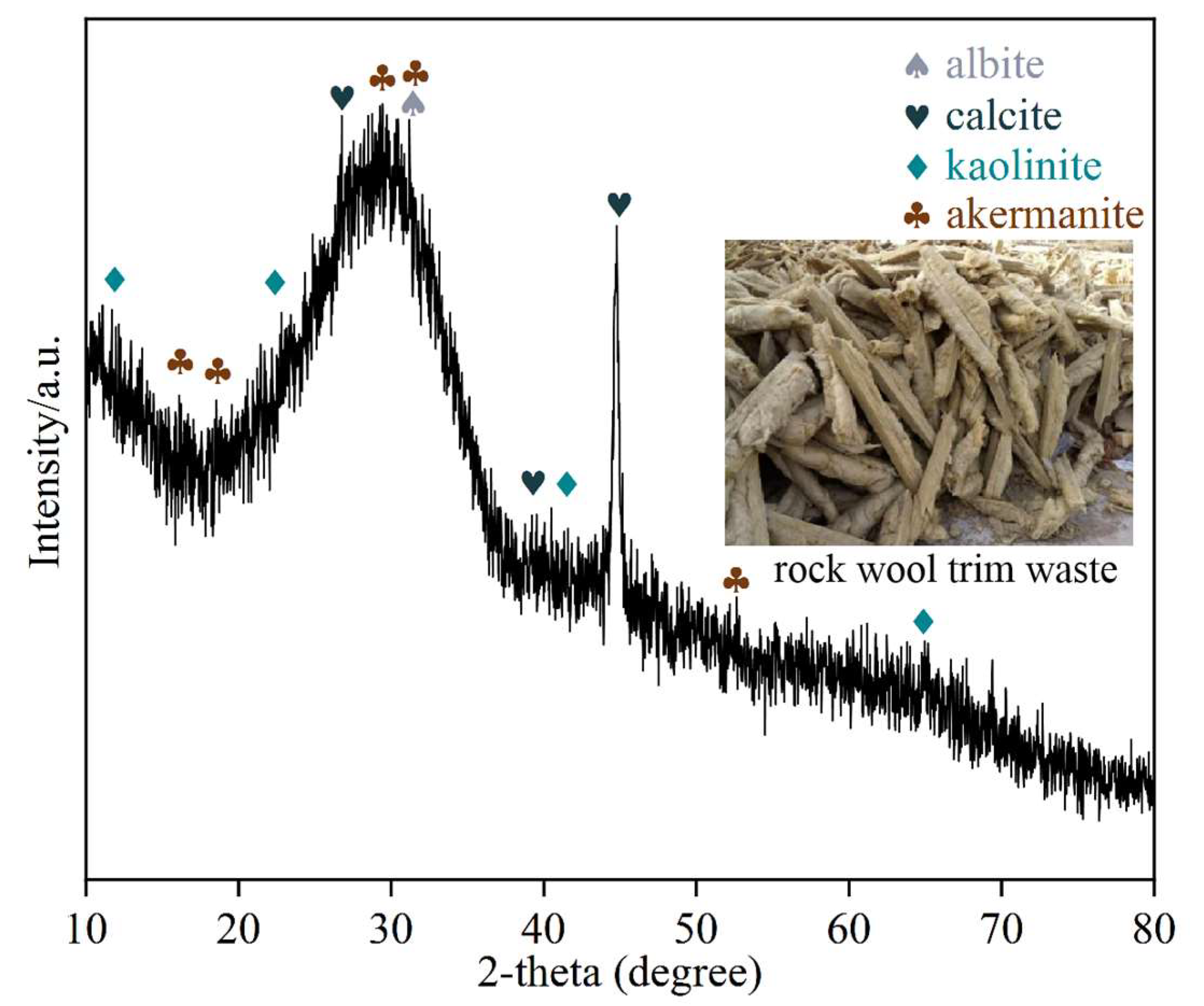
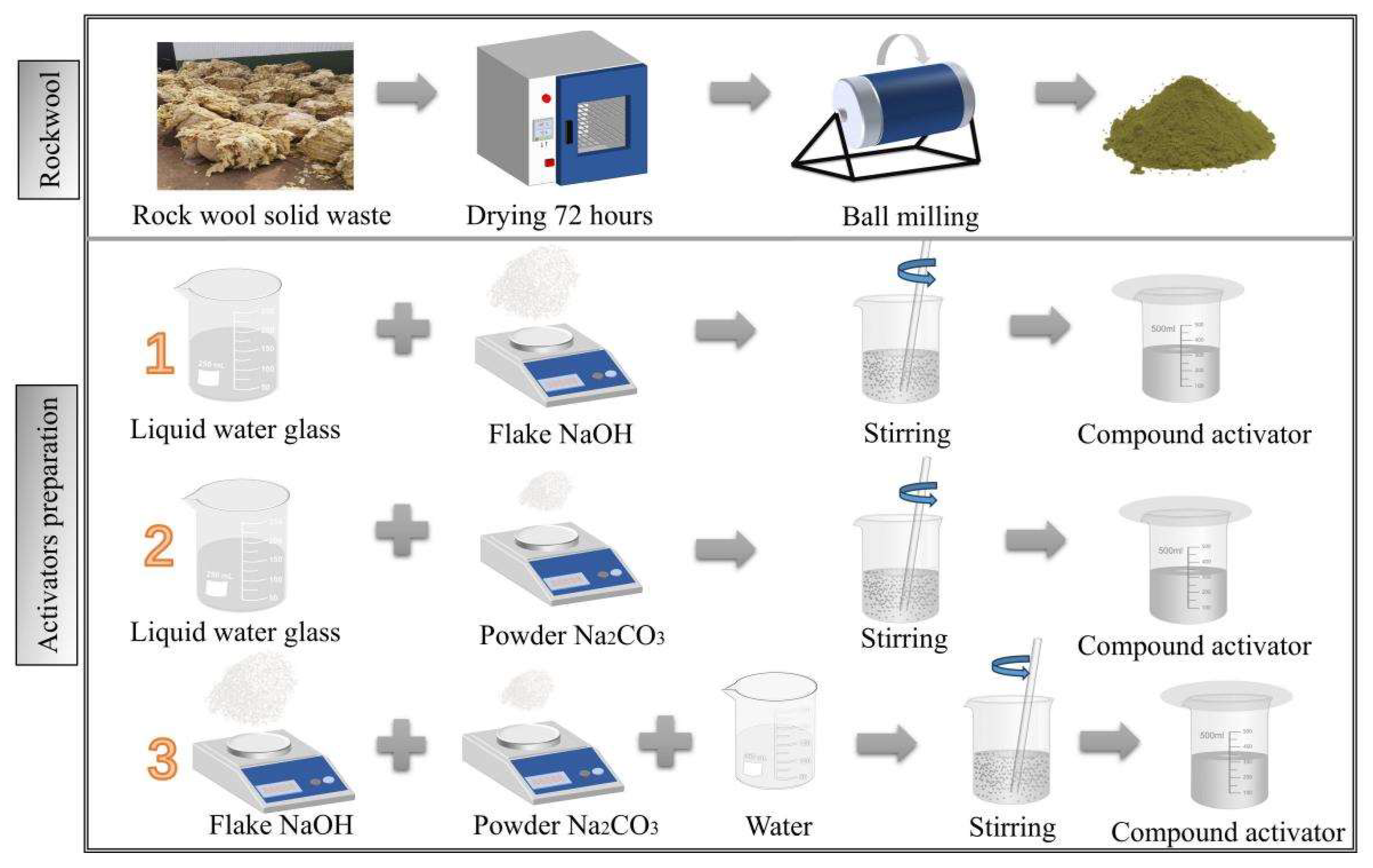
1. Introduction
Derived from the high-temperature melting (≥1500 °C) of igneous substrates such as basalt, rock wool (RW) constitutes an inorganic fibrous material extensively employed in the construction sectors, due to its superior thermal insulation efficiency and mechanical integrity [
1,
2]. The conventional landfilling of bulk RW-associated waste streams generated during production and deployment not only represents unsustainable land appropriation, but also exacerbates anthropogenic environmental risks, including particulate emission and soil alkalization [
3,
4]. Made up of geometrically irregular byproducts from the precision cutting of RW boards, rock wool trim waste (RWTW) primarily consists of flocculent fibrous matrices with persistent surface-adhered phenolic resin residues [
5].
