The Role of Chemical Activation in Strengthening Iron Ore Tailings Supplementary Cementitious Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Design
2.2.1. Mechanochemical Activation
2.2.2. Sample Preparation
2.3. Experimental Methods
3. Results
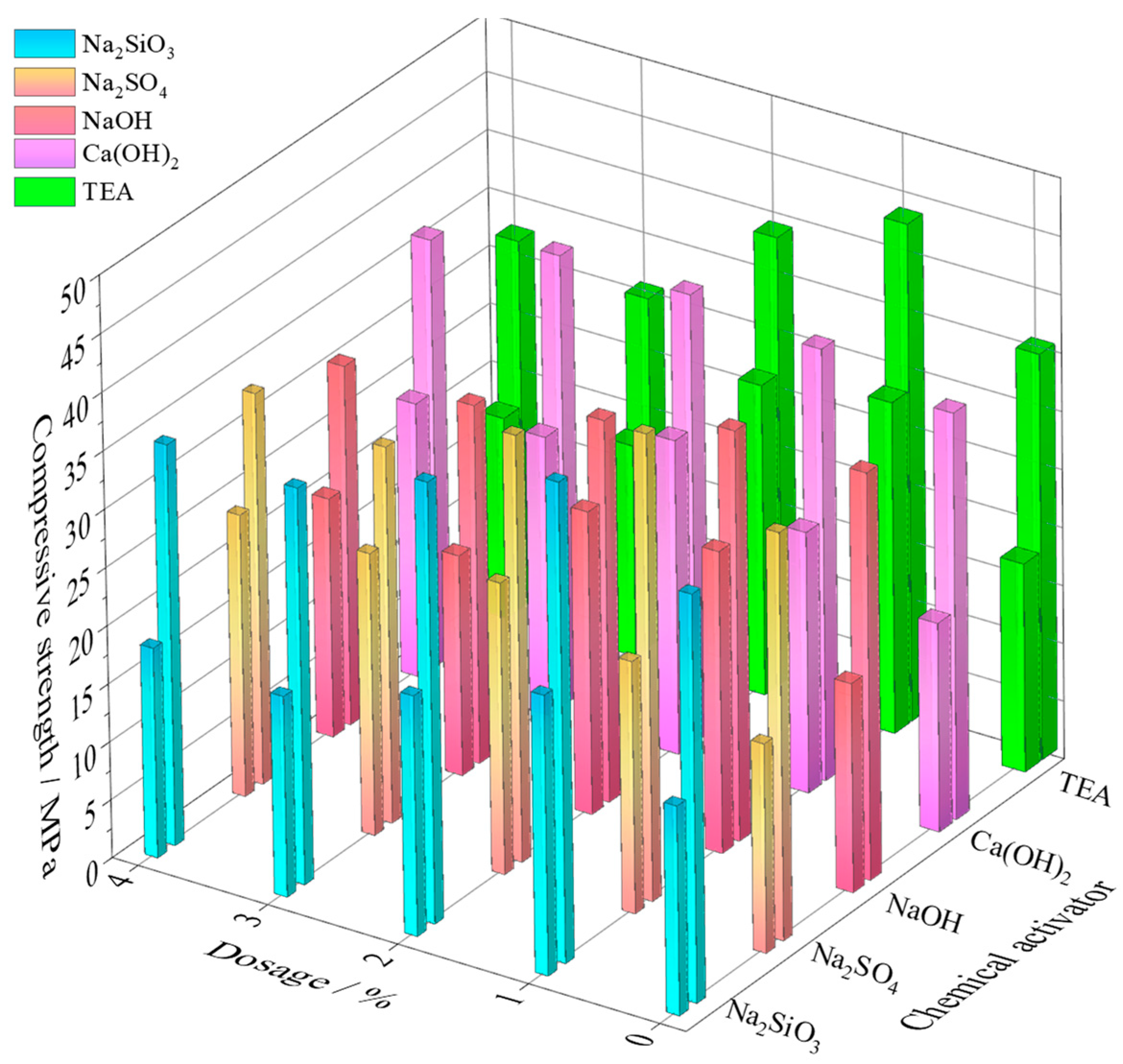
3.1. Compressive Strength of IOTs
3.1.1. Inorganic Chemical Activator Dosage Effect
