiO3 alkali activators have a significant impact on the mineral phase composition, surface morphology, and degree of hydration of the filling material. Notably, there are distinct differences in the regulation of the hydration reaction by CaO and Na2SiO3, and the curing age is an important factor influencing the material’s properties. Furthermore, under the influence of single alkali activators, the compressive strength of the filling material follows a trend of first increasing and then decreasing as the activator content increases. The optimal CaO content is between 6% and 9%, and the optimal Na2SiO3 content is 2%. When using composite alkali activators, the compressive strength also increases and then decreases as the ratio of CaO to Na2SiO3 increases, with the optimal ratio being 3:1. Additionally, the compressive strength of the filling material increases with the curing age, showing a positive correlation. To address practical application needs, the optimal parameters for the gangue-based filling material derived from this study are recommended as follows: the composite CaO-Na2SiO3 activator should adopt a mass ratio of 3:1 with a total dosage fixed at 12 wt% of coal gangue, while single-component activators perform best at 6–9% CaO or around 2% Na2SiO3. For on-site construction, the curing age of the filling body should not be less than 28 days to ensure sufficient development of compressive strength through complete geopolymerization–mineralization. These parameters not only maximize the utilization of mine-derived coal gangue to reduce transportation costs but also guarantee the material’s load-bearing capacity to adapt to underground mining environments, providing clear operational guidance for green backfill mining. This study clarified the geopolymerization–mineralization pathway and regulatory mechanism of optimal parameters. The obtained empirical relationships (e.g., activator ratio vs. strength, curing age vs. hydration degree) serve as core input parameters for subsequent correlation model construction, aligning phased results with long-term goals. These findings provide theoretical support for the formulation optimization of gangue-based solid waste filling materials and have practical significance in promoting the resource utilization of mining waste.
Author Contributions
All authors contributed to the study conception and design. J.F.: writing—review and editing, methodology, data curation, investigation, validation, conceptualization. G.B.: experiment design, sample preparation, data analysis (e.g., auxiliary XRD/SEM test operation), and preliminary result collation. X.Z.: writing—original draft, formal analysis, resources, project administration. K.N.: investigation, data curation, validation, methodology support. L.W.: formal analysis, resources, supervision. Y.L.: writing—review and editing, methodology, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Research and Development Task Special Project of Xinjiang Uygur Autonomous Region—Department Joint Project [grant number 2022B01051-3], the Xinjiang Uygur Autonomous Region Key Research and Development Program Project [grant number 2023B01009-2], the National Natural Science Foundation of China (NSFC)—Regional Science and Technology Development Fund [52364021], the Central Guidance Fund for Local Science and Technology Development Projects [ZYYD2024JD16], the Natural Science Foundation of Xinjiang Uygur Autonomous Region [2023D01B22], and the Independent Research Project of the Key Laboratory of Green Mining of Coal Resources in Xinjiang, Ministry of Education [grant number KLXGY-Z2412].
Data Availability Statement
All data included in this study are available upon request by contacting the corresponding author.
Conflicts of Interest
Author Guangqing Bao was employed by the company China Construction Fourth Engineering Division Corp Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bo, L.; Yang, S.; Liu, Y.; Zhang, Z.; Wang, Y.; Wang, Y. Coal mine solid waste backfill process in China: Current status and challenges. Sustainability 2023, 15, 13489. [Google Scholar] [CrossRef]
- Wang, C.; Liu, C.; Zhang, L.; Wang, C.; Xu, S.; Yang, J. Exploring calcined coal gangue fines as the total substitute of fly ash in the production of alkali-activated slag/fly ash materials. Case Stud. Constr. Mater. 2022, 17, e01332. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, J.; Taheri, A.; Zhou, N.; Li, Z.; Li, M. Control effect of coal mining solid-waste backfill for ground surface movement in slice mining: A case study of the Nantun Coal Mine. Environ. Sci. Pollut. Res. 2023, 30, 27270–27288. [Google Scholar] [CrossRef]
- Zhao, K.; Yu, X.; Zhu, S.; Yan, Y.; Zhou, Y.; He, Z.; Song, Y.; Huang, M. Acoustic emission investigation of cemented paste backfill prepared with tantalum—niobium tailings. Constr. Build. Mater. 2020, 237, 117523. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, K.; He, X.; Zhao, X.; Wei, Z.; He, S. Research status of comprehensive utilization of coal-based solid waste (CSW) and key technologies of filling mining in China: A review. Sci. Total Environ. 2024, 926, 171855. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jing, H.; Yin, Q.; Meng, B.; Han, G. Strength and ultrasonic properties of cemented waste rock backfill considering confining pressure, dosage and particle size effects. Constr. Build. Mater. 2020, 242, 118132. [Google Scholar] [CrossRef]