Unlike conventional alkali-activated precursors such as fly ash and slag, rock wool solid waste possesses unique three-dimensional fibrous networks that provide intrinsic mechanical reinforcement through crack deflection and fiber bridging mechanisms in geopolymer composites [
6]. Chemical analysis by Wang et al. [
7] revealed that rock wool slag contains elevated SiO
2 and Al
2O
3 concentrations, collectively constituting 72–78 wt% of the reactive fraction. This composition promotes alkaline dissolution kinetics, forming cementitious phases with a higher binding capacity than conventional precursors. Notably, Nguyen et al. [
8] reported up to 50% compressive strength enhancement in mortars where rock wool completely replaced conventional precursors (100 wt%), utilizing either 2.5 M NaOH/Na
2SiO
3 or 10 M NaOH activators. This performance enhancement originates from synergistic multiscale reinforcement: micro-scale fiber–matrix interlocking via surface-adhered geopolymeric gels, and macro-scale stress redistribution through spatially continuous fiber networks. Complementary kinetic analyses by Yliniemi et al. [
9] revealed accelerated dissolution kinetics in NaOH- or sodium silicate-activated rock wool systems, manifesting as reduced setting durations and elevated hydration exotherms at 40 °C. These reactive systems primarily form amorphous calcium (alumino)silicate hydrate (C-A-S-H) and sodium aluminosilicate hydrate (N-A-S-H) gels, with minor crystalline phases including hydrotalcite-like layered double hydroxides identified via TEM-EDS mapping.
Current research on alkali-activated rock wool systems systematically overlooks the detrimental effects of organic resin contaminants, particularly phenolic adhesives, which compromise material viability through multiple degradation pathways [
10]. Specifically, the residual phenolic resins in RWTW substantially prolong setting durations and trigger near-total 28-day compressive strength degradation (>95% reduction relative to resin-free controls), consequently invalidating its utility as high-performance inorganic cementitious matrices [
11,
12,
13,
14,
15,
16]. Upon alkaline exposure, thermosetting binders—initially spray-deposited during RW manufacturing—liberate toxic volatiles (carbon monoxide, formaldehyde, and phenolic compounds) through thermo-alkaline decomposition, thereby intensifying recycling complexities [
17]. Lemougna et al. [
12] quantitatively verified that phenol-contaminated glass wool diminishes comminution efficiency by 40–60% and impedes strength evolution (e.g., achieving merely 60 MPa after 28 days versus >80 MPa in resin-free counterparts). Consequently, pretreatment protocols for adhesive elimination constitute a prerequisite for viable RWTW valorization. Resin extraction methodologies and their corresponding advantages/constraints are systematically catalogued in
Table 1.
Despite advances in RWTW recycling, current methods fail to resolve the triple challenge: (i) thermal treatments (≥600 °C) emit 3.2 kg CO
2/kg resin decomposed, while degrading Si/Al reactivity by 18–25%, (ii) chemical solvents generate 5–8 L toxic effluent per kg of RWTW processed, requiring costly remediation, and (iii) conventional milling achieves particle refinement but ignores resin encapsulation, allowing for organic leaching during alkali activation. This work bridges these gaps via ambient-temperature mechanochemistry—simultaneously degrading resin, preserving reactivity, and eliminating secondary waste. Thermal treatment decomposes organic constituents through high-temperature incineration (>600 °C), yet invariably generates pyrolytic volatile emissions (e.g., VOCs, quantified as 3 wt% mass loss), secondary contamination via PAHs’ adsorption onto fiber surfaces, and deleterious phase transformations, yielding molten agglomerates and fly ash particulates [
18]. Chemical techniques, though enabling partial dissolution via acid/alkaline/organic solvent immersion, necessitate exorbitant reagent consumption while adversely impacting subsequent alkali activation processes: selective Si/Al leaching diminishes precursor reactivity (18–25% reduction) and persistent solvent residues impede polycondensation kinetics [
19]. Conversely, mechanochemical milling exploits shear/compressive forces to generate localized stress concentrations within resin’s molecular architectures, preferentially cleaving labile C−O bonds (bond energy ≈ 360 kJ/mol) while conserving mineral phase integrity. Hu et al. [
20] substantiated that the cryogenic comminution of resin-coated particulates to a >120 mesh size fraction (
D50 < 125 μm) disrupts cross-linked networks, attenuating resin stabilization signatures by 40% (residual stabilization efficiency: 60%). Given its negligible ecological burden and preservation of compositional fidelity, the present investigation adopts mechanochemical milling as the principal organic extraction strategy.
While preliminary studies have explored RWTW incorporation within alkali-activated systems, substantial mechanistic knowledge deficits persist. Specifically, the regulatory mechanisms through which organic residues (e.g., phenolic resins) in RWTW modulate hydration pathways remain inadequately elucidated. Such residues potentially disrupt alkali activation kinetics, modify hydration product nucleation/microstructure, and consequently compromise macroscopic material performance. This investigation employs RWTW as the principal aluminosilicate source, integrating sequential ball milling pretreatment and alkali activation protocols. Four formulations of alkali-activated binders were synthesized from RWTW pretreated at varied ball milling durations (30, 60, 90, and 120 min). RWTW particle size distributions were characterized via vacuum sieving coupled with laser diffraction analysis. Complementary analytical techniques—including scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FT-IR)—were deployed to interrogate the effects of the milling duration on RWTW’s surface morphology, particle gradation, and chemical activation potential. Furthermore, the ball milling duration modulation of hydration kinetics and reaction product microarchitecture was resolved through isothermal calorimetry and high-resolution SEM. Mechanochemical milling selectively cleaves binders’ covalent bonds while maintaining fibers’ compositional integrity, thereby enabling efficient resin degradation under moderate energy conditions. This methodology establishes a mechanistic framework for eco-efficient RWTW valorization. This study aims to develop an eco-efficient mechanochemical ball milling pretreatment that selectively degrades phenolic resin binders in RWTW while preserving mineral reactivity, enabling its valorization in high-performance RWACCs. This approach effectively addresses three critical industrial priorities by enabling the direct valorization of RWTW (~3% of global insulation waste [
5]) within construction composites, thereby reducing landfill burden; mitigating toxicity through the encapsulation of resin-derived organics, which eliminates VOC emissions during recycling and resolves a key safety hazard [
17]; and offering inherent scalability via its ambient-temperature ball milling process, which avoids the need for energy-intensive pyrolysis (≥600 °C) or complex solvent handling, thus robustly aligning with circular economy mandates [
3,
18], potentially diverting ~1.2 million tons/year of rock wool waste [
5].
3. Results and Discussion
3.1. Effect of Ball Milling Duration on RWTW Properties
3.1.1. RWTW Fineness
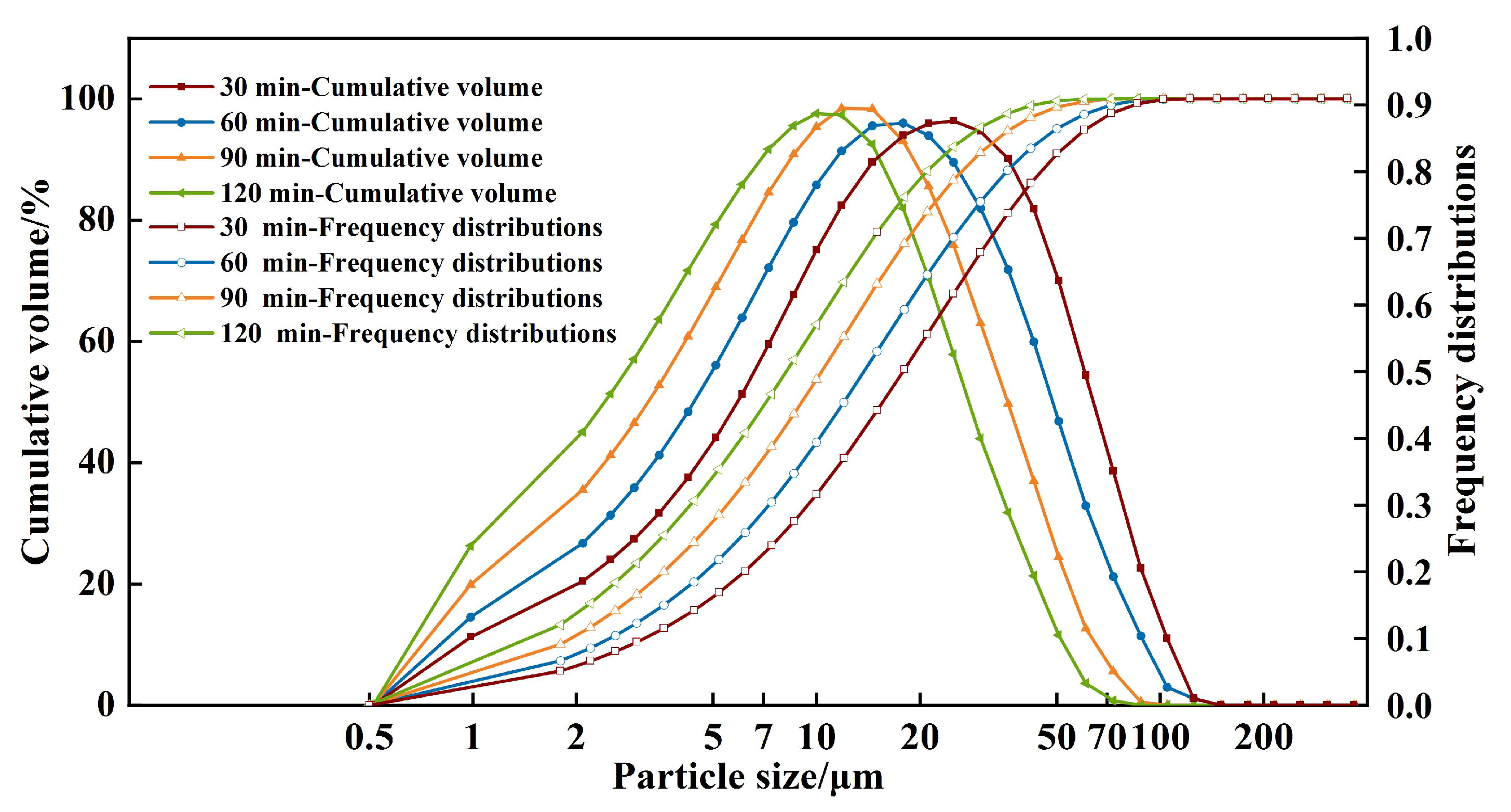
Figure 3 illustrates the particle size distribution of RWTW after mechanochemical ball milling. The duration of ball milling exerted a considerable influence on the granulometric profile, a relationship effectively modeled by the RRB distribution. Prolonged milling intervals resulted in a progressive reduction in the particle diameter, thereby confirming the efficacy of mechanical activation via ball milling in facilitating particle comminution and refinement.