3.1.2. Organic Chemical Activator Dosage Effect
3.2. XRD Characterization
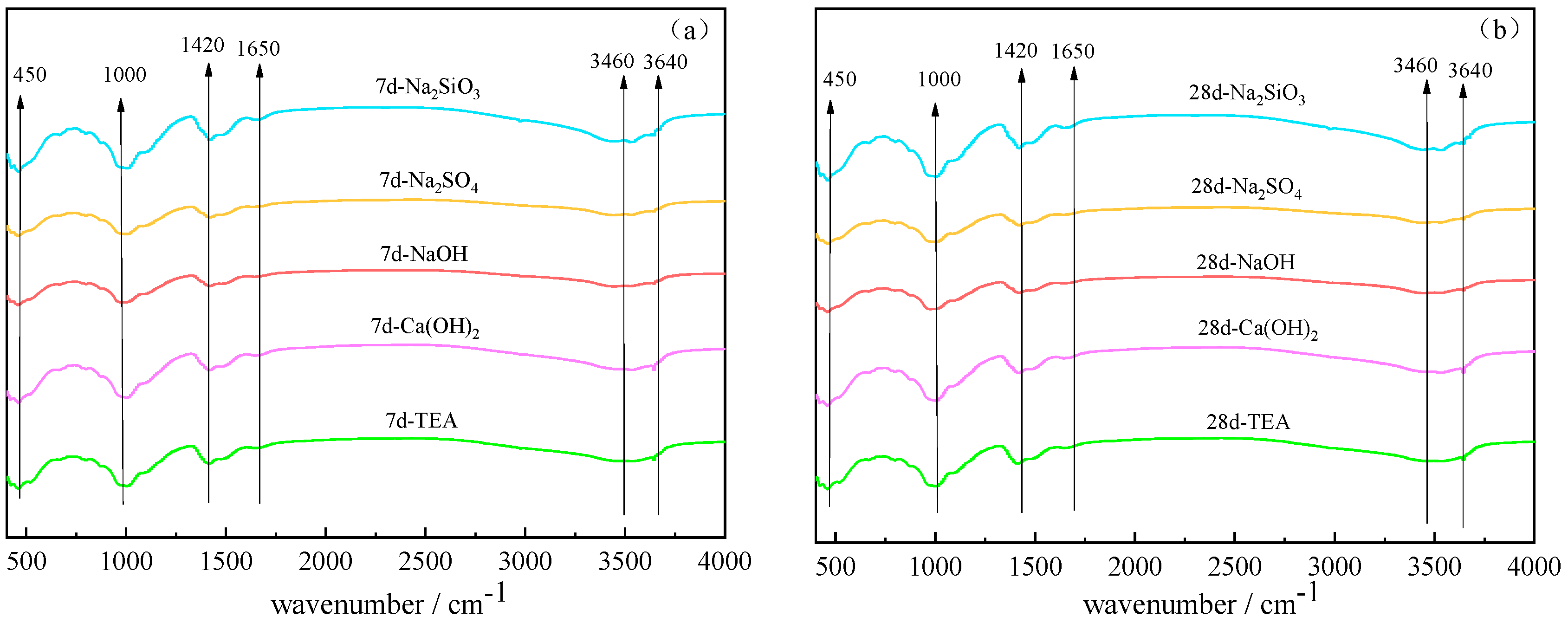
3.3. FTIR Analyses
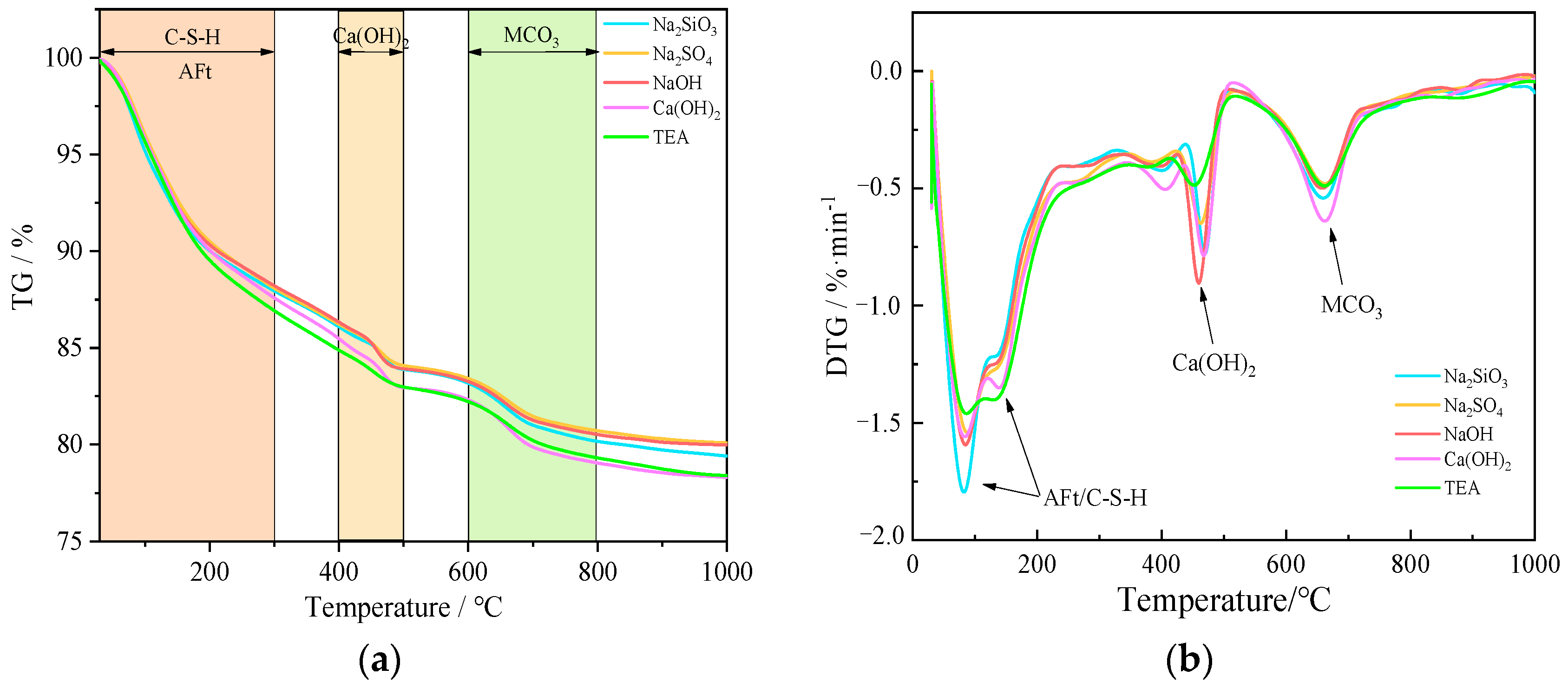
3.4. TG–DTG Analyses
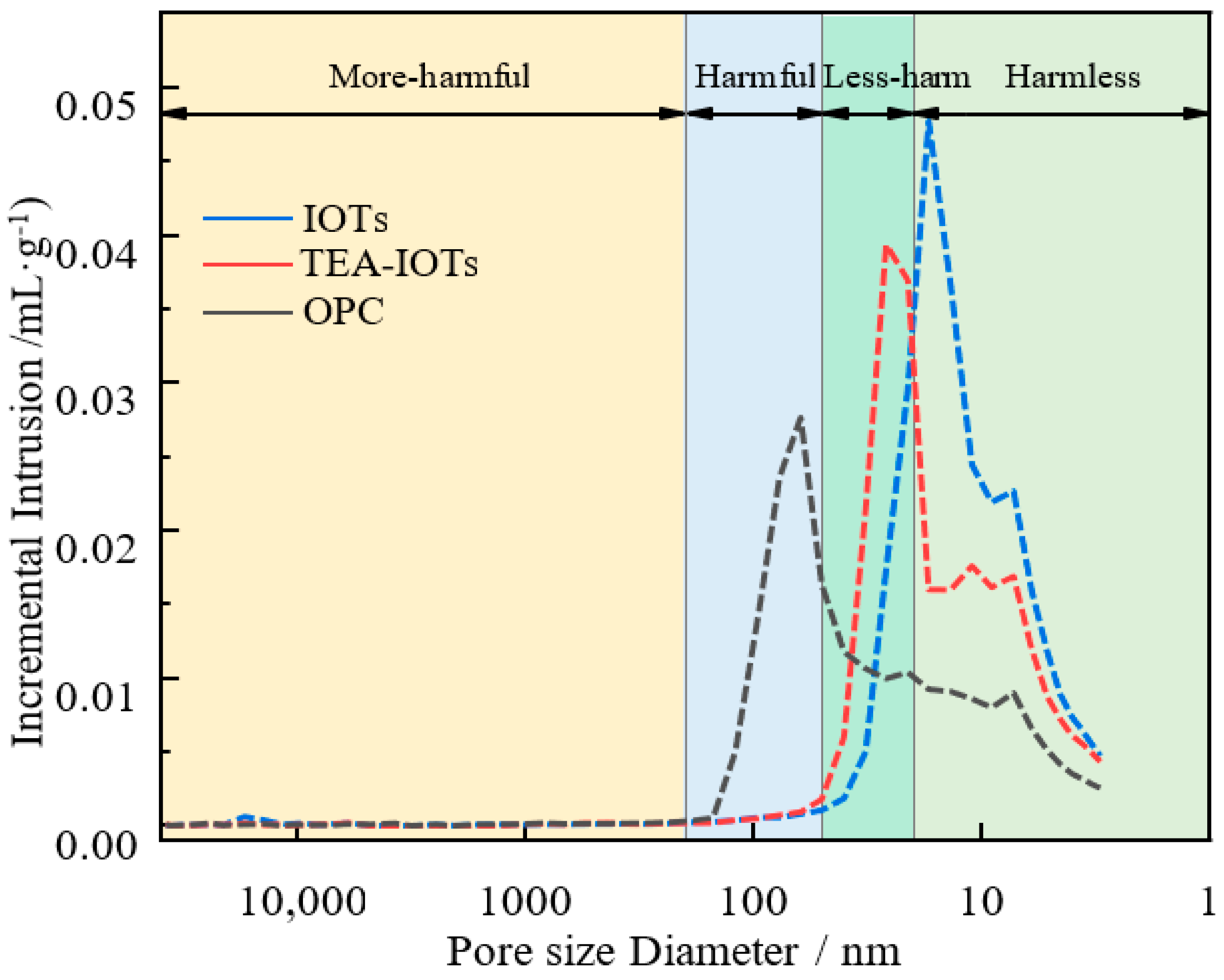
3.5. MIP Analyses
3.6. SEM Analyses
4. Discussion
4.1. Mechanism of Mechanical-Chemical Activation Coupling
4.2. Analysis of the Mechanism of Action of Chemical Activation
5. Conclusions
- (1)
- Compared to the five chemical additives, TEA maximized the compressive strength of IOTs SCMs. When TEA is used at a 1% dosage, it increases the strength of IOTs at 7 days to 28.6 MPa, and at 28 days to 42.9 MPa, comparable to OPC 42.5.
- (2)
- SCMs provide additional nucleation sites for hydration products. The addition of TEA enhances the amount of amorphous and non-crystalline CH in the system, thereby promoting the reaction of volcanic ash and counteracting the inhibitory effect of TEA on C3S. The interaction of the TEA complexation reaction with the volcanic ash reaction represents the main reason for the positive development of the late strength of IOTs SCMs.