- Yang, S.; Xing, X.; Su, S.; Wang, F.; Ivanov, I. Experimental Study on Rheological Properties and Strength Variation of High Concentration Cemented Unclassified Tailings Backfill. Adv. Mater. Sci. Eng. 2020, 2020, 6360131. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Y.; Zuo, X.; Hong, M. Time-dependent rheological and mechanical properties of silica fume modified cemented tailings backfill in low temperature environment. Cem. Concr. Compos. 2020, 114, 103804. [Google Scholar] [CrossRef]
- Guo, L.; Liu, J.; Zhou, M.; An, S. Effect of an alkali activators on the compressive strength and reaction mechanism of coal gangue-slag-fly ash geopolymer grouting materials. Constr. Build. Mater. 2024, 426, 136012. [Google Scholar] [CrossRef]
- Zhong, M.; Meng, J.; Ning, B.; Na, F.; Cui, T.; Shi, X.; Cui, T. Preparation and alkali excitation mechanism of coal gangue-iron ore tailings non-sintering ceramsite. Constr. Build. Mater. 2024, 426, 136209. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, T.; Ai, Q.; Mao, M.; Li, J.; Yang, Q. Performance of coal gangue concrete with fly ash and ground-granulated blast slag: Rheology, mechanical properties and microstructure. Constr. Build. Mater. 2024, 427, 136250. [Google Scholar] [CrossRef]
- Reddy, B.S.; Siempu, R. Studies on the Mechanical Properties of Ternary Blended Geopolymer Concrete using Fly ash, GGBS and Alccofine. J. Phys. Conf. Ser. 2024, 2779, 012072. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, S.; Li, W.; Li, Z.; Fan, J.; Shen, Z. Study of mechanical properties and durability of alkali-activated coal gangue-slag concrete. Materials 2020, 13, 5576. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Jiang, W.; Zhang, C.; Li, J.; Wu, S.; Wang, X.; Xu, Y.; Wang, W.; Feng, M. Preparation of solid-waste-based pervious concrete for pavement: A two-stage utilization approach of coal gangue. Constr. Build. Mater. 2022, 319, 125962. [Google Scholar] [CrossRef]
- Bian, Z.; Jin, G.; Ji, T. Effect of combined activator of Ca(OH)2 and Na2CO3 on workability and compressive strength of alkali-activated ferronickel slag System. Cem. Concr. Compos. 2021, 123, 104179. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, H.; Wu, C.; Chen, H.; Sun, J.; Liu, J. Study on compressive strength and durability of alkali-activated coal gangue-slag concrete and its mechanism. Powder Technol. 2020, 368, 112–124. [Google Scholar] [CrossRef]
- Feng, D.; Wang, J.; Wang, Y.; Xiao, X.; Hou, W.; Liang, S. Alkali-activated geopolymer materials prepared from coal gangue and municipal solid waste incineration byproducts. J. Build. Eng. 2023, 80, 108074. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Kang, D.; Fang, C.; Jiao, Y.; Mi, S. Experimental study on subgrade material of calcium silicate slag. Materials 2022, 15, 2304. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Non-metallic minerals and their deposits. In Geology and Mineral Resources; Springer: Singapore, 2025; pp. 563–652. [Google Scholar]
- Pang, S.; Li, J.; Xie, F.; Wang, G.; Fan, H.; Zhu, K. Research on improving the flexural performance of alkali-activated geopolymer cemented coal gangue through layered addition of fibers. J. Build. Eng. 2024, 96, 110549. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Pan, Z.; Chen, W.; Muhammad, F.; Zhang, B.; Li, L. Geopolymerization of coal gangue via alkali-activation: Dependence of mechanical properties on alkali activators. Buildings 2024, 14, 787. [Google Scholar] [CrossRef]
- Qin, Y.; Qu, C.; Ma, C.; Zhou, L. One-part alkali-activated materials: State of the art and perspectives. Polymers 2022, 14, 5046. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Chen, Q.; Duan, Y.; Liu, B.; Wang, B. Silica polymerization driving opposite effects of pH on aqueous carbonation using crystalline and amorphous calcium silicates. Inorg. Chem. 2024, 63, 4574–4582. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E. Stope depth effect on field behaviour and performance of cemented paste backfills. Int. J. Min. Reclam. Environ. 2018, 32, 273–296. [Google Scholar] [CrossRef]
- Cao, S.; Song, W.; Yilmaz, E. Influence of structural factors on uniaxial compressive strength of cemented tailings backfill. Constr. Build. Mater. 2018, 174, 190–201. [Google Scholar] [CrossRef]
- Rao, G.A. Generalization of Abrams’ law for cement mortars. Cem. Concr. Res. 2001, 31, 495–502. [Google Scholar] [CrossRef]
- Pane, I.; Hansen, W. Investigation of blended cement hydration by isothermal calorimetry and thermal analysis. Cem. Concr. Res. 2005, 35, 1155–1164. [Google Scholar] [CrossRef]
- He, N.; Zhang, L.; Zhuang, X.; Huang, H.; Yuan, Z. Preparation and Performance of Geopolymer Pervious Concrete in Red Mud Slag Base. In Advances in Frontier Research on Engineering Structures; Advances in transdisciplinary engineering; IOS Press: Amsterdam, The Netherlands, 2023; pp. 346–354. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).