Quantitative granulometric analysis utilized the median particle diameter (
D50) and the 90th percentile diameter (
D90) as primary metrics. The experimental
D50 values exhibited a sequential reduction from 15.63 µm (30 min) to 12.02 µm (60 min), 9.08 µm (90 min), and finally 7.15 µm (120 min) as the milling duration increased. These results indicate that prolonged milling simultaneously enhanced comminution efficiency and reduced the polydispersity of the particle size distribution. However, the comminution kinetics, quantified by the rate of temporal
D50 reduction, displayed two distinct kinetic regimes. An initial primary fragmentation phase (0–60 min) was characterized by a pronounced reduction in particle diameter. Conversely, a subsequent secondary refinement phase (60–120 min) featured a significant attenuation in comminution efficiency. This kinetic transition is mechanistically attributed to competing phenomena: agglomeration (driven by elevated surface energy) and strain (hardening within the silicate matrices) [
25]. The reduction in particle size is anticipated to increase the specific surface area, consequently accelerating the subsequent alkaline activation reaction.
Table 3 synthesizes the RRB parameters—characteristic particle diameter (
De) and uniformity coefficient (
n)—derived from the granulometric analysis of RWTW processed at varied milling durations. The characteristic diameter
De diminished asymptotically to 5.02 μm, concomitant with
n maximizing at 1.89 following 120 min of milling. An increase in the
n value indicates an improvement in particle uniformity, which may promote the uniformity of the reaction interface. These findings establish that extended milling simultaneously drives particle refinement toward the submicron order (
De < 6.18 μm) and enhances monodispersity (
n > 1.74), despite incipient agglomeration effects beyond 90 min.
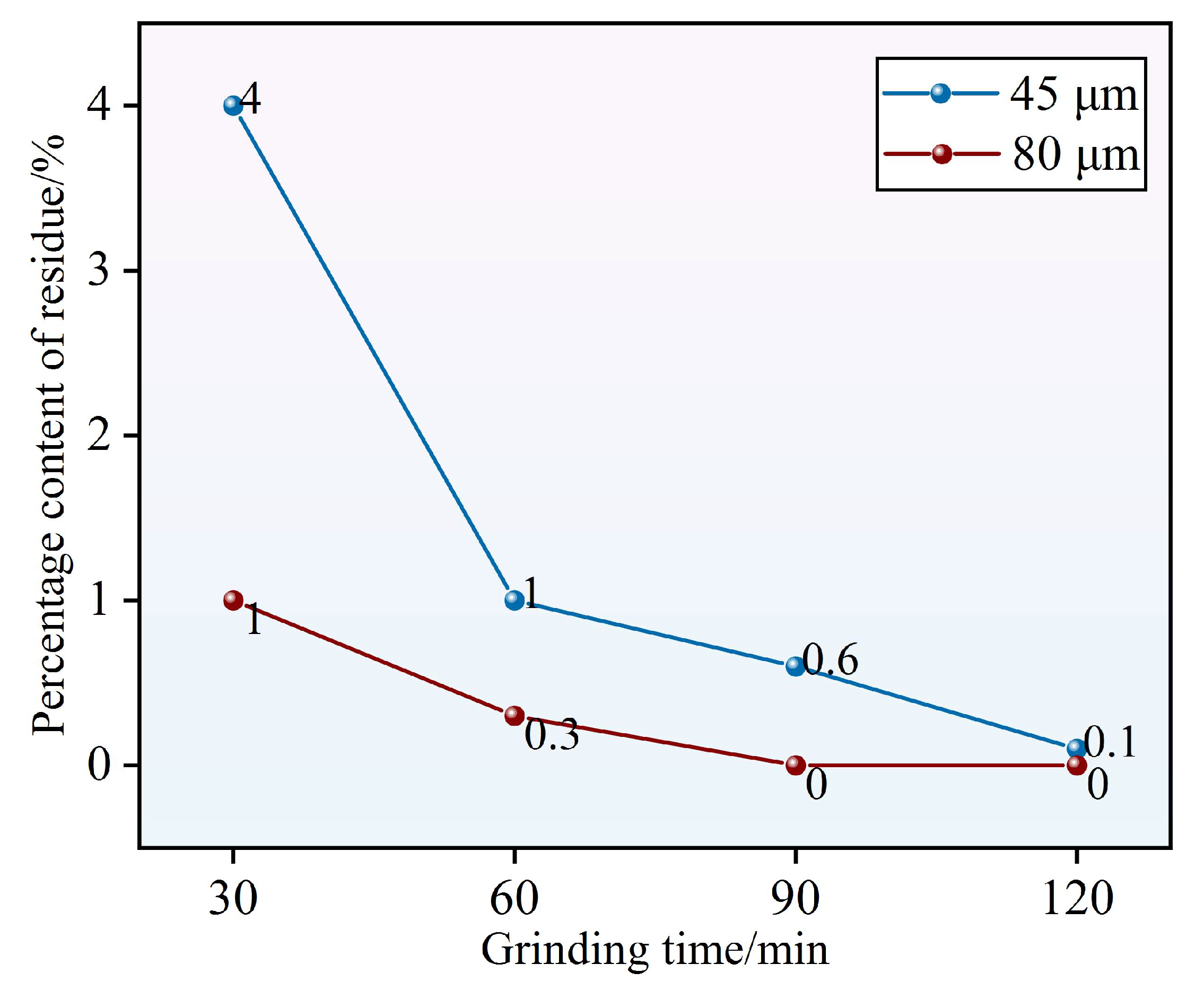
3.1.2. RWTW Sieve Residue
Figure 4 illustrates the granulometric evolution of RWTW, as characterized by cumulative residue fractions across selected sieve apertures following incremental ball milling durations. The residue fractions demonstrated a monotonic decrease with extended milling, thereby confirming the efficacy of mechanical comminution in achieving progressive particle refinement. At an 80 μm aperture, the cumulative residue decreased from 1% (30 min) to 0.3% (60 min) and was completely eliminated after 90 min of milling. Correspondingly, the residue at the 45 μm aperture diminished from 4% to 0.6%, and ultimately to 0.1% after 120 min of processing. This corresponds to a 92.5% reduction in residue within the initial 60 min, equivalent to a fragmentation efficiency of 1.54% per minute, which indicates intensive particle disintegration during the primary comminution phase. Beyond the 60 min mark, the kinetics of residue reduction attenuated markedly (to 0.11% per minute), while particle diameters asymptotically approached a comminution equilibrium, as indicated by an RRB distribution modulus (
n) converging to 1.74. This decrease in efficiency is consistent with the established theory of the fragmentation–agglomeration equilibrium [
26], where van der Waals-driven particle coalescence becomes the dominant mechanism over mechanical fragmentation at particle scales below 10 μm.