- (3)
- The use of the chemical additive in the IOTs reduces the harmful pores and carbonate content in the cement samples. Chemical activation of the IOTs has impacted the optimization of both non-damaging and damaging porosities. This activation process revitalizes the hydration process, leading to more rapid hydration and increased pore formation around the hydration products, thereby affecting the optimization of pore structures.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, N.; Tang, B.; Liu, X. Cementitious activity of iron ore tailing and its utilization in cementitious materials, bricks and concrete. Constr. Build. Mater. 2021, 288, 123022. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Gu, X.; Nehdi, M.L.; Marani, A.; Zhang, L. Utilization of iron ore tailings with high volume in green concrete. J. Build. Eng. 2023, 72, 106585. [Google Scholar] [CrossRef]
- Li, Y.; Sheng, J.; Li, W.; Yu, M.; Zheng, X.; Wang, F. Effect of a novel spherical tailings aggregate on the macro- and mesoscopic properties of pervious concrete. Cem. Concr. Compos. 2023, 144, 105311. [Google Scholar] [CrossRef]
- Zhao, S.; Fan, J.; Sun, W. Utilization of iron ore tailings as fine aggregate in ultra-high performance concrete. Constr. Build. Mater. 2014, 50, 540–548. [Google Scholar] [CrossRef]
- Shettima, A.U.; Hussin, M.W.; Ahmad, Y.; Mirza, J. Evaluation of iron ore tailings as replacement for fine aggregate in concrete. Constr. Build. Mater. 2016, 120, 72–79. [Google Scholar] [CrossRef]
- Manjunatha, M.; Seth, D.; Kvgd, B.; Roy, S.; Tangadagi, R.B. Utilization of industrial-based PVC waste powder in self-compacting concrete: A sustainable building material. J. Clean. Prod. 2023, 428, 139428. [Google Scholar] [CrossRef]
- Xiang, J.; Qiu, J.; Zhao, Y.; Zheng, P.; Peng, H.; Fei, X. Rheology, mechanical properties, and hydration of synergistically activated coal gasification slag with three typical solid wastes. Cem. Concr. Compos. 2024, 147, 105418. [Google Scholar] [CrossRef]
- Liu, K.; Wang, S.; Quan, X.; Jing, W.; Xu, J.; Zhao, N.; Liu, B.; Ying, H. Industrial byproduct Iron ore tailings as ecofriendly materials in the utilization of cementitious composites. Constr. Build. Mater. 2023, 372, 130813. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Wang, Q.; Han, D.; Li, Z. Iron ore tailings, phosphate slags, and lithium slags as ternary supplementary cementitious materials for concrete: Study on compression strength and microstructure. Mater. Today Commun. 2023, 36, 106644. [Google Scholar] [CrossRef]