3.1.3. RWTW Reactivity
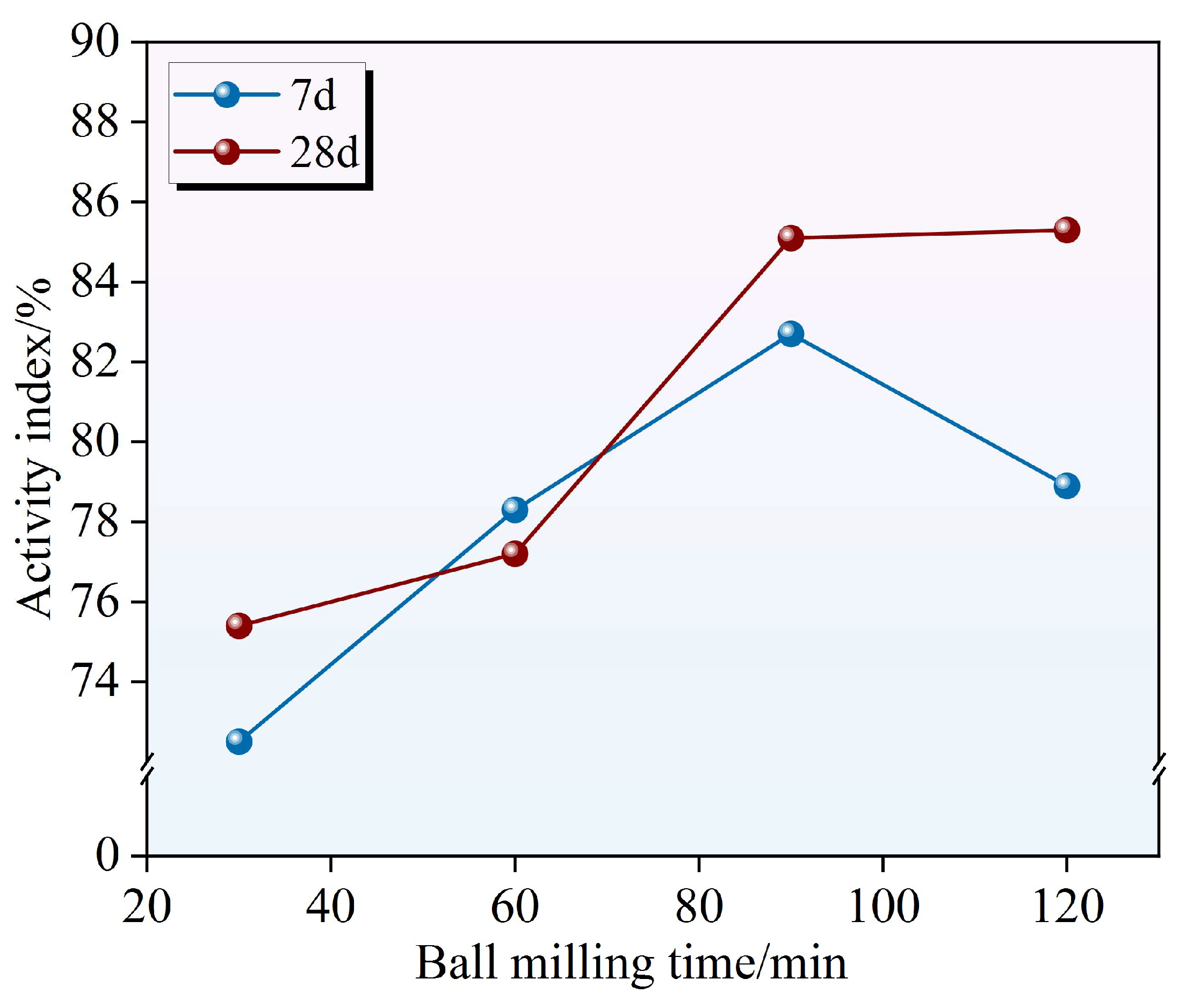
Figure 5 illustrates the mechanochemical activation profile of RWTW reactivity as a function of the ball milling duration, revealing distinct time-dependent hydration kinetics. The hydraulic activity indices exhibited a monotonic increase with an extended milling duration, culminating in a 28-day reactivity that exceeded the 7-day performance by 8.7%. After 30 min of milling, the 7-day activity index reached 72.5%, falling below the 75% threshold required for classification as a highly reactive supplementary cementitious material. An extended milling duration (60 min) increased the index to 78.3%, confirming the enhancement of dissolution through mechanochemical activation. Beyond the optimal duration (120 min), reactivity exhibited a marginal decline to 78.9%, indicating the onset of mechanochemically induced degradation. This slight reduction suggests that excessive milling may alter the surface properties or crystalline structure of RWTW, consequently impairing its reactivity [
26]. Consequently, a narrow optimal milling window (60–90 min) maximizes reactivity, while prolonged overmilling triggers detrimental phase transformations that compromise material performance.
The evolution of late-age (28-day) reactivity paralleled the early-age trends, but exhibited a significantly amplified magnitude. The 28-day indices were measured at 75.4% (30 min) and 77.2% (60 min), indicative of a characteristically delayed pozzolanic response. This trend demonstrates a fundamental trade-off in early activation: abbreviated milling prioritizes the initial dissolution kinetics at the expense of long-term reaction completeness. Following extended milling (90/120 min), the 28-day indices were elevated to 85.1% and 85.3%, asymptotically approaching the theoretical reactivity ceiling of 86%. The enhancement in late-age reactivity originates from progressive pozzolanic reactions, where silico-aluminous phases undergo dissolution and subsequent reprecipitation with portlandite (Ca(OH)
2) to form strength-imparting C-A-S-H gels (Ca/Si ≈ 1.2, Al/Si ≈ 0.3) through epitaxial growth on residual fibers [
25]. This microstructural evolution enhances the degree of gel polymerization, thereby augmenting the measured reactivity. These observations are consistent with and corroborate Liu’s mechanoactivation model for aluminosilicate precursors [
25].
3.1.4. RWTW Chemical Composition
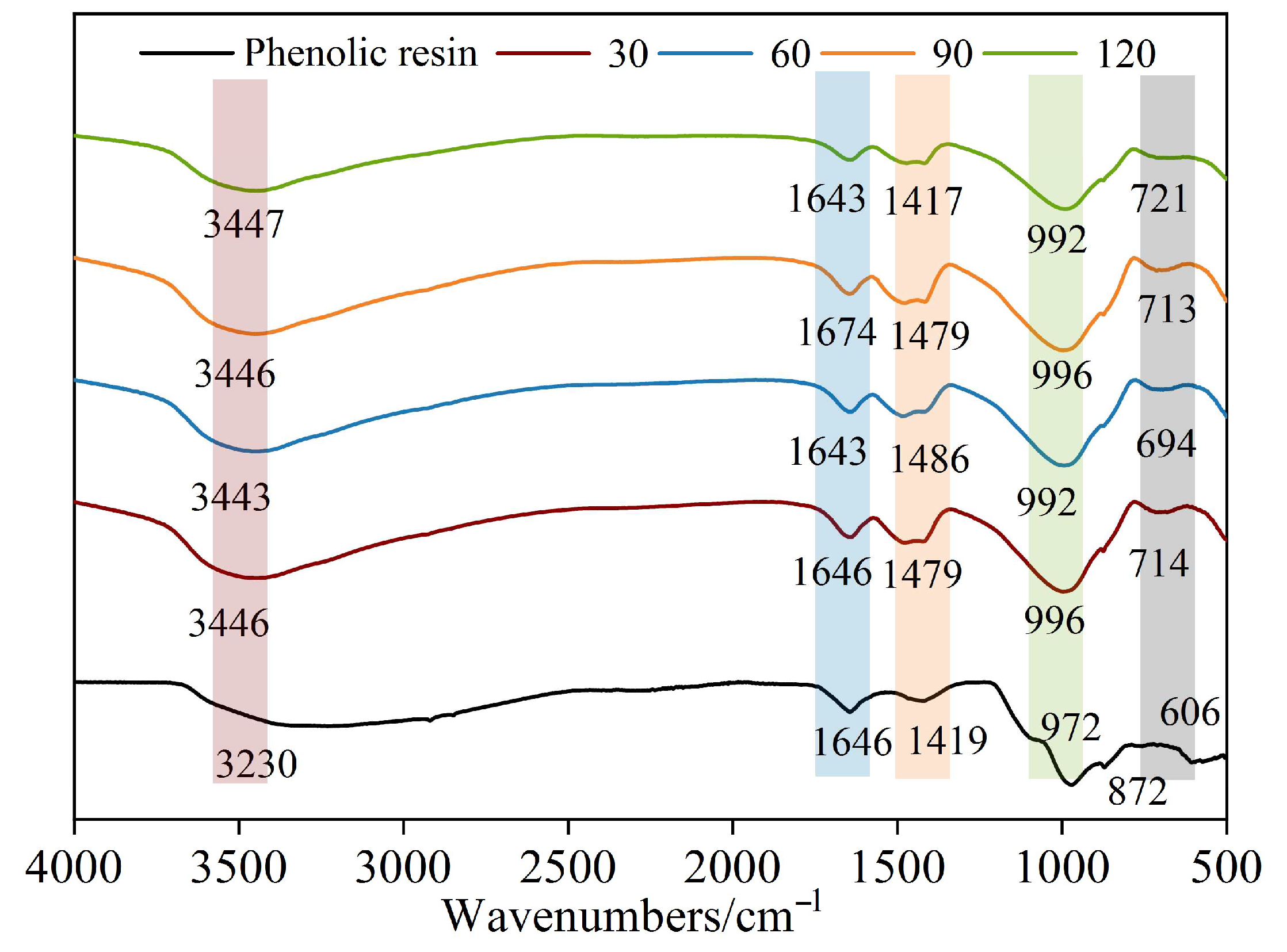
Figure 6 presents the comparative FT-IR spectra of RWTW at four distinct particle sizes and its residual organic fraction. The broad absorption band observed within the 3200–3500 cm
−1 range is attributed to O−H stretching vibrations, indicating the presence of hydroxyl groups (−OH) or adsorbed water [
27]. The intensity of this band progressively increased with an extended ball milling duration. This enhancement likely correlates with the increased specific surface area and the greater abundance of surface hydroxyl groups, confirming that ball milling modifies the surface chemical characteristics of RWTW. The absorption peak located between 996 and 992 cm
−1 originates from Si−O stretching vibrations in silicate mineral phases [
27]. A significant intensification of this peak was observed with an increasing milling time, signifying the enhanced exposure and interaction of the silicate phases, rendering their characteristic features more pronounced. This finding aligns with the observed trend in RWTW particle size reduction. Characteristic peaks within the 1500–1400 cm
−1 wavenumber region correspond to C=C skeletal vibrations of aromatic rings [
28]. The intensity of these peaks remained largely unchanged across different milling durations, suggesting that the molecular structure of organic components within the rock wool (e.g., phenolic resin) remained stable during milling. However, a gradual release of these organics into the system may occur. Prolonged milling might facilitate the partial release or transformation of specific structural elements within the phenolic resin, while its core aromatic backbone structure appears to be preserved. Absorption bands within the 1646–1674 cm
−1 range are associated with C=O stretching vibrations, indicating the potential presence of carboxylic acid groups (−COOH) or esterification products [
29]. The intensity of these bands did not exhibit significant variation with milling time. Nevertheless, the heightened reactivity of surface organics induced by extended milling could potentially lead to an increase in the number of organic acids or polar functional groups.
The FT-IR spectrum of the isolated organic fraction (black curve) reveals key features: the broad peak at 3446 cm
−1 stems from O−H stretching vibrations in phenolic rings; the peak at 3230 cm
−1 may be associated with unsaturated C−H bonds in aromatic structures [
29,
30]; the absorption bands between 1479 and 1419 cm
−1 reflect the skeletal vibrations of aromatic rings; and the C=O peak at 1646 cm
−1 confirms the presence of minor oxidation or esterification products. These characteristic peaks collectively identify the organic residue as resinous material interacting with the mineral components, while its overall aromatic framework remains largely intact. The concomitant presence of the Si−O absorption peak at 992 cm
−1 provides further evidence that the primary organic component is phenolic resin, consistent with findings from prior studies [
28].
3.1.5. RWTW Microstructure
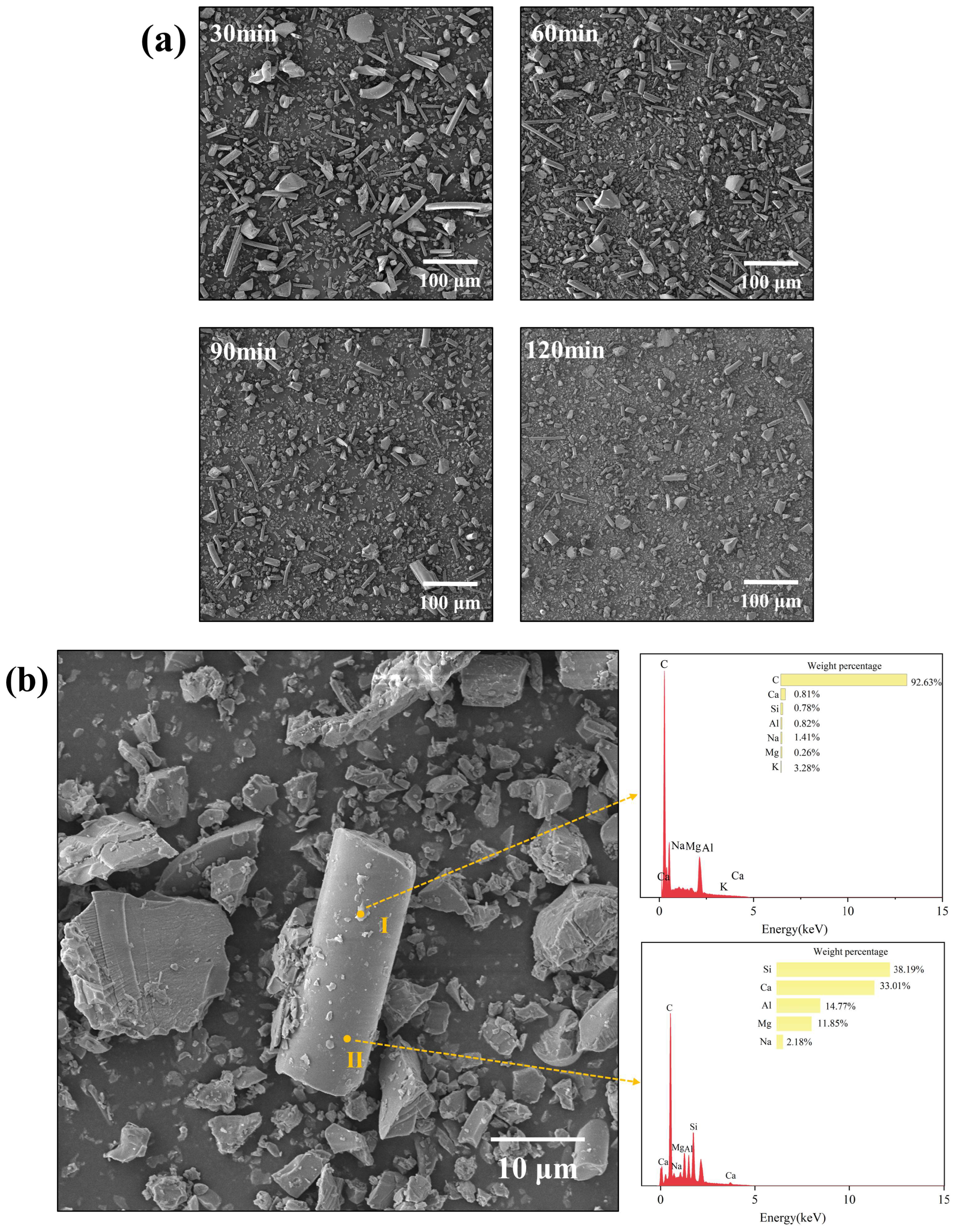
Figure 7 presents representative scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDS) micrographs of RWTW particles subjected to varying ball milling durations.
Figure 7a illustrates the morphological evolution of RWTW particles across progressive milling durations (30, 60, 90, and 120 min), revealing distinct comminution-induced microstructural transitions. Mechanochemically activated RWTW exhibits a bimodal morphological architecture, comprising both angular particulate fragments (resulting from mechanical fracture) and pristine cylindrical fibers with aspect ratios ranging from 3:1 to 10:1. The fibrous constituents maintained their topological integrity, exhibiting smooth surfaces and regular cylindrical morphologies, whereas the particulate domains displayed characteristic conchoidal fracture patterns indicative of brittle fragmentation in silicate materials. This morphological dichotomy originates from the melt-blown manufacturing process, where centrifugal fiberization at approximately 1400 °C generates intrinsically anisotropic microstructures. Progressive milling reduced the dimensions of particulate domains from >200 μm to <10 μm while simultaneously enhancing their geometric isotropy.
Figure 7b integrates high-resolution SEM with EDS elemental mapping of RWTW milled for 120 min, revealing distinct phase-specific chemical distributions. At Point I (fiber surface deposit), the carbon content reached 92.63 wt%, confirming the dominance of organic residues. This carbonaceous deposit is attributed to the pyrolysis byproducts of phenolic resins resulting from binder degradation during high-temperature fiberization processes. EDS analysis confirmed the presence of sodium (Na), aluminum (Al), silicon (Si), potassium (K), and calcium (Ca) within the RWTW particles. Sodium likely originates from residual chemical additives or mineral salts present in the raw materials. Aluminum and magnesium (Mg) primarily derive from silicate minerals or intentional additives incorporated during manufacturing. Sodium originates from fluxing agents (Na
2CO
3) added during smelting to reduce the melting point by approximately 200 °C. Silicon, as the primary constituent of rock wool, exhibited reduced abundance at Point I, likely due to surface coverage by organic residues. Aluminum and magnesium derive from basalt precursors (albite: NaAlSi
3O
8; diopside: NaAlSi
3O
8), with magnesium functioning as a network modifier in the silicate glass matrix. Potassium and calcium are attributed to residual chemical compounds from the manufacturing process. Potassium and calcium can be traced to feldspathic impurities (orthoclase: KAlSi
3O
8) and limestone flux (CaCO
3) present in the raw materials. Point analysis at Location II, positioned on a smooth surface region of an RWTW particle, revealed a composition predominantly comprising silicon (38.19 wt%), followed by calcium (33.01 wt%), aluminum (14.77 wt%), and magnesium (11.85 wt%). This elemental signature is characteristic of silicate minerals that constitute the primary inorganic matrix of RWTW, which coexists with residual organic phases [
27].