- Durdziński, P.T.; Ben Haha, M.; Bernal, S.A.; De Belie, N.; Gruyaert, E.; Lothenbach, B.; Menéndez Méndez, E.; Provis, J.L.; Schöler, A.; Stabler, C.; et al. Outcomes of the RILEM round robin on degree of reaction of slag and fly ash in blended cements. Mater. Struct. 2017, 50, 135. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Almada, B.S.; Alves da Silva Neto, G.; Fraga do Prado, D.; Paulino Aguilar, M.T.; Silva Garcia, D.C.; Brigolini Silva, G.J.; José dos Santos, W. Evaluation of the microstructure and micromechanics properties of structural mortars with addition of iron ore tailings. J. Build. Eng. 2023, 63, 105405. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, F.; Li, W.; Liu, R.; Li, G.; Wei, J. Test research on the effects of mechanochemically activated iron tailings on the compressive strength of concrete. Constr. Build. Mater. 2016, 118, 164–170. [Google Scholar] [CrossRef]
- Liu, B.; Meng, H.; Pan, G.; Zhou, H.; Li, D. Relationship between the fineness and specific surface area of iron tailing powder and its effect on compressive strength and drying shrinkage of cement composites. Constr. Build. Mater. 2022, 357, 129421. [Google Scholar] [CrossRef]
- Duarte, M.S.; Almada, B.S.; José dos Santos, W.; Lima Bessa, S.A.; Cesar da Silva Bezerra, A.; Paulino Aguilar, M.T. Influence of mechanical treatment and magnetic separation on the performance of iron ore tailings as supplementary cementitious material. J. Build. Eng. 2022, 59, 105099. [Google Scholar] [CrossRef]
- Goulart Bezerra, C.; Abelha Rocha, C.A.; Siqueira, I.S.d.; Toledo Filho, R.D. Feasibility of iron-rich ore tailing as supplementary cementitious material in cement pastes. Constr. Build. Mater. 2021, 303, 124496. [Google Scholar] [CrossRef]
- Liu, J.; Ge, X.; Liu, P.; Song, G.; Hu, Z. Experimental study on the preparation of cementitious materials from iron ore tailings by activation. Constr. Build. Mater. 2023, 385, 131409. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Z.; Cheng, Z.; Zhang, H. Effects of wet grinding combined with chemical activation on the activity of iron tailings powder. Case Stud. Constr. Mater. 2022, 17, e01385. [Google Scholar] [CrossRef]
- Carvalho, A.R.d.; Calderón-Morales, B.R.d.S.; Borba Júnior, J.C.; Oliveira, T.M.d.; Silva, G.J.B. Proposition of geopolymers obtained through the acid activation of iron ore tailings with phosphoric acid. Constr. Build. Mater. 2023, 403, 133078. [Google Scholar] [CrossRef]
- Cheah, C.B.; Tan, L.E.; Ramli, M. Recent advances in slag-based binder and chemical activators derived from industrial by-products—A review. Constr. Build. Mater. 2021, 272, 121657. [Google Scholar] [CrossRef]
- Xu, Z.; Li, W.; Sun, J.; Hu, Y.; Xu, K.; Ma, S.; Shen, X. Research on cement hydration and hardening with different alkanolamines. Constr. Build. Mater. 2017, 141, 296–306. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, S.; Zhang, Z.; Xue, J.; Guan, X. Effect of triethanolamine on the chloride binding capacity of cement paste with a high volume of fly ash. Constr. Build. Mater. 2022, 315, 125612. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Zhang, X.; Liu, W.; Liao, G.; Xu, M. Influence of triethanolamine on mechanical strength and hydration performance of blended cement containing fly ash, limestone and slag. J. Build. Eng. 2021, 44, 102879. [Google Scholar] [CrossRef]
- Heren, Z.; Ölmez, H. The influence of ethanolamines on the hydration and mechanical properties of portland cement. Cem. Concr. Res. 1996, 26, 701–705. [Google Scholar] [CrossRef]
- Cheung, J.; Jeknavorian, A.; Roberts, L.; Silva, D. Impact of admixtures on the hydration kinetics of Portland cement. Cem. Concr. Res. 2011, 41, 1289–1309. [Google Scholar] [CrossRef]
- Ghafari, E.; Ghahari, S.; Feys, D.; Khayat, K.; Baig, A.; Ferron, R. Admixture compatibility with natural supplementary cementitious materials. Cem. Concr. Compos. 2020, 112, 103683. [Google Scholar] [CrossRef]
- Kourounis, S.; Tsivilis, S.; Tsakiridis, P.E.; Papadimitriou, G.D.; Tsibouki, Z. Properties and hydration of blended cements with steelmaking slag. Cem. Concr. Res. 2007, 37, 815–822. [Google Scholar] [CrossRef]
- Yang, X.; Wu, S.; Xu, S.; Chen, B.; Chen, D.; Wang, F.; Jiang, J.; Fan, L.; Tu, L. Effects of GBFS content and curing methods on the working performance and microstructure of ternary geopolymers based on high-content steel slag. Constr. Build. Mater. 2024, 410, 134128. [Google Scholar] [CrossRef]
- Jiang, J.; Ye, Z.; Wu, J.; Yang, Q.; Li, Q.; Kong, X. Impact of triethanolamine on the hydration of Portland cement in the presence of high pozzolanic activity supplementary cementitious materials. Cem. Concr. Compos. 2024, 147, 105435. [Google Scholar] [CrossRef]
- Ilić, B.; Radonjanin, V.; Malešev, M.; Zdujić, M.; Mitrović, A. Effects of mechanical and thermal activation on pozzolanic activity of kaolin containing mica. Appl. Clay Sci. 2016, 123, 173–181. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Gu, X.; Nehdi, M.L.; Zhang, L.V. Mechanochemical activation of iron ore tailing-based ternary supplementary cementitious materials. Constr. Build. Mater. 2022, 346, 128420. [Google Scholar] [CrossRef]
- Saeki, T.; Monteiro, P.J.M. A model to predict the amount of calcium hydroxide in concrete containing mineral admixtures. Cem. Concr. Res. 2005, 35, 1914–1921. [Google Scholar] [CrossRef]
- Piao, C.; Wang, D.; Zhang, L.; Wang, C.; Liu, H. Influence of chemical mechanical coupling effect on iron ore tailings cementitious activity. Yingyong Jichu Yu Gongcheng Kexue Xuebao/J. Basic Sci. Eng. 2016, 24, 1100–1109. [Google Scholar] [CrossRef]
- Han, J.; Wang, K.; Shi, J.; Wang, Y. Mechanism of triethanolamine on Portland cement hydration process and microstructure characteristics. Constr. Build. Mater. 2015, 93, 457–462. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Kong, X.M.; Lu, Z.C.; Lu, Z.B.; Qing, Z.; Dong, B.Q.; Feng, X. Influence of triethanolamine on the hydration product of portlandite in cement paste and the mechanism. Cem. Concr. Res. 2016, 87, 64–76. [Google Scholar] [CrossRef]