3.2. RWACCs’ Specimen Properties
3.2.1. RWACCs’ Paste Viscosity
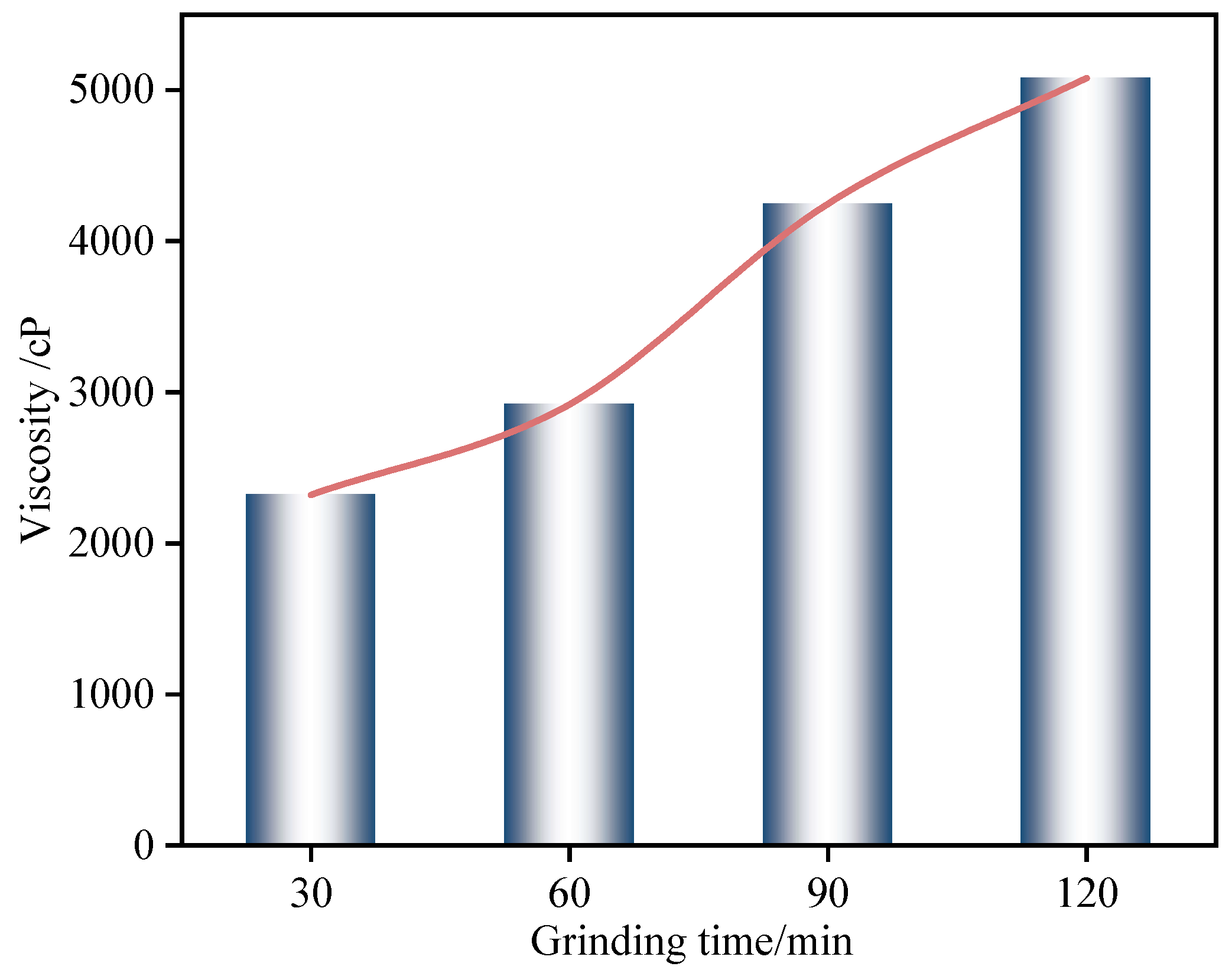
The variation in paste viscosity for RWACC specimens with different ball milling durations is presented in
Figure 8. The viscosity exhibited a continuous increase with a prolonged milling time. The measured viscosities corresponding to milling times of 30, 60, 90, and 120 min were 2320.48, 2919.42, 4247.60, and 5080.00 centipoise (cP), respectively. This progressive rise in viscosity primarily stems from the reduction in the RWTW’s particle size and the consequent increase in the specific surface area induced by extended milling. These factors enhance the intermolecular interactions (e.g., van der Waals forces and hydrogen bonding) between particles, promoting the formation of a denser paste microstructure that impedes flow.
Concurrently, prolonged ball milling accelerates alkali activation reaction kinetics. This acceleration fosters the rapid generation of hydration products and the development of a flocculated network structure. These microstructural changes further amplify the interaction forces between reaction products, thereby contributing significantly to the observed increase in viscosity.
The fixed water content directly induced a 119% viscosity increase (2320→5080 cP,
Figure 8) despite the 54% reduction in
D50, primarily because finer particles increased the specific surface area by ~130%, elevating the water demand for surface wetting and amplifying the interparticle friction and van der Waals forces, collectively causing significant workability loss (flow spread reduction from 185 mm to 102 mm). This fundamental trade-off confirms that while milling enhances reactivity, industrial processing necessitates future water optimization to balance rheology and performance.
3.2.2. RWACCs’ Setting Times
Figure 9 illustrates the influence of the ball milling duration on the setting time of RWACC specimens. A prolonged milling time resulted in a marked reduction in both the initial and final setting time. Specifically, specimens prepared with RWTW milled for 30 min exhibited an initial setting time of 70 min and a final setting time of 104 min. When the milling duration was increased to 60 min, these times decreased significantly to 38 min (initial) and 63 min (final). This acceleration in setting is attributed to the progressive refinement of rock wool particles and the concomitant increase in specific surface area induced by extended milling. These changes enhance alkali activation reaction kinetics and accelerate the formation of hydration products [
31]. The resulting flocculated microstructure promotes rapid setting and the hardening of the paste.
A further reduction occurred after 120 min of milling, yielding an initial setting time of 20 min and a final setting time of 39 min. Notably, all RWACC setting times were substantially shorter than the standard requirements for Ordinary Portland Cement (OPC) (initial setting ≥ 45 min; final setting ≤ 390 min). The fibrous and particulate structure of ball-milled RWTW presents a potential challenge: fibrous rock wool components may induce premature surface stiffening during alkali activation. This manifests as the surface stiffening of the paste while the interior remains inadequately hardened, leading to anomalously shortened setting times and potentially compromising performance [
32].
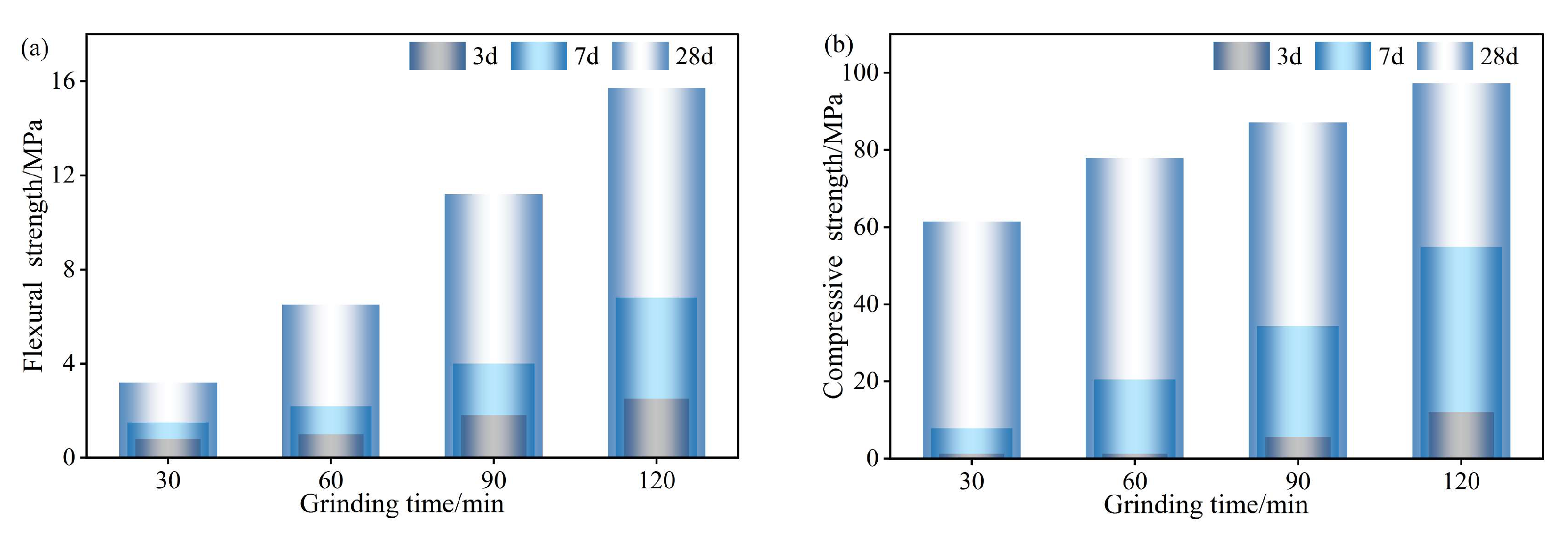
3.2.3. RWACCs’ Flexural and Compressive Strengths
Figure 10 presents the development of flexural and compressive strengths in RWACC specimens as functions of the ball milling duration. At 3 days, specimens milled for 120 min demonstrated the highest flexural and compressive strengths, measuring 2.51 MPa and 12.12 MPa, respectively. In contrast, the 30 min and 60 min milling durations yielded significantly lower strengths, with flexural values of 0.80 MPa and 1.04 MPa, and compressive values of 1.18 MPa and 1.32 MPa, respectively. A significant enhancement in strength was observed with an extended curing time, evident at both the 7-day and 28-day intervals. Notably, the specimens milled for 120 min attained remarkable 28-day flexural and compressive strengths of 15.73 MPa and 97.27 MPa, respectively, significantly outperforming all other formulations. This compressive strength (97.27 MPa) not only substantially exceeds that of conventional Ordinary Portland Cement (OPC) mortars (typically 40–50 MPa [
33]), but also rivals that of high-performance alkali-activated systems (e.g., 70–90 MPa for slag/fly ash blends [
23,
34]), underscoring the viability of RWTW as a competitive structural material.