- Ramachandran, V.S. Influence of triethanolamine on the hydration characteristics of tricalcium silicate. J. Appl. Chem. Biotechnol. 1972, 22, 1125–1138. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Jansen, D.; Zhang, C.; Wang, J.; Pang, X.; Yin, J. Towards a further understanding of cement hydration in the presence of triethanolamine. Cem. Concr. Res. 2020, 132, 106041. [Google Scholar] [CrossRef]
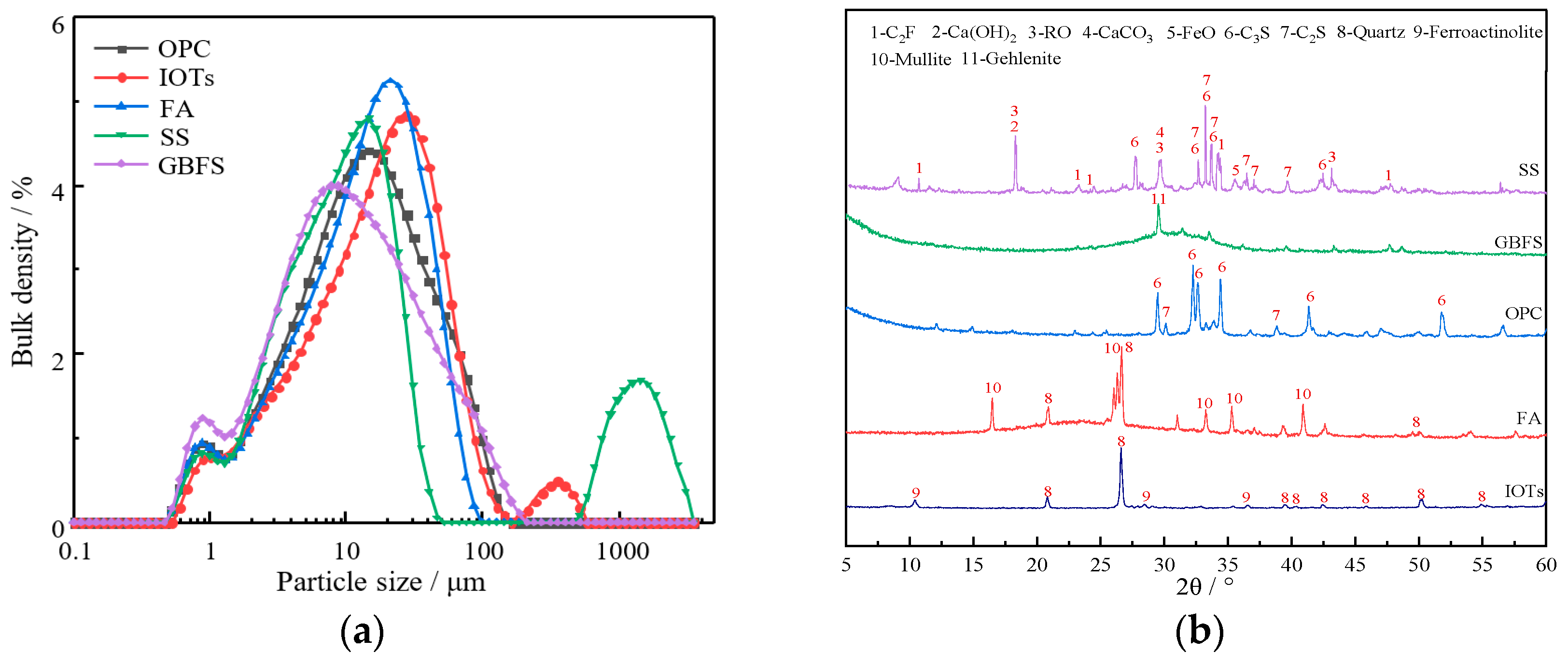


| Chemical Composition (wt. %) | Materials | ||||
|---|---|---|---|---|---|
| OPC | IOTs | SS | FA | GBFS | |
| SiO2 | 28.16 | 70.46 | 15.20 | 60.20 | 34.50 |
| Al2O3 | 7.44 | 0.91 | 2.53 | 29.39 | 17.70 |
| CaO | 54.86 | 2.68 | 42.65 | 2.49 | 34.00 |
| Fe2O3 | 2.76 | 14.54 | 27.54 | 3.78 | 1.03 |
| MgO | 2.37 | 11.29 | 6.05 | 0.51 | 6.01 |
| SO3 | 2.32 | 0.12 | 0.12 | 0.26 | 1.64 |
| Specific surface area (m2/g) | 1.01 | 0.83 | 0.97 | 1.02 | 1.23 |
| OPC | IOTs | SS | FA | GBFS | ISO Sand | Water | w/b |
|---|---|---|---|---|---|---|---|
| 315 | 45 | 30 | 40 | 20 | 1350 | 225 | 0.5 |
| Serial Number | Dehydrated Amount of Aft, C-S-H/C-(A)-S-H | Dehydrated Amount of CH | CaCO3 Amount of Decomposition | Total Loss | Ca(OH)2 Content |
|---|---|---|---|---|---|
| Na2iSO3 | 8.92% | 2.92% | 3.01% | 20.6% | 20.8% |
| Na2SO4 | 8.92% | 2.81% | 2.7% | 19.9% | 19.68% |
| NaOH | 8.92% | 3.07% | 2.73% | 20.0% | 19.82% |
| Ca(OH)2 | 9.34% | 3.15% | 3.27% | 21.6% | 22.28% |
| TEA | 9.53% | 2.66% | 2.90% | 21.7% | 19.41% |
| Serial Number | Pore Size Distribution mL/g | Porosity | |||
|---|---|---|---|---|---|
| <20 nm | 20–50 nm | 50–200 nm | >200 nm | ||
| OPC | 0.07000 | 0.00764 | 0.00574 | 0.00002 | 28% |
| IOTs | 0.16220 | 0.01523 | 0.00030 | 0.00001 | 25.6% |
| TEA-IOTs | 0.12514 | 0.01856 | 0.00051 | 0.00001 | 23.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Gu, X.; Cheng, B.; Wang, Q.; Liu, J.; Ge, X.; Yin, S. The Role of Chemical Activation in Strengthening Iron Ore Tailings Supplementary Cementitious Materials. Buildings 2024, 14, 963. https://doi.org/10.3390/buildings14040963
Hu Z, Gu X, Cheng B, Wang Q, Liu J, Ge X, Yin S. The Role of Chemical Activation in Strengthening Iron Ore Tailings Supplementary Cementitious Materials. Buildings. 2024; 14(4):963. https://doi.org/10.3390/buildings14040963
Chicago/Turabian StyleHu, Zhihang, Xiaowei Gu, Baojun Cheng, Qing Wang, Jianping Liu, Xiaowei Ge, and Shiqi Yin. 2024. "The Role of Chemical Activation in Strengthening Iron Ore Tailings Supplementary Cementitious Materials" Buildings 14, no. 4: 963. https://doi.org/10.3390/buildings14040963
APA StyleHu, Z., Gu, X., Cheng, B., Wang, Q., Liu, J., Ge, X., & Yin, S. (2024). The Role of Chemical Activation in Strengthening Iron Ore Tailings Supplementary Cementitious Materials. Buildings, 14(4), 963. https://doi.org/10.3390/buildings14040963