The observed strength progression is mechanistically attributed to particle refinement: prolonged milling reduces the particle size of RWTW, thereby increasing the specific surface area available for reaction with the alkaline activator. This enhancement promotes accelerated hydration kinetics and facilitates the formation of a dense, continuous gel network—fundamental determinants that enable early strength development. The subsequent strength development across all formulations is attributed to the progressive accumulation of hydration products and concomitant microstructural densification of the gel matrix over time. However, an excessive milling duration involves a critical trade-off: ultra-fine particles may induce increased material brittleness. As demonstrated by Tao et al. [
33], extreme particle refinement can introduce excessive structural defects that compromise both tensile strength and fracture toughness. Consequently, while the moderate extension of the milling duration enhances the compressive strength and reduces the setting time, meticulous optimization is required to prevent overmilling and maintain adequate material ductility [
33,
34].
3.2.4. RWACCs’ Organic Matter Leaching
Figure 11 illustrates the leaching behavior of a reddish substance from RWACC specimens subjected to varying ball milling durations. The extent of leaching diminished progressively with an increased milling time, accompanied by a visible lightening of the reddish hue. Specimens milled for 30 min exhibited an approximately 2 mm-thick distinct red oily film on their surface. This film was virtually absent after 90 min of milling, while only trace amounts of reddish residue remained on specimens milled for 120 min. This phenomenon is mechanistically linked to the interaction with the alkali activator and surface deposition processes, with the reddish oily film originating from inherent organic components within the RWTW.
The formation of the reddish oily film likely involves dual mechanisms:
(1) The alkaline dissolution of unreacted phenolic compounds (e.g., free phenols) or low-molecular-weight oligomers derived from incompletely cured phenolic resin present in the RWTW [
7]. When exposed to highly alkaline solutions such as waterglass prior to complete curing, these uncured components can leach out. Their inherent hydrophobicity and reddish-brown coloration facilitate the formation of a distinct oily film that migrates to the paste surface;
(2) The alkali-activated leaching of metal ions (e.g., Fe and Al) from the RWTW via OH
− ions in the activator. Subsequent redox processes involving Fe
2+/Fe
3+ can lead to the precipitation of reddish-brown iron oxides (e.g., Fe
2O
3 and Fe
3O
4) [
35]. Concurrently, the gelation of sodium silicate (waterglass) under alkaline conditions promotes the formation of aluminosilicate gel structures, which accelerate the co-deposition of these iron oxides.
During paste setting and hardening, the evolving stable gel matrix confines the mobility of the leached species, resulting in the surface-localized deposition of the reddish film. Prolonged ball milling effectively mitigates both the leaching of organic constituents and the deposition of metal oxides, thereby suppressing the formation and surfacing of the reddish oily film.
3.2.5. RWACCs’ Hydration Reactions
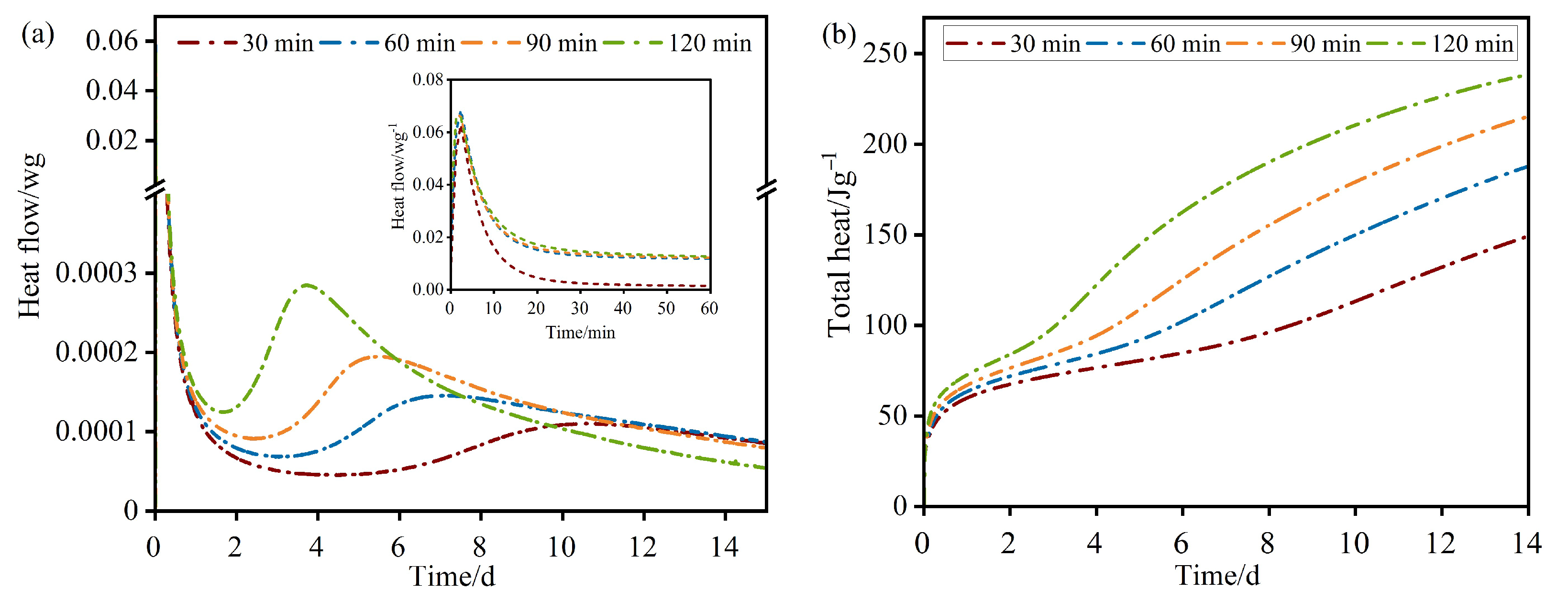
The hydration heat evolution curves for RWACC specimens across the four milling durations are presented in
Figure 12. All specimens exhibited two distinct exothermic peaks characteristic of alkali-activated systems [
28]. Notably, as the milling duration increased from 30 min to 120 min, these exothermic peaks progressively shifted towards the origin of the time axis (
Y-axis), indicating accelerated reaction kinetics.
The hydration process can be delineated into five distinct stages based on heat evolution profiles:
(1) Rapid reaction stage (≤20 min): Alkaline constituents (e.g., Na+, OH−, and silicate oligomers) from the sodium silicate solution rapidly react with surface-active species on RWTW particles (e.g., Ca2+, SiO44−, and AlO45−), generating an intense, short-duration exothermic peak. This stage involves rapid dissolution and initial gel nucleation;
(2) Dormant period (1–3 days): Reaction rates diminish significantly, yielding reduced heat output. Active mineral components continue dissolving, forming precursor species for C–S–H and N–A–S–H gels. However, the initial reaction products form a passivating layer on particle surfaces, impeding ion diffusion and reactant contact. This kinetic deceleration stage is analogous to the dormant period observed in OPC hydration;
(3) Acceleration stage (3–7 days): Marked by the second prominent exothermic peak, this stage represents the primary heat liberation phase of alkali activation. Critically, prolonged milling induced an earlier onset and higher intensity of this peak, correlating directly with increased particle fineness and reduced setting times. The highly alkaline environment accelerates dissolution and polycondensation reactions, driving the massive formation of C–S–H and N–A–S–H gels. The narrowing and intensification of the peak with milling duration reflects accelerated reaction kinetics due to an increased specific surface area and enhanced activator accessibility;
(4) Deceleration stage (>7 days): Heat evolution gradually declines as the reaction rates become diffusion-controlled. The depletion of reactants and the formation of a continuous, flocculated gel network progressively restrict ion mobility, slowing further reactions. Continued slow growth and the microstructural densification of the gel matrix occur at reduced heat liberation rates;
(5) Stabilization stage: Heat evolution approaches the baseline, signifying the near completion of the reactive processes. Residual slow reactions may involve minor unreacted phases, contributing negligibly to cumulative heat. This stage governs long-term strength development and durability through ongoing gel maturation and pore refinement.
3.2.6. Optimization of Ball Milling Duration via Weighted Sum Method
To quantitatively determine the optimum milling duration, a linear weighted sum analysis was performed using five key parameters (
Table 4). Weight assignments reflected the performance priorities:
D50 (
w1 = 0.20), reactivity index (
w2 = 0.25), flexural strength (
w3 = 0.15), compressive strength (
w4 = 0.35), and milling time inverse (
w5 = 0.05). Each parameter was normalized (0–1 scale) relative to the dataset extremes [
36,
37].
The composite score was calculated in Equation (3):
where
Sj represents the comprehensive score of the evaluated object,
i denotes the evaluation index serial number (a total of five indicators),
Xij represents the original data on the i-th indicator,
Xi,min is the minimum value of the i-th indicator,
Xi,max is the maximum value of the i-th indicator, and
wi represents the weight of the i-th indicator. The results demonstrate that 120 min of milling achieves the highest composite score (0.92), primarily due to its superior mechanical properties (97.27 MPa compressive strength) and reactivity (85.3% index), outweighing its longer processing time. Thus, 120 min is recommended as the optimum duration.
3.2.7. RWACCs’ Microstructural Evolution
Figure 13 presents SEM images illustrating microstructural changes in RWACC specimens across milling durations. Prolonged milling induced significant alterations in the hydration products and matrix morphology:
- (1)
Hydration products primarily comprised amorphous three-dimensional networks resembling cotton-like clusters. These C–A–S–H gel structures interconnected to partially fill pores [
27]. Incompletely hydrated RWTW particles exhibited exposed surfaces with visible pores and microcracks, indicating limited reaction progression;
- (2)
Particle refinement accelerated hydration kinetics, yielding denser interfacial zones and reduced porosity. Observed microcracks originated from either expansion stresses during alkali activation or volumetric changes in hydration products. Yellow-outlined regions highlight active reaction zones, where prolonged milling enhanced particle interactions. Dominant C–A–S–H gel formation continued, though incipient cracking posed potential compromises to mechanical integrity [
38];
- (3)
Reduced crack widths suggested the stabilization of hydration-induced strains. These features likely resulted from localized structural instability during gel expansion. Enhanced reaction progression in 90 min specimens manifested through advanced C–A–S–H gel formation—consistent with calorimetry data (
Figure 12). Hexagonal Ca(OH)
2 platelets (arrowed) provided partial pore filling [
39];
- (4)
Substantially improved hydration yielded denser microstructures with fewer voids. Spheroidal aluminosilicate particles (1–10 μm diameter) facilitated paste workability, while their hydration products enhanced matrix consolidation. However, excessive reaction kinetics induced the stress concentration, generating localized microcracks despite overall densification [
34].
Figure 13.
28-day microscopic morphology of RWACC specimens under different ball milling times. (a,a’) 30 min; (b,b’) 60 min; (c,c’) 90 min; and (d,d’) 120 min.
Figure 13.
28-day microscopic morphology of RWACC specimens under different ball milling times. (a,a’) 30 min; (b,b’) 60 min; (c,c’) 90 min; and (d,d’) 120 min.
Progressive particle refinement (30→120 min) promoted hydration product formation, reduced porosity/cracking, and improved mechanical performance. Optimal milling balanced enhanced reactivity against risks of over-reaction-induced defects.
A comprehensive analysis of the experimental results reveals a profound and consistent correlation between the ball milling duration and the properties of both the RWTW precursor and the resultant RWACC, underscoring the central role of mechanochemical pretreatment. An extended milling time served as the primary driver, initiating a cascade of effects: it directly dictated the reduction in particle size (D50) and the enhancement of particle uniformity (n). This refinement in granulometry was the fundamental factor that consequently increased the specific surface area and reactivity of the RWTW, which, in turn, accelerated the alkali activation kinetics. This acceleration was unequivocally demonstrated by the shift in hydration heat evolution peaks towards earlier times and the significant reduction in both the initial and final setting times. The combination of enhanced reactivity and accelerated gel formation directly translated into superior mechanical performance, with the 120 min-milled specimens achieving exceptional 28-day flexural and compressive strengths due to a denser microstructure. Furthermore, the intensified mechanical energy input facilitated the encapsulation of organic residues within the geopolymer matrix, effectively mitigating the leaching of reddish oily substances. However, this positive correlation was accompanied by a critical trade-off: the increased fineness and surface energy led to a substantial rise in paste viscosity, highlighting an important consideration for practical workability. Therefore, the optimal milling duration emerges as a balance between maximizing reactivity and mechanical strength while managing the resulting rheological challenges for future industrial applications.
3.3. Sustainability Metrics and Circular Economy Impact
The environmental and economic superiority of mechanochemical pretreatment was quantified through comparative life cycle assessment (
Table 5). Key findings demonstrate that ball milling reduces energy consumption by 89% and carbon emissions by 94% versus conventional pyrolysis, while completely eliminating VOC emissions. These metrics establish a carbon-negative pathway for RWTW valorization [
40].
3.4. Limitations and Future Research Perspectives
While this study successfully demonstrates the proof of concept for mechanochemical resin degradation and its positive impact on early-age properties, several limitations inherent to its scope must be acknowledged. These limitations, however, define critical pathways for future work:
(1) The trade-off between particle fineness and water demand was identified. The fixed water-to-binder ratio used to isolate the milling effect resulted in significantly increased viscosity, which would impair the workability of mortars or concretes on an industrial scale. Future research must focus on optimizing superplasticizers or mix designs tailored for these ultra-fine, reactive particles, in order to ensure practical applicability;
(2) This study established the effective encapsulation of organic residues but did not evaluate the long-term stability of this encapsulation under harsh service environments. A comprehensive durability assessment—including resistance to acid/sulfate attack, carbonation, freeze–thaw cycles, and, crucially, the long-term leaching behavior of encapsulated organics—is essential before field application can be considered;
(3) As noted, excessive milling may induce brittleness. Future investigations should include fracture mechanics tests (e.g., fracture toughness and characteristic length) to fully understand the impact of ultra-fine particles on the tensile and ductile performance of the composite.
Addressing these points will be the focus of our subsequent research, in order to translate this promising laboratory-scale technology into a viable, sustainable solution for construction.
4. Conclusions
This study demonstrates that mechanochemical ball milling pretreatment is a highly effective and sustainable strategy for upgrading RWTW into high-performance RWACCs. The key conclusions are summarized as follows:
(1) Prolonged ball milling (up to 120 min) effectively cleaves phenolic resin C–O bonds while preserving the mineral phase integrity of RWTW. This results in a significant reduction in the median particle size (D50 = 7.15 μm) and improved uniformity (n = 1.89), enhancing both the specific surface area and reactivity;
(2) The 120 min-milled RWTW achieved a 28-day strength activity index of 85.3%, meeting ASTM C618 standards for pozzolanic materials. The corresponding RWACC specimens exhibited exceptional mechanical properties, with flexural and compressive strengths of 15.73 and 97.27 MPa, respectively, surpassing conventional OPC and many alkali-activated systems;
(3) Extended milling shortened the setting time and promoted the rapid formation of C−A−S−H and N−A−S−H gels, leading to a denser matrix and reduced porosity. This was corroborated via calorimetry and SEM analyses, which revealed accelerated hydration and improved microstructural continuity;
(4) The mechanochemical process mitigated the leaching of phenolic residues and metal oxides, preventing the formation of surface oil films and eliminating VOC emissions. This addresses critical environmental and safety concerns in RWTW recycling;
(5) Compared to conventional pyrolysis, the proposed method reduces energy consumption by 89% and CO2 emissions by 94%, while completely avoiding secondary pollution. This aligns with circular economy principles and supports the valorization of ~1.2 million tons/year of global rock wool waste.
This work not only provides a viable pathway for the high-value recycling of RWTW, but also establishes a mechanochemical framework for managing organic-contaminated inorganic wastes. Future research should focus on optimizing water demand and workability, evaluating long-term durability under harsh conditions, and scaling up the process for industrial adoption. Multi-criteria decision-making approaches could further refine the balance between performance, economics, and sustainability